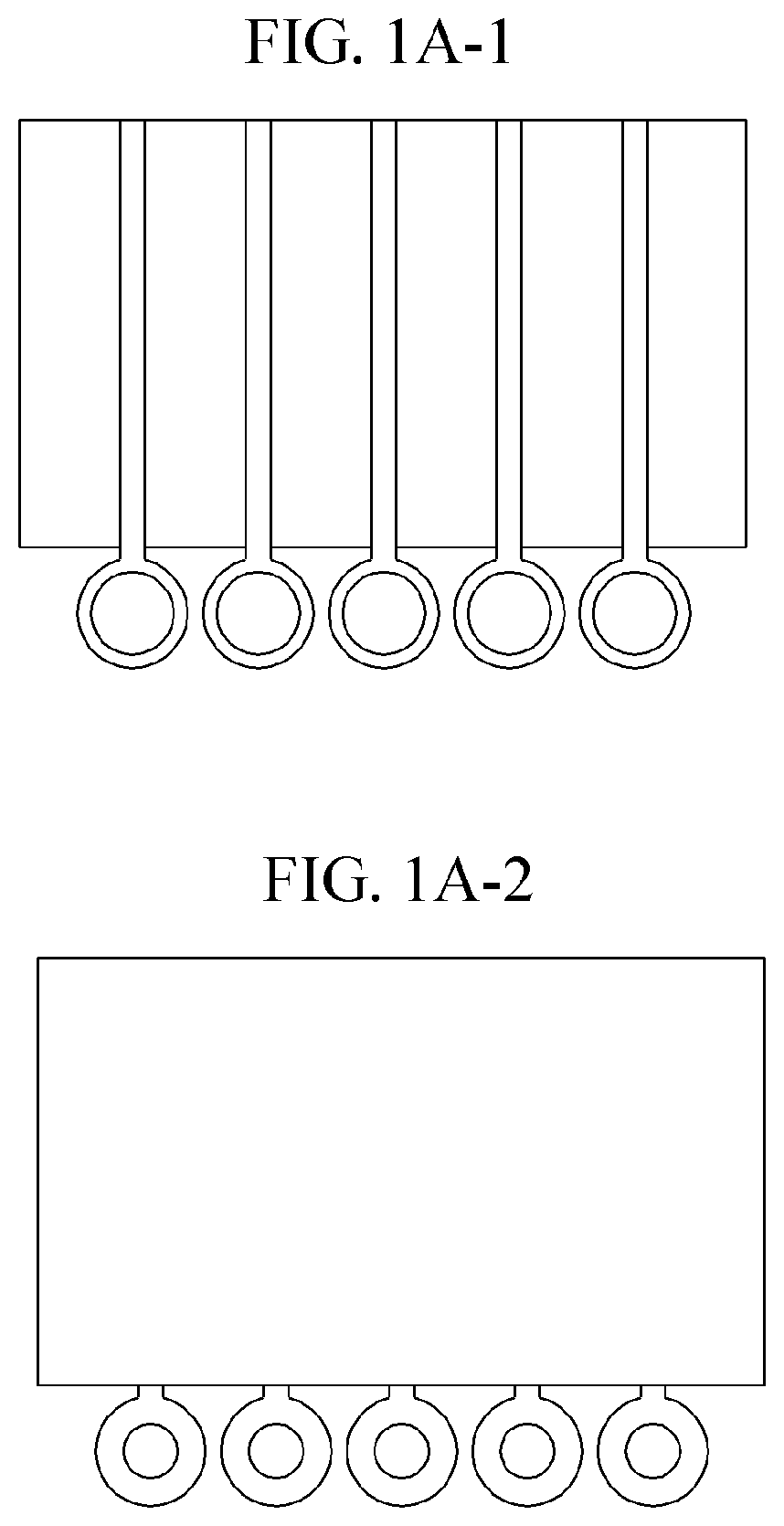
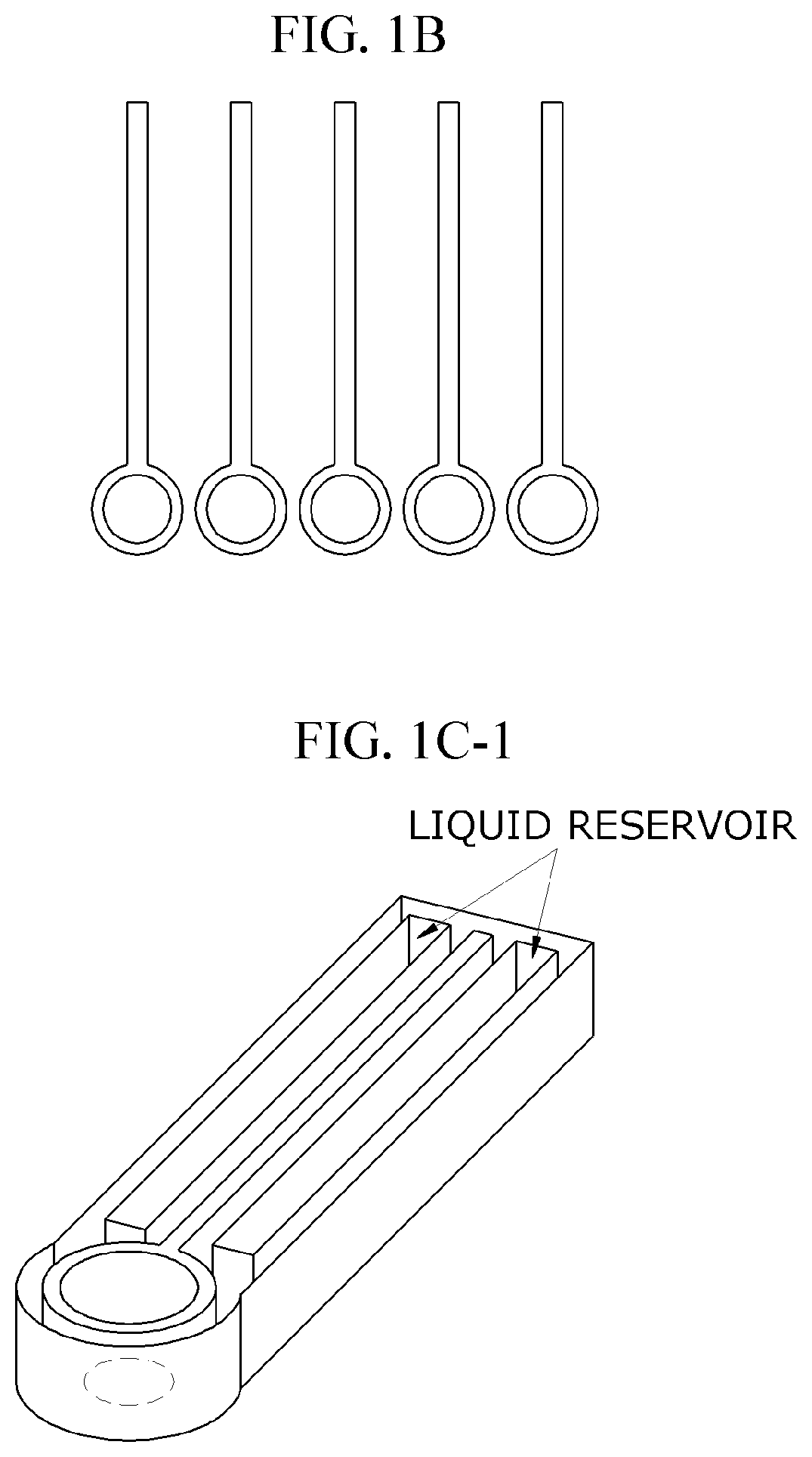
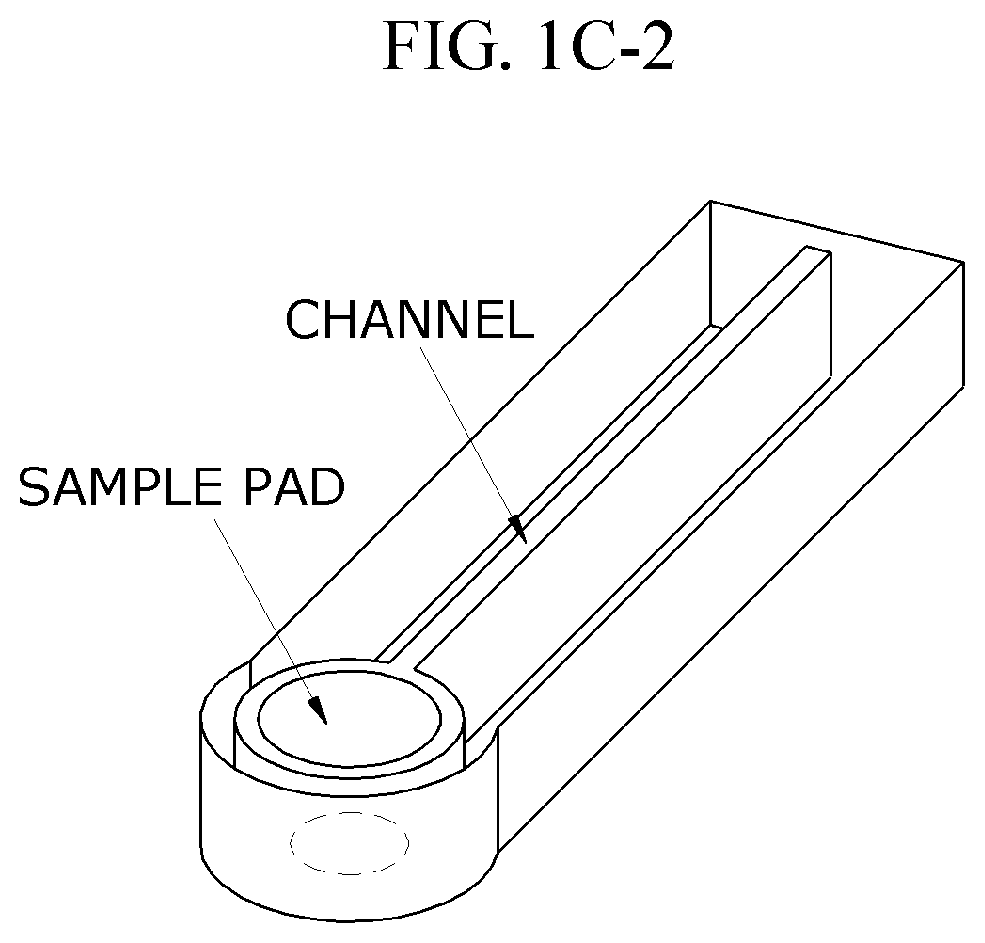
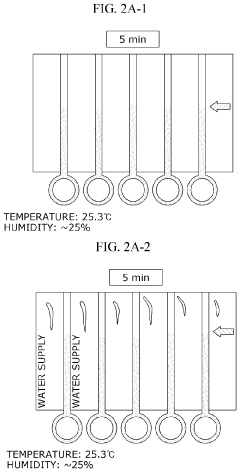
Characterization of Flow Dynamics in Microfluidic ELISA Devices
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Flow Dynamics Background and Objectives
Microfluidic Enzyme-Linked Immunosorbent Assay (ELISA) represents a significant advancement in diagnostic technology, combining the sensitivity and specificity of traditional ELISA with the advantages of microfluidic systems. The evolution of this technology spans over four decades, beginning with the development of conventional ELISA in the 1970s, followed by the emergence of microfluidics in the 1990s, and culminating in their integration in the early 2000s.
