Optimization of Reagent Mixing in Microfluidic ELISA Chips
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Reagent Mixing Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has evolved significantly since its inception in the 1970s, transforming from traditional plate-based formats to advanced microfluidic platforms. This evolution represents a paradigm shift in diagnostic capabilities, offering enhanced sensitivity, reduced sample volumes, and accelerated analysis times. Microfluidic ELISA technology integrates the principles of traditional immunoassays with the advantages of microscale fluid manipulation, creating powerful diagnostic tools with broad applications in healthcare, environmental monitoring, and biomedical research.
The optimization of reagent mixing within microfluidic ELISA chips addresses a fundamental challenge in this technology's development trajectory. Efficient mixing is critical for ensuring uniform distribution of reagents, maximizing binding interactions, and ultimately improving assay sensitivity and reproducibility. Historical approaches to this challenge have progressed from simple diffusion-based mixing to more sophisticated techniques involving active and passive mixing strategies, each with distinct advantages and limitations.
Current technological trends indicate a growing emphasis on developing mixing strategies that maintain the benefits of microfluidic platforms while overcoming inherent limitations related to laminar flow conditions. The field is witnessing convergence between microfluidics, materials science, and computational modeling to create innovative solutions that enhance mixing efficiency without compromising other performance parameters.
The primary objective of optimizing reagent mixing in microfluidic ELISA chips is to achieve rapid, homogeneous distribution of reagents throughout the reaction chambers while minimizing sample and reagent consumption. This optimization aims to reduce analysis time, improve detection limits, and enhance reproducibility—factors critical for clinical and point-of-care applications. Additionally, effective mixing strategies must be compatible with mass production techniques to facilitate widespread adoption.
Secondary objectives include developing mixing mechanisms that maintain the structural integrity of biological molecules, operate with minimal external power requirements, and integrate seamlessly with existing detection systems. The ideal solution would balance mixing efficiency with practical considerations such as fabrication complexity, operational simplicity, and cost-effectiveness.
The technological trajectory suggests that future developments will likely focus on adaptive mixing systems that can adjust parameters based on sample characteristics, reagent properties, and assay requirements. Integration with artificial intelligence for real-time optimization represents an emerging frontier that could revolutionize how mixing processes are controlled and monitored in microfluidic ELISA platforms.
Understanding the historical context, current challenges, and future directions of reagent mixing optimization provides essential groundwork for developing innovative solutions that can advance microfluidic ELISA technology toward its full potential in diverse application domains.
The optimization of reagent mixing within microfluidic ELISA chips addresses a fundamental challenge in this technology's development trajectory. Efficient mixing is critical for ensuring uniform distribution of reagents, maximizing binding interactions, and ultimately improving assay sensitivity and reproducibility. Historical approaches to this challenge have progressed from simple diffusion-based mixing to more sophisticated techniques involving active and passive mixing strategies, each with distinct advantages and limitations.
Current technological trends indicate a growing emphasis on developing mixing strategies that maintain the benefits of microfluidic platforms while overcoming inherent limitations related to laminar flow conditions. The field is witnessing convergence between microfluidics, materials science, and computational modeling to create innovative solutions that enhance mixing efficiency without compromising other performance parameters.
The primary objective of optimizing reagent mixing in microfluidic ELISA chips is to achieve rapid, homogeneous distribution of reagents throughout the reaction chambers while minimizing sample and reagent consumption. This optimization aims to reduce analysis time, improve detection limits, and enhance reproducibility—factors critical for clinical and point-of-care applications. Additionally, effective mixing strategies must be compatible with mass production techniques to facilitate widespread adoption.
Secondary objectives include developing mixing mechanisms that maintain the structural integrity of biological molecules, operate with minimal external power requirements, and integrate seamlessly with existing detection systems. The ideal solution would balance mixing efficiency with practical considerations such as fabrication complexity, operational simplicity, and cost-effectiveness.
The technological trajectory suggests that future developments will likely focus on adaptive mixing systems that can adjust parameters based on sample characteristics, reagent properties, and assay requirements. Integration with artificial intelligence for real-time optimization represents an emerging frontier that could revolutionize how mixing processes are controlled and monitored in microfluidic ELISA platforms.