The flow dynamics within microfluidic ELISA devices constitute a critical aspect that directly impacts assay performance, sensitivity, and reproducibility. Understanding these dynamics involves the complex interplay of fluid mechanics, surface chemistry, and biomolecular interactions at the microscale. Recent technological trends indicate a shift towards more sophisticated flow control mechanisms, including passive capillary systems, active pneumatic controls, and hybrid approaches that optimize reagent delivery and washing efficiency.
The primary technical objective of characterizing flow dynamics in microfluidic ELISA devices is to establish quantitative relationships between flow parameters and assay outcomes. This includes mapping velocity profiles, shear stress distributions, and concentration gradients across microchannels and reaction chambers. Additionally, there is a growing emphasis on understanding how these parameters influence antibody-antigen binding kinetics, non-specific adsorption, and signal generation.
Another crucial objective involves developing predictive models that can accurately simulate flow behavior under various operational conditions. These models aim to optimize channel geometries, surface treatments, and flow rates to enhance assay sensitivity while minimizing sample and reagent consumption. The integration of computational fluid dynamics (CFD) with experimental validation has emerged as a powerful approach for achieving this goal.
From a broader perspective, the characterization of flow dynamics seeks to address fundamental challenges in microfluidic ELISA, such as bubble formation, channel clogging, and surface fouling. These phenomena can significantly compromise assay performance and reliability, particularly in point-of-care applications where robust operation is essential.
The technological trajectory suggests an increasing convergence with adjacent fields, including materials science for advanced substrate development, nanotechnology for enhanced signal amplification, and artificial intelligence for automated flow optimization. This interdisciplinary approach is expected to yield more integrated, efficient, and user-friendly microfluidic ELISA platforms capable of meeting the demands of next-generation diagnostic applications.
Ultimately, comprehensive characterization of flow dynamics aims to establish standardized protocols and design principles that can guide the development of microfluidic ELISA devices with improved performance, reproducibility, and clinical utility across diverse application domains, from infectious disease diagnostics to cancer biomarker detection and environmental monitoring.
The flow dynamics within microfluidic ELISA devices constitute a critical aspect that directly impacts assay performance, sensitivity, and reproducibility. Understanding these dynamics involves the complex interplay of fluid mechanics, surface chemistry, and biomolecular interactions at the microscale. Recent technological trends indicate a shift towards more sophisticated flow control mechanisms, including passive capillary systems, active pneumatic controls, and hybrid approaches that optimize reagent delivery and washing efficiency.
The primary technical objective of characterizing flow dynamics in microfluidic ELISA devices is to establish quantitative relationships between flow parameters and assay outcomes. This includes mapping velocity profiles, shear stress distributions, and concentration gradients across microchannels and reaction chambers. Additionally, there is a growing emphasis on understanding how these parameters influence antibody-antigen binding kinetics, non-specific adsorption, and signal generation.
Another crucial objective involves developing predictive models that can accurately simulate flow behavior under various operational conditions. These models aim to optimize channel geometries, surface treatments, and flow rates to enhance assay sensitivity while minimizing sample and reagent consumption. The integration of computational fluid dynamics (CFD) with experimental validation has emerged as a powerful approach for achieving this goal.
From a broader perspective, the characterization of flow dynamics seeks to address fundamental challenges in microfluidic ELISA, such as bubble formation, channel clogging, and surface fouling. These phenomena can significantly compromise assay performance and reliability, particularly in point-of-care applications where robust operation is essential.
The technological trajectory suggests an increasing convergence with adjacent fields, including materials science for advanced substrate development, nanotechnology for enhanced signal amplification, and artificial intelligence for automated flow optimization. This interdisciplinary approach is expected to yield more integrated, efficient, and user-friendly microfluidic ELISA platforms capable of meeting the demands of next-generation diagnostic applications.