Understanding the historical context, current challenges, and future directions of reagent mixing optimization provides essential groundwork for developing innovative solutions that can advance microfluidic ELISA technology toward its full potential in diverse application domains.
Market Analysis for Microfluidic Immunoassay Technologies
The global microfluidic immunoassay market has experienced substantial growth, valued at approximately $2.1 billion in 2022 and projected to reach $4.3 billion by 2028, representing a compound annual growth rate (CAGR) of 12.7%. This growth is primarily driven by increasing demand for point-of-care testing, advancements in healthcare infrastructure, and the rising prevalence of chronic diseases requiring frequent diagnostic testing.
The microfluidic ELISA segment specifically holds a significant market share due to its advantages in sensitivity, specificity, and reduced sample volume requirements compared to traditional ELISA methods. The optimization of reagent mixing in these chips addresses a critical market need for improved assay performance and reliability, potentially expanding applications across clinical diagnostics, pharmaceutical research, and food safety testing.
Healthcare facilities represent the largest end-user segment, accounting for approximately 45% of the market share. Research institutions follow at 30%, with pharmaceutical and biotechnology companies comprising 20%. The remaining 5% is distributed among food safety testing laboratories and other applications. This distribution highlights the diverse market applications for optimized microfluidic ELISA technologies.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%), Asia-Pacific (20%), and the rest of the world (10%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.3% during the forecast period, driven by increasing healthcare expenditure, growing awareness about early disease diagnosis, and expanding research activities in countries like China, Japan, and India.
Key market drivers include the growing demand for rapid and accurate diagnostic solutions, increasing prevalence of infectious diseases and chronic conditions, technological advancements in microfluidic fabrication, and the shift toward personalized medicine. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities.
Challenges in market adoption include high initial development costs, technical complexities in manufacturing optimized mixing systems, regulatory hurdles, and competition from established traditional immunoassay methods. The average cost of developing a new microfluidic ELISA platform ranges from $2-5 million, creating barriers for smaller companies entering the market.
Consumer trends indicate growing preference for portable, user-friendly diagnostic devices with minimal sample requirements and rapid results. Healthcare providers increasingly value systems that can be integrated with existing laboratory information systems and electronic health records, creating opportunities for developers focusing on connectivity and data management solutions alongside technical optimization of reagent mixing.
The microfluidic ELISA segment specifically holds a significant market share due to its advantages in sensitivity, specificity, and reduced sample volume requirements compared to traditional ELISA methods. The optimization of reagent mixing in these chips addresses a critical market need for improved assay performance and reliability, potentially expanding applications across clinical diagnostics, pharmaceutical research, and food safety testing.
Healthcare facilities represent the largest end-user segment, accounting for approximately 45% of the market share. Research institutions follow at 30%, with pharmaceutical and biotechnology companies comprising 20%. The remaining 5% is distributed among food safety testing laboratories and other applications. This distribution highlights the diverse market applications for optimized microfluidic ELISA technologies.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%), Asia-Pacific (20%), and the rest of the world (10%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 15.3% during the forecast period, driven by increasing healthcare expenditure, growing awareness about early disease diagnosis, and expanding research activities in countries like China, Japan, and India.
Key market drivers include the growing demand for rapid and accurate diagnostic solutions, increasing prevalence of infectious diseases and chronic conditions, technological advancements in microfluidic fabrication, and the shift toward personalized medicine. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities.
Challenges in market adoption include high initial development costs, technical complexities in manufacturing optimized mixing systems, regulatory hurdles, and competition from established traditional immunoassay methods. The average cost of developing a new microfluidic ELISA platform ranges from $2-5 million, creating barriers for smaller companies entering the market.
Consumer trends indicate growing preference for portable, user-friendly diagnostic devices with minimal sample requirements and rapid results. Healthcare providers increasingly value systems that can be integrated with existing laboratory information systems and electronic health records, creating opportunities for developers focusing on connectivity and data management solutions alongside technical optimization of reagent mixing.