Ultimately, comprehensive characterization of flow dynamics aims to establish standardized protocols and design principles that can guide the development of microfluidic ELISA devices with improved performance, reproducibility, and clinical utility across diverse application domains, from infectious disease diagnostics to cancer biomarker detection and environmental monitoring.
Market Analysis for Microfluidic ELISA Applications
The global microfluidic ELISA market is experiencing robust growth, driven by increasing demand for point-of-care testing and personalized medicine. Current market valuations place this sector at approximately 1.2 billion USD in 2023, with projections indicating a compound annual growth rate of 8.7% through 2030. This growth trajectory is supported by the expanding applications of microfluidic ELISA in clinical diagnostics, pharmaceutical research, and academic institutions.
Healthcare diagnostics represents the largest application segment, accounting for nearly 45% of the market share. The ability of microfluidic ELISA devices to provide rapid, sensitive, and multiplexed detection of biomarkers has positioned them as valuable tools in early disease detection and monitoring. Particularly notable is their adoption in oncology, infectious disease testing, and autoimmune disorder diagnostics.
Pharmaceutical and biotechnology companies constitute the second-largest market segment, utilizing microfluidic ELISA platforms for drug discovery, development, and validation processes. The reduced sample volume requirements and enhanced sensitivity of these devices align perfectly with the industry's push toward more efficient research methodologies and cost reduction strategies.
Regionally, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about advanced diagnostic technologies in countries like China, Japan, and India.
Key market drivers include technological advancements in flow dynamics optimization, increasing prevalence of chronic and infectious diseases, growing demand for point-of-care diagnostics, and rising investments in healthcare infrastructure. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid and accurate diagnostic tools.
Challenges facing market expansion include high initial investment costs, technical complexities in device fabrication, and regulatory hurdles. Additionally, standardization issues and the need for specialized training present barriers to widespread adoption in resource-limited settings.
Emerging trends indicate a shift toward integrated systems that combine sample preparation, analysis, and result interpretation in a single platform. There is also growing interest in smartphone-compatible microfluidic ELISA devices, which could significantly expand the accessibility of advanced diagnostics in remote and underserved regions.
The competitive landscape features both established medical device manufacturers and innovative startups. Strategic collaborations between technology developers and clinical partners are becoming increasingly common, accelerating the translation of research innovations into commercially viable products.
Healthcare diagnostics represents the largest application segment, accounting for nearly 45% of the market share. The ability of microfluidic ELISA devices to provide rapid, sensitive, and multiplexed detection of biomarkers has positioned them as valuable tools in early disease detection and monitoring. Particularly notable is their adoption in oncology, infectious disease testing, and autoimmune disorder diagnostics.
Pharmaceutical and biotechnology companies constitute the second-largest market segment, utilizing microfluidic ELISA platforms for drug discovery, development, and validation processes. The reduced sample volume requirements and enhanced sensitivity of these devices align perfectly with the industry's push toward more efficient research methodologies and cost reduction strategies.
Regionally, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about advanced diagnostic technologies in countries like China, Japan, and India.
Key market drivers include technological advancements in flow dynamics optimization, increasing prevalence of chronic and infectious diseases, growing demand for point-of-care diagnostics, and rising investments in healthcare infrastructure. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid and accurate diagnostic tools.
Challenges facing market expansion include high initial investment costs, technical complexities in device fabrication, and regulatory hurdles. Additionally, standardization issues and the need for specialized training present barriers to widespread adoption in resource-limited settings.
Emerging trends indicate a shift toward integrated systems that combine sample preparation, analysis, and result interpretation in a single platform. There is also growing interest in smartphone-compatible microfluidic ELISA devices, which could significantly expand the accessibility of advanced diagnostics in remote and underserved regions.
The competitive landscape features both established medical device manufacturers and innovative startups. Strategic collaborations between technology developers and clinical partners are becoming increasingly common, accelerating the translation of research innovations into commercially viable products.
Current Challenges in Microfluidic Flow Characterization
Despite significant advancements in microfluidic ELISA technology, several critical challenges persist in accurately characterizing flow dynamics within these miniaturized systems. The microscale dimensions of microfluidic channels create unique fluid behavior that differs substantially from macroscale systems, making traditional flow characterization methods inadequate. Surface tension and viscous forces dominate at this scale, resulting in laminar flow regimes that require specialized measurement approaches.
One primary challenge is the limited spatial resolution of current imaging techniques. When attempting to visualize flow patterns in microchannels with dimensions of 10-100 μm, conventional optical microscopy reaches its fundamental limits. While confocal microscopy offers improved resolution, it struggles with capturing real-time flow dynamics due to scanning speed limitations. Advanced techniques like micro-particle image velocimetry (μPIV) show promise but require expensive equipment and complex data processing algorithms.
The integration of sensing elements presents another significant obstacle. Incorporating flow sensors directly into microfluidic ELISA devices without disrupting the flow patterns remains problematic. Current approaches using external sensors often fail to capture localized flow phenomena at critical reaction zones, leading to incomplete characterization of the system. Miniaturized pressure sensors and thermal flow meters have been developed, but their integration compromises device simplicity and increases manufacturing complexity.
Material-fluid interactions further complicate flow characterization efforts. The surface properties of PDMS, glass, and other common microfluidic materials significantly influence flow behavior through phenomena like electroosmotic flow and surface adsorption. These effects vary with buffer composition, pH, and protein concentration—all critical parameters in ELISA protocols. Quantifying these interactions requires multiphysics modeling approaches that are computationally intensive and often rely on simplifying assumptions.
Temporal resolution presents an additional challenge, particularly for transient flow phenomena during sample loading or washing steps. Current characterization methods struggle to capture rapid changes in flow patterns that occur within milliseconds. This limitation is especially problematic for optimizing critical ELISA steps where timing precision directly impacts assay sensitivity and specificity.
Computational modeling approaches face validation difficulties due to the complex multiphysics nature of microfluidic ELISA systems. Models must simultaneously account for fluid dynamics, mass transport, surface interactions, and biochemical reactions. The lack of standardized validation protocols makes it difficult to assess model accuracy across different device geometries and operating conditions, limiting the predictive power of simulation-based approaches.
One primary challenge is the limited spatial resolution of current imaging techniques. When attempting to visualize flow patterns in microchannels with dimensions of 10-100 μm, conventional optical microscopy reaches its fundamental limits. While confocal microscopy offers improved resolution, it struggles with capturing real-time flow dynamics due to scanning speed limitations. Advanced techniques like micro-particle image velocimetry (μPIV) show promise but require expensive equipment and complex data processing algorithms.
The integration of sensing elements presents another significant obstacle. Incorporating flow sensors directly into microfluidic ELISA devices without disrupting the flow patterns remains problematic. Current approaches using external sensors often fail to capture localized flow phenomena at critical reaction zones, leading to incomplete characterization of the system. Miniaturized pressure sensors and thermal flow meters have been developed, but their integration compromises device simplicity and increases manufacturing complexity.
Material-fluid interactions further complicate flow characterization efforts. The surface properties of PDMS, glass, and other common microfluidic materials significantly influence flow behavior through phenomena like electroosmotic flow and surface adsorption. These effects vary with buffer composition, pH, and protein concentration—all critical parameters in ELISA protocols. Quantifying these interactions requires multiphysics modeling approaches that are computationally intensive and often rely on simplifying assumptions.
Temporal resolution presents an additional challenge, particularly for transient flow phenomena during sample loading or washing steps. Current characterization methods struggle to capture rapid changes in flow patterns that occur within milliseconds. This limitation is especially problematic for optimizing critical ELISA steps where timing precision directly impacts assay sensitivity and specificity.
Computational modeling approaches face validation difficulties due to the complex multiphysics nature of microfluidic ELISA systems. Models must simultaneously account for fluid dynamics, mass transport, surface interactions, and biochemical reactions. The lack of standardized validation protocols makes it difficult to assess model accuracy across different device geometries and operating conditions, limiting the predictive power of simulation-based approaches.
Current Methodologies for Flow Dynamics Characterization
01 Flow control mechanisms in microfluidic ELISA devices
Various flow control mechanisms are employed in microfluidic ELISA devices to optimize assay performance. These include passive capillary flow systems, pressure-driven flows, and electrokinetic methods that precisely control reagent movement through microchannels. Advanced valve systems and flow regulators enable sequential delivery of samples and reagents, ensuring proper incubation times and washing steps critical for ELISA sensitivity and specificity. These mechanisms help maintain consistent flow rates and minimize cross-contamination between reagents.- Flow control mechanisms in microfluidic ELISA devices: Various flow control mechanisms are employed in microfluidic ELISA devices to optimize assay performance. These include valves, pumps, and channel designs that regulate fluid movement through the system. Precise control of flow dynamics ensures proper mixing of reagents, adequate incubation times, and efficient washing steps, which are critical for accurate and sensitive ELISA results. Advanced flow control systems can automate the entire ELISA process, reducing manual intervention and improving reproducibility.
- Channel geometry and design for optimal flow dynamics: The geometry and design of microchannels significantly impact flow dynamics in microfluidic ELISA devices. Specific channel configurations, such as serpentine channels, herringbone structures, or gradient generators, can enhance mixing efficiency and control residence time. Channel dimensions, aspect ratios, and surface properties affect flow behavior, reagent distribution, and binding kinetics. Optimized channel designs minimize dead volumes, reduce diffusion limitations, and improve overall assay sensitivity and reproducibility.
- Detection and quantification methods in microfluidic ELISA: Microfluidic ELISA devices incorporate various detection methods to quantify analytes under specific flow conditions. These include optical detection systems (fluorescence, chemiluminescence, colorimetric), electrochemical sensors, and label-free detection techniques. The integration of these detection methods with controlled flow dynamics allows for real-time monitoring of binding events and signal amplification. Advanced detection systems can achieve higher sensitivity and lower detection limits compared to conventional ELISA methods, while requiring smaller sample volumes.