Current Challenges in Microfluidic Reagent Mixing
Despite significant advancements in microfluidic ELISA chip technology, reagent mixing remains one of the most challenging aspects in these miniaturized systems. The fundamental issue stems from the laminar flow regime that dominates at the microscale, where Reynolds numbers are typically less than 100. This laminar flow characteristic inherently prevents turbulent mixing, which is the primary mechanism for rapid reagent mixing in conventional laboratory settings. Consequently, mixing in microfluidic channels relies predominantly on molecular diffusion, a significantly slower process that can become a rate-limiting step in ELISA protocols.
The geometric constraints of microfluidic channels further exacerbate mixing challenges. With typical channel dimensions in the range of 10-500 micrometers, the surface area-to-volume ratio is substantially higher than in macroscale systems. This results in dominant surface forces and viscous effects that can impede efficient mixing. Additionally, the small volumes involved (typically nanoliters to microliters) make it difficult to implement conventional mixing strategies without significant sample loss or dilution.
Temperature gradients and surface tension effects present additional complications in microfluidic ELISA systems. Even minor temperature variations across the chip can create localized convection currents that disrupt controlled mixing processes. Similarly, surface tension at liquid-air interfaces can generate Marangoni flows that introduce unpredictability into mixing dynamics, particularly at reagent introduction points.
The timing precision required for ELISA protocols adds another layer of complexity. Many immunoassay reactions are kinetically sensitive, requiring precise control over reagent exposure times. Inefficient mixing can lead to concentration gradients within reaction chambers, resulting in spatially heterogeneous reaction kinetics and ultimately compromising assay sensitivity and reproducibility. This is particularly problematic for quantitative ELISA applications where consistent reaction conditions are essential for accurate analyte quantification.
Material compatibility issues further constrain mixing solution options. Many active mixing approaches require integration of external components such as electrodes or actuators, which must be compatible with both the chip substrate materials and the biological reagents. Certain mixing strategies may introduce localized heating, electrical fields, or mechanical stresses that could potentially denature antibodies or affect antigen-antibody binding kinetics, directly impacting assay performance.
Scaling and manufacturing considerations also present significant challenges. Complex mixing structures that perform well in laboratory prototypes often prove difficult to mass-produce reliably. Features such as herringbone ridges, serpentine channels, or obstacle arrays require precise fabrication techniques that may increase production costs and reduce manufacturing yield, limiting commercial viability.
The geometric constraints of microfluidic channels further exacerbate mixing challenges. With typical channel dimensions in the range of 10-500 micrometers, the surface area-to-volume ratio is substantially higher than in macroscale systems. This results in dominant surface forces and viscous effects that can impede efficient mixing. Additionally, the small volumes involved (typically nanoliters to microliters) make it difficult to implement conventional mixing strategies without significant sample loss or dilution.
Temperature gradients and surface tension effects present additional complications in microfluidic ELISA systems. Even minor temperature variations across the chip can create localized convection currents that disrupt controlled mixing processes. Similarly, surface tension at liquid-air interfaces can generate Marangoni flows that introduce unpredictability into mixing dynamics, particularly at reagent introduction points.
The timing precision required for ELISA protocols adds another layer of complexity. Many immunoassay reactions are kinetically sensitive, requiring precise control over reagent exposure times. Inefficient mixing can lead to concentration gradients within reaction chambers, resulting in spatially heterogeneous reaction kinetics and ultimately compromising assay sensitivity and reproducibility. This is particularly problematic for quantitative ELISA applications where consistent reaction conditions are essential for accurate analyte quantification.
Material compatibility issues further constrain mixing solution options. Many active mixing approaches require integration of external components such as electrodes or actuators, which must be compatible with both the chip substrate materials and the biological reagents. Certain mixing strategies may introduce localized heating, electrical fields, or mechanical stresses that could potentially denature antibodies or affect antigen-antibody binding kinetics, directly impacting assay performance.
Scaling and manufacturing considerations also present significant challenges. Complex mixing structures that perform well in laboratory prototypes often prove difficult to mass-produce reliably. Features such as herringbone ridges, serpentine channels, or obstacle arrays require precise fabrication techniques that may increase production costs and reduce manufacturing yield, limiting commercial viability.
Current Approaches to Optimize Reagent Mixing in Microchips
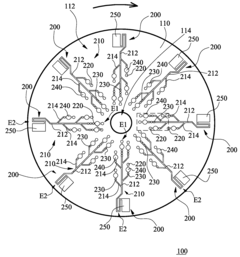
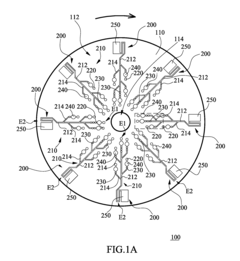
01 Microfluidic mixing mechanisms for ELISA reagents
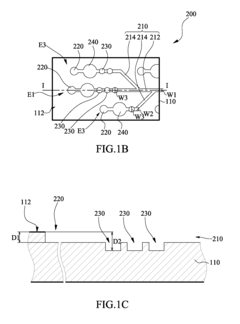
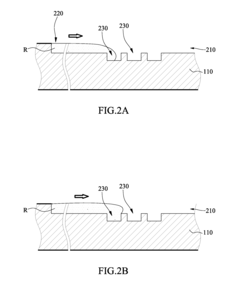
Various microfluidic mixing mechanisms can be integrated into ELISA chips to enhance reagent mixing efficiency. These include passive mixers utilizing channel geometries like serpentine channels or herringbone structures that create chaotic advection, and active mixers employing external forces such as acoustic waves, magnetic fields, or electrokinetic effects. These mixing strategies help overcome diffusion limitations in laminar flow conditions, ensuring homogeneous distribution of reagents and improving assay sensitivity and reproducibility.- Microfluidic mixing mechanisms for ELISA reagents: Various microfluidic mixing mechanisms can be integrated into ELISA chips to enhance reagent mixing efficiency. These include passive mixers utilizing channel geometries like serpentine channels, herringbone structures, or obstacles that create chaotic advection. Active mixing methods employ external forces such as acoustic waves, magnetic fields, or electrokinetic effects to improve mixing performance. These mechanisms ensure homogeneous distribution of reagents, which is critical for consistent and sensitive ELISA results.
- Integrated reagent storage and automated delivery systems: Microfluidic ELISA chips can incorporate integrated reagent storage compartments and automated delivery systems. These systems may include pre-loaded reagent chambers, blister packs, or dried reagent spots that can be reconstituted on-chip. Automated delivery mechanisms control the precise timing and volume of reagent release through valves, pumps, or capillary forces. This integration minimizes manual handling, reduces contamination risks, and enables point-of-care applications with simplified workflows.
- Multi-chamber designs for sequential ELISA reactions: Multi-chamber microfluidic designs facilitate sequential ELISA reactions by controlling reagent flow through different reaction zones. These designs incorporate chambers for sample preparation, antibody binding, washing, enzyme reactions, and detection. The chambers are interconnected through microchannels with precisely controlled dimensions to manage flow rates and residence times. This architecture enables complete ELISA protocols to be performed with minimal user intervention while maintaining high sensitivity and reproducibility.
- Surface modification techniques for enhanced biomolecule immobilization: Surface modification techniques can be applied to microfluidic ELISA chips to enhance biomolecule immobilization and reduce non-specific binding. These include chemical treatments to create functional groups, plasma treatments to alter surface energy, or coating with capture proteins like streptavidin. Optimized surfaces improve antibody orientation and accessibility, leading to higher sensitivity and lower detection limits. These modifications also help maintain reagent activity during mixing and washing steps.
- Digital microfluidics for droplet-based ELISA: Digital microfluidics enables droplet-based ELISA by manipulating discrete droplets containing reagents on an array of electrodes. This approach allows precise control over droplet generation, transport, mixing, splitting, and merging through electrowetting forces. Droplet-based systems offer advantages including reduced reagent consumption, faster reaction kinetics, and multiplexed analysis capabilities. The technology enables parallel processing of multiple samples and can be integrated with various detection methods for quantitative analysis.
02 Automated reagent delivery and sequential mixing systems
Automated systems for precise delivery and sequential mixing of ELISA reagents in microfluidic chips enable controlled reaction timing and reduced manual intervention. These systems incorporate programmable pumps, valves, and flow controllers to manage the introduction and mixing of sample, antibodies, washing buffers, and detection reagents. The automation ensures consistent timing between reaction steps, minimizes cross-contamination, and allows for multiplexed assays with different reagent combinations across multiple detection chambers.Expand Specific Solutions03 On-chip reagent storage and release mechanisms
Innovative approaches for storing and releasing ELISA reagents directly on microfluidic chips eliminate the need for external reagent handling. These include dried reagent spots that dissolve upon fluid introduction, encapsulated reagents in dissolvable films or hydrogels, and compartmentalized storage chambers with breakable seals. Such integration simplifies assay protocols, reduces user error, ensures reagent stability during storage, and enables point-of-care applications where laboratory infrastructure is unavailable.Expand Specific Solutions04 Digital microfluidics for droplet-based ELISA
Digital microfluidic platforms manipulate discrete droplets containing ELISA reagents rather than continuous flows. These systems use electrowetting, acoustic forces, or surface tension gradients to control individual droplet movement, merging, mixing, and splitting operations. The droplet-based approach offers advantages including reduced reagent consumption, faster reaction kinetics due to high surface-to-volume ratios, and flexibility in assay design through programmable droplet manipulation sequences.Expand Specific Solutions05 Integrated detection systems for microfluidic ELISA
Microfluidic ELISA chips incorporate various detection systems directly integrated with the mixing and reaction chambers. These include optical detection methods such as fluorescence, chemiluminescence, and colorimetric measurements, as well as electrochemical and impedance-based sensing approaches. The integration of detection components with reagent mixing systems enables real-time monitoring of reactions, quantitative analysis without external equipment, and enhanced sensitivity through optimized signal collection geometries.Expand Specific Solutions
Leading Companies in Microfluidic ELISA Technology
The microfluidic ELISA chip reagent mixing optimization market is in a growth phase, with increasing adoption across biomedical research and clinical diagnostics. The global market size is expanding rapidly, driven by demand for point-of-care testing and lab-on-chip technologies. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include Emulate Inc., specializing in organ-on-chip platforms; Roche Diagnostics Operations, leveraging its diagnostic expertise; and academic powerhouses like University of California and École Polytechnique Fédérale de Lausanne driving fundamental research. Industrial players such as Toshiba, Samsung Electronics, and Konica Minolta bring manufacturing scale, while specialized firms like Tecan Trading AG and Oxford Nanoimaging provide targeted solutions. The competitive landscape features collaboration between academic institutions and industry partners to overcome technical challenges in fluid dynamics, surface chemistry, and automation.
The Regents of the University of California
Technical Solution: The University of California has developed advanced microfluidic ELISA platforms utilizing passive mixing techniques through serpentine channel designs and herringbone structures. Their technology incorporates Dean flow principles in curved microchannels to enhance reagent mixing efficiency without external power requirements. The system employs precision-controlled flow rates and optimized channel geometries to create controlled vortices that significantly reduce diffusion limitations. Their latest innovations include integrated pneumatic valves for precise reagent metering and timing control, allowing for automated multi-step ELISA protocols with minimal user intervention. The platform achieves detection sensitivities in the pg/mL range while reducing sample volumes to under 10 μL and total assay times to less than 30 minutes - a substantial improvement over conventional ELISA methods.
Strengths: Exceptional mixing efficiency through passive mechanisms reduces power requirements and simplifies device operation. The integrated valve system enables precise timing control for complex multi-step assays. Weaknesses: The complex microfabrication requirements may increase manufacturing costs, and the system may require specialized expertise for operation and maintenance.
EMULATE INC
Technical Solution: Emulate has pioneered Organ-on-Chip technology with integrated microfluidic ELISA capabilities specifically optimized for reagent mixing efficiency. Their platform features a two-layer microfluidic system separated by a porous membrane, with the upper channel containing cultured cells and the lower channel delivering reagents. The company has developed proprietary surface treatments that minimize non-specific binding while enhancing antibody-antigen interactions. Their mixing optimization incorporates controlled pulsatile flow patterns that mimic physiological conditions while enhancing reagent mixing through controlled turbulence. The system utilizes precision micropumps to create oscillatory flows that significantly improve mixing efficiency without damaging biological samples. This approach has demonstrated up to 85% reduction in reagent consumption while maintaining or improving detection sensitivity compared to conventional methods.
Strengths: The biomimetic approach provides physiologically relevant conditions while simultaneously optimizing reagent mixing, offering unique advantages for drug development applications. The system's compatibility with live cell analysis enables real-time monitoring of cellular responses. Weaknesses: The specialized equipment requirements and higher initial investment costs may limit accessibility for some laboratories.
Key Patents and Research in Microfluidic Mixing Optimization
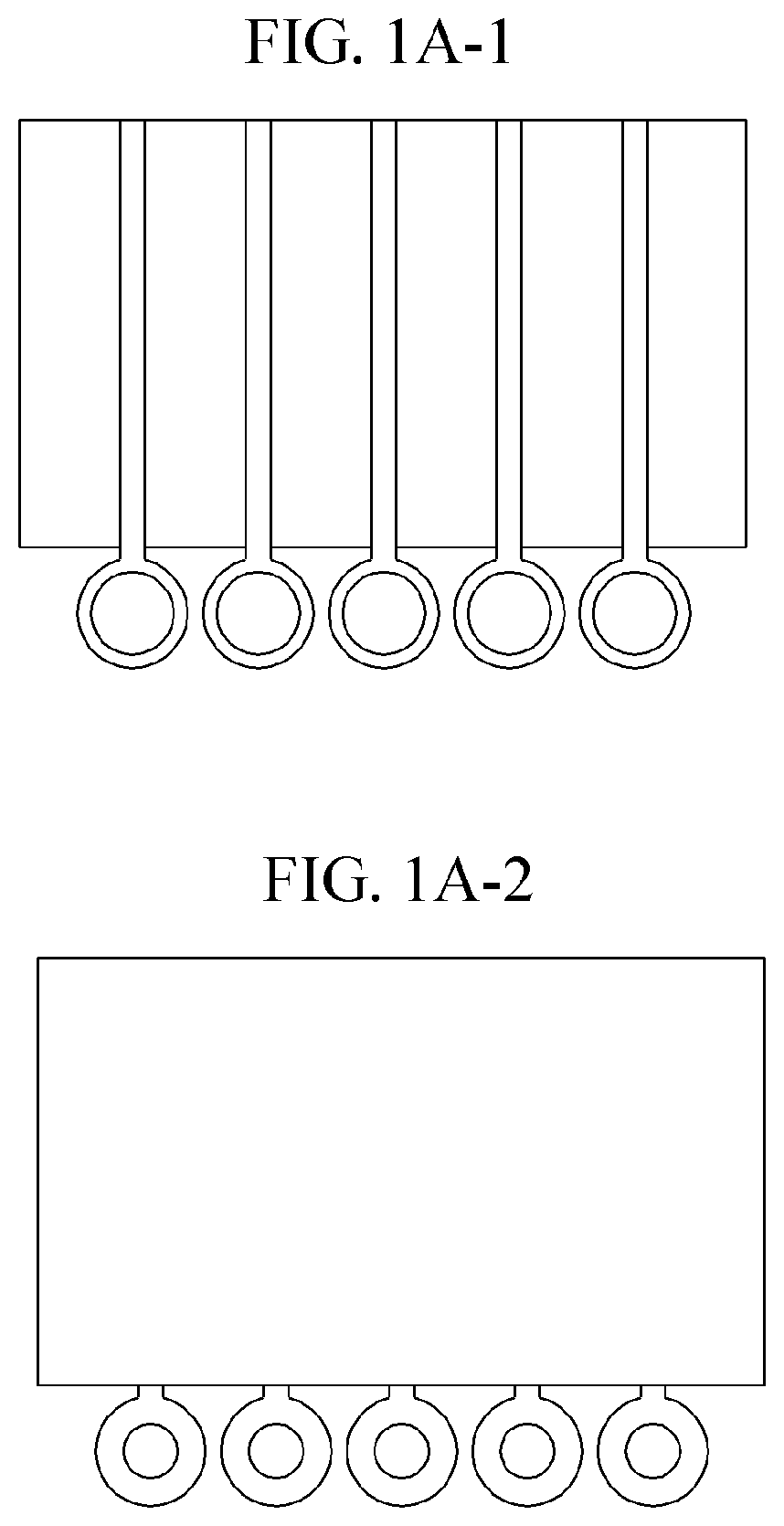
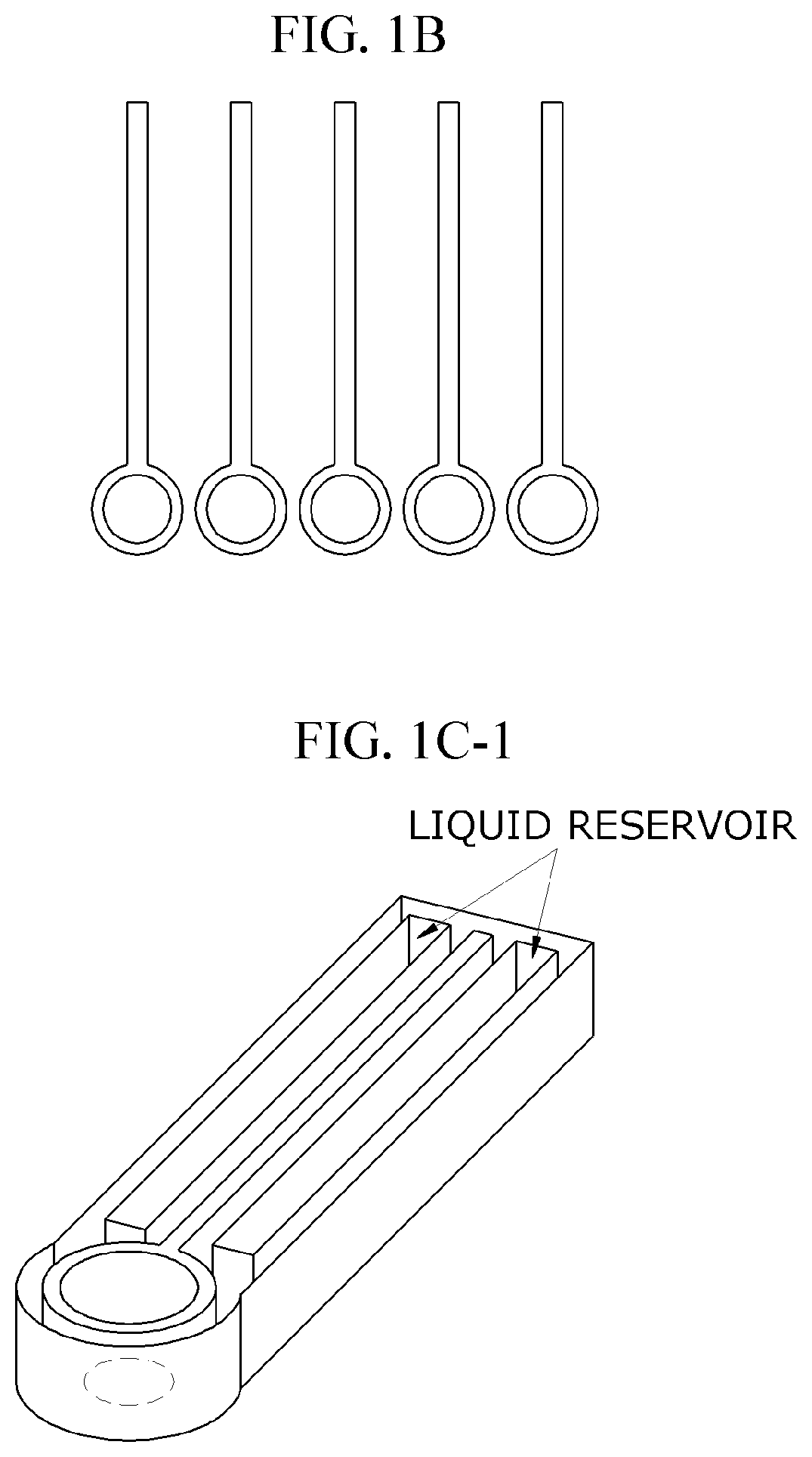