- Sample preparation and reagent delivery systems: Efficient sample preparation and reagent delivery are crucial aspects of microfluidic ELISA flow dynamics. Integrated systems for sample processing, including filtration, concentration, and dilution, help prepare samples for analysis. Controlled reagent delivery ensures precise timing and concentration of antibodies, enzymes, and substrates throughout the assay. These systems often incorporate gradient generators, droplet formation, or sequential injection techniques to optimize reagent consumption and enhance assay performance under dynamic flow conditions.
- Integration and automation of microfluidic ELISA platforms: Fully integrated microfluidic ELISA platforms combine sample preparation, reagent handling, incubation, washing, and detection into automated systems with optimized flow dynamics. These lab-on-a-chip devices enable point-of-care diagnostics with minimal user intervention. Integration strategies include multiplexing capabilities for simultaneous detection of multiple analytes, temperature control systems, and compatibility with external analytical instruments. Advanced platforms incorporate feedback control mechanisms that adjust flow parameters in real-time based on assay conditions, improving reliability and performance.
02 Channel design and geometry for optimized flow dynamics
The design and geometry of microchannels significantly impact flow dynamics in microfluidic ELISA devices. Innovations include tapered channels, serpentine structures, and specialized junction configurations that enhance mixing efficiency and control flow velocities. Surface treatments and modifications alter hydrophobicity/hydrophilicity properties to influence fluid behavior. Strategic placement of reaction chambers and detection zones optimizes antibody-antigen interactions while minimizing reagent consumption. These design considerations improve assay sensitivity and reproducibility.Expand Specific Solutions03 Integration of detection systems with flow dynamics
Advanced detection systems are integrated with microfluidic ELISA platforms to enable real-time monitoring of flow dynamics and assay results. These include optical detection methods such as fluorescence and chemiluminescence that are synchronized with fluid flow rates. Electrochemical sensors embedded within microchannels provide continuous measurement capabilities. Some designs incorporate flow-focusing techniques to enhance signal detection sensitivity. The integration of these detection systems with precisely controlled flow dynamics allows for quantitative analysis with improved limits of detection.Expand Specific Solutions04 Automated fluid handling and multiplexing capabilities
Microfluidic ELISA devices incorporate automated fluid handling systems that enable precise control over multiple reagent streams simultaneously. These systems utilize programmable pumps, digital microfluidics, and droplet manipulation techniques to coordinate complex assay protocols. Multiplexing capabilities allow for parallel processing of multiple samples or detection of multiple analytes within a single device. Advanced flow sequencing algorithms optimize reagent delivery timing and washing efficiency, reducing manual intervention and improving reproducibility across tests.Expand Specific Solutions05 Novel materials and fabrication techniques for flow optimization
Innovative materials and fabrication methods are employed to enhance flow dynamics in microfluidic ELISA devices. These include stimuli-responsive polymers that can dynamically alter channel properties, paper-based microfluidics that utilize capillary action for reagent transport, and 3D-printed structures with complex internal geometries. Surface modifications with nanomaterials improve protein binding while maintaining optimal flow characteristics. These material innovations enable more efficient mixing, reduced non-specific binding, and enhanced control over fluid behavior throughout the assay process.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic ELISA device market is in a growth phase, characterized by increasing adoption across clinical diagnostics and research applications. The market size is projected to expand significantly due to rising demand for point-of-care testing solutions and miniaturized analytical platforms. From a technical maturity perspective, the field shows varied development levels among key players. Research institutions like Centre National de la Recherche Scientifique and Massachusetts Institute of Technology are advancing fundamental flow dynamics research, while companies such as Boehringer Ingelheim microParts and Leica Microsystems CMS GmbH are commercializing refined solutions. Industrial Technology Research Institute and Japan Science & Technology Agency are bridging research-to-market gaps through collaborative innovation. The competitive landscape reveals a mix of academic institutions, established biomedical companies, and specialized microfluidics firms working to optimize flow dynamics for enhanced sensitivity, reproducibility, and throughput in ELISA applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed sophisticated microfluidic ELISA platforms that emphasize precise control over diffusion-based processes to enhance assay sensitivity. Their technology incorporates carefully designed microchannel networks that create controlled diffusion interfaces between sample and reagent streams, optimizing binding kinetics while minimizing reagent consumption. CNRS researchers have characterized flow dynamics using advanced imaging techniques including confocal microscopy with fluorescent tracers to visualize concentration gradients and mixing patterns at the microscale. Their platforms feature innovative capillary-driven flow control mechanisms that eliminate the need for external pumps, making devices more portable and accessible. CNRS has pioneered the development of surface treatments that maintain consistent hydrophilicity/hydrophobicity patterns, ensuring reproducible flow characteristics across multiple assays. Their characterization studies have established detailed relationships between channel dimensions, flow rates, and assay performance, enabling optimization for specific clinical applications. The research group has also developed mathematical models that accurately predict reagent transport and binding kinetics within microfluidic channels.
Strengths: Excellent passive flow control without external power requirements; sophisticated understanding of diffusion-based processes; good potential for low-cost, portable applications. Weaknesses: Somewhat slower assay times compared to active-flow systems; more sensitive to environmental conditions like temperature and humidity; limited dynamic range in some applications.
President & Fellows of Harvard College
Technical Solution: Harvard has developed sophisticated microfluidic ELISA platforms that focus on precise manipulation of nanoliter-scale fluid volumes. Their technology utilizes advanced fabrication techniques to create multi-layered microfluidic structures with precisely controlled channel dimensions and surface properties. Harvard researchers have characterized flow dynamics using particle image velocimetry and fluorescence-based flow visualization techniques to map velocity profiles and mixing patterns at microscale. Their platforms incorporate gradient generators that create controlled concentration profiles of reagents, enabling multiplexed assays within a single device. Harvard has also pioneered the integration of electrokinetic flow control mechanisms that allow for rapid switching between different flow regimes without mechanical valves. Their characterization work has revealed critical insights into how surface interactions and channel geometry affect protein binding kinetics in confined microfluidic environments, leading to optimized ELISA protocols with enhanced sensitivity.
Strengths: Exceptional precision in handling extremely small sample volumes; sophisticated multiplexing capabilities; advanced integration with detection systems. Weaknesses: Higher technical complexity requiring specialized expertise; potentially longer development time for new assays; higher initial investment costs compared to traditional systems.
Key Technical Innovations in Microfluidic Flow Control
Lateral-flow microfluidic chip and flow velocity control method thereof
PatentInactiveUS20190374940A1
Innovation
- Increasing vapor pressure around specific channels in the microfluidic chip by using a separate vapor supply device or a liquid reservoir adjacent to the channel, which suppresses fluid evaporation and accelerates flow velocity, allowing for efficient sequential reactions without additional processes or equipment.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Standardization and Validation Protocols for Microfluidic ELISA
The standardization and validation of microfluidic ELISA systems represent critical steps toward ensuring reliable, reproducible results across different laboratories and experimental conditions. Current protocols exhibit significant variability, hampering widespread adoption of these promising technologies in clinical and research settings.
Standardization efforts must address multiple parameters simultaneously. Flow rate calibration procedures require precise measurement techniques using reference standards to establish baseline performance metrics. Temperature control protocols need validation across the operational range (typically 20-40°C), as enzymatic reactions in ELISA are highly temperature-dependent, with optimal activity generally occurring at 37°C.
Sample preparation standardization presents particular challenges due to matrix effects from biological samples. Protocols must specify acceptable sample viscosity ranges and pre-treatment steps to minimize interference with flow dynamics. Validation studies indicate that sample dilution ratios between 1:5 and 1:20 typically provide optimal balance between sensitivity and flow consistency.
Reagent stability under microfluidic conditions requires specific validation approaches beyond traditional ELISA. Shear forces in microchannels can affect protein conformation and antibody binding efficiency. Standard operating procedures should include shelf-life determination under dynamic flow conditions rather than static storage alone.
Quality control measures must incorporate both internal and external standards. Reference materials with certified analyte concentrations enable inter-laboratory comparison and system validation. Statistical process control methods, including Levey-Jennings charts and Westgard rules, should be adapted specifically for microfluidic platforms to monitor system performance over time.
Validation protocols should follow a tiered approach. Initial qualification establishes fundamental performance characteristics including limits of detection, quantification ranges, and precision metrics. Intermediate validation compares results against reference methods, typically conventional plate-based ELISA. Advanced validation examines robustness across different operators, reagent lots, and environmental conditions.
Regulatory considerations vary by application domain. Clinical applications require compliance with ISO 13485 for medical devices and potentially FDA or equivalent regulatory approval pathways. Research applications benefit from adherence to Good Laboratory Practice (GLP) standards even when not strictly required.
Documentation frameworks must capture all critical parameters affecting flow dynamics, including channel dimensions, surface treatments, pump specifications, and environmental conditions during testing. Standardized reporting formats facilitate comparison across different microfluidic ELISA implementations and support meta-analysis of performance data.
Standardization efforts must address multiple parameters simultaneously. Flow rate calibration procedures require precise measurement techniques using reference standards to establish baseline performance metrics. Temperature control protocols need validation across the operational range (typically 20-40°C), as enzymatic reactions in ELISA are highly temperature-dependent, with optimal activity generally occurring at 37°C.
Sample preparation standardization presents particular challenges due to matrix effects from biological samples. Protocols must specify acceptable sample viscosity ranges and pre-treatment steps to minimize interference with flow dynamics. Validation studies indicate that sample dilution ratios between 1:5 and 1:20 typically provide optimal balance between sensitivity and flow consistency.
Reagent stability under microfluidic conditions requires specific validation approaches beyond traditional ELISA. Shear forces in microchannels can affect protein conformation and antibody binding efficiency. Standard operating procedures should include shelf-life determination under dynamic flow conditions rather than static storage alone.
Quality control measures must incorporate both internal and external standards. Reference materials with certified analyte concentrations enable inter-laboratory comparison and system validation. Statistical process control methods, including Levey-Jennings charts and Westgard rules, should be adapted specifically for microfluidic platforms to monitor system performance over time.
Validation protocols should follow a tiered approach. Initial qualification establishes fundamental performance characteristics including limits of detection, quantification ranges, and precision metrics. Intermediate validation compares results against reference methods, typically conventional plate-based ELISA. Advanced validation examines robustness across different operators, reagent lots, and environmental conditions.
Regulatory considerations vary by application domain. Clinical applications require compliance with ISO 13485 for medical devices and potentially FDA or equivalent regulatory approval pathways. Research applications benefit from adherence to Good Laboratory Practice (GLP) standards even when not strictly required.
Documentation frameworks must capture all critical parameters affecting flow dynamics, including channel dimensions, surface treatments, pump specifications, and environmental conditions during testing. Standardized reporting formats facilitate comparison across different microfluidic ELISA implementations and support meta-analysis of performance data.
Integration with Point-of-Care Diagnostic Systems
The integration of microfluidic ELISA devices with point-of-care (POC) diagnostic systems represents a significant advancement in healthcare technology, enabling rapid and accurate testing in resource-limited settings. This convergence leverages the precise flow dynamics characterization in microfluidic platforms to enhance diagnostic capabilities at the patient's location, reducing the need for centralized laboratory facilities.
Current POC diagnostic systems incorporating microfluidic ELISA technology demonstrate remarkable versatility across various healthcare environments. These integrated systems utilize optimized flow dynamics to ensure consistent sample delivery, reagent mixing, and waste removal—critical factors for reliable test results. The miniaturization achieved through microfluidic technology enables portable devices that maintain laboratory-quality performance while requiring minimal sample volumes and reagents.
Flow dynamics characterization has been instrumental in developing microfluidic-POC integration strategies that address common challenges in field diagnostics. By precisely controlling fluid behavior at the microscale, engineers have created systems capable of performing complex immunoassays with minimal user intervention. These advancements have led to automated sample preparation, precise reagent delivery, and controlled incubation times—all essential for reliable ELISA performance in non-laboratory settings.
Recent technological innovations have focused on enhancing the robustness of these integrated systems through adaptive flow control mechanisms. These mechanisms can compensate for environmental variations that might otherwise affect test reliability, such as temperature fluctuations or operator handling differences. Smart microfluidic platforms now incorporate feedback systems that monitor flow parameters in real-time, adjusting operational parameters to maintain optimal assay conditions.
The integration pathway typically involves modular design approaches where characterized microfluidic ELISA components interface with detection systems, power sources, and user interfaces. This modular architecture allows for customization based on specific diagnostic needs while maintaining the core benefits of controlled flow dynamics. Standardized connection protocols are emerging to facilitate this integration, enabling interoperability between components from different manufacturers.
Energy efficiency considerations have become increasingly important as these systems move toward true field deployment. Flow dynamics optimization has contributed significantly to reducing power requirements by minimizing pumping needs and enabling passive flow mechanisms where appropriate. This optimization has extended device operational time while reducing overall system complexity and cost.
Data connectivity represents another crucial aspect of integration, with modern systems incorporating wireless transmission capabilities to share test results with healthcare information systems. The characterization of flow dynamics has enabled precise timing predictions for test completion, allowing these systems to optimize data transmission schedules and power usage while maintaining result integrity.
Current POC diagnostic systems incorporating microfluidic ELISA technology demonstrate remarkable versatility across various healthcare environments. These integrated systems utilize optimized flow dynamics to ensure consistent sample delivery, reagent mixing, and waste removal—critical factors for reliable test results. The miniaturization achieved through microfluidic technology enables portable devices that maintain laboratory-quality performance while requiring minimal sample volumes and reagents.
Flow dynamics characterization has been instrumental in developing microfluidic-POC integration strategies that address common challenges in field diagnostics. By precisely controlling fluid behavior at the microscale, engineers have created systems capable of performing complex immunoassays with minimal user intervention. These advancements have led to automated sample preparation, precise reagent delivery, and controlled incubation times—all essential for reliable ELISA performance in non-laboratory settings.
Recent technological innovations have focused on enhancing the robustness of these integrated systems through adaptive flow control mechanisms. These mechanisms can compensate for environmental variations that might otherwise affect test reliability, such as temperature fluctuations or operator handling differences. Smart microfluidic platforms now incorporate feedback systems that monitor flow parameters in real-time, adjusting operational parameters to maintain optimal assay conditions.
The integration pathway typically involves modular design approaches where characterized microfluidic ELISA components interface with detection systems, power sources, and user interfaces. This modular architecture allows for customization based on specific diagnostic needs while maintaining the core benefits of controlled flow dynamics. Standardized connection protocols are emerging to facilitate this integration, enabling interoperability between components from different manufacturers.
Energy efficiency considerations have become increasingly important as these systems move toward true field deployment. Flow dynamics optimization has contributed significantly to reducing power requirements by minimizing pumping needs and enabling passive flow mechanisms where appropriate. This optimization has extended device operational time while reducing overall system complexity and cost.
Data connectivity represents another crucial aspect of integration, with modern systems incorporating wireless transmission capabilities to share test results with healthcare information systems. The characterization of flow dynamics has enabled precise timing predictions for test completion, allowing these systems to optimize data transmission schedules and power usage while maintaining result integrity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!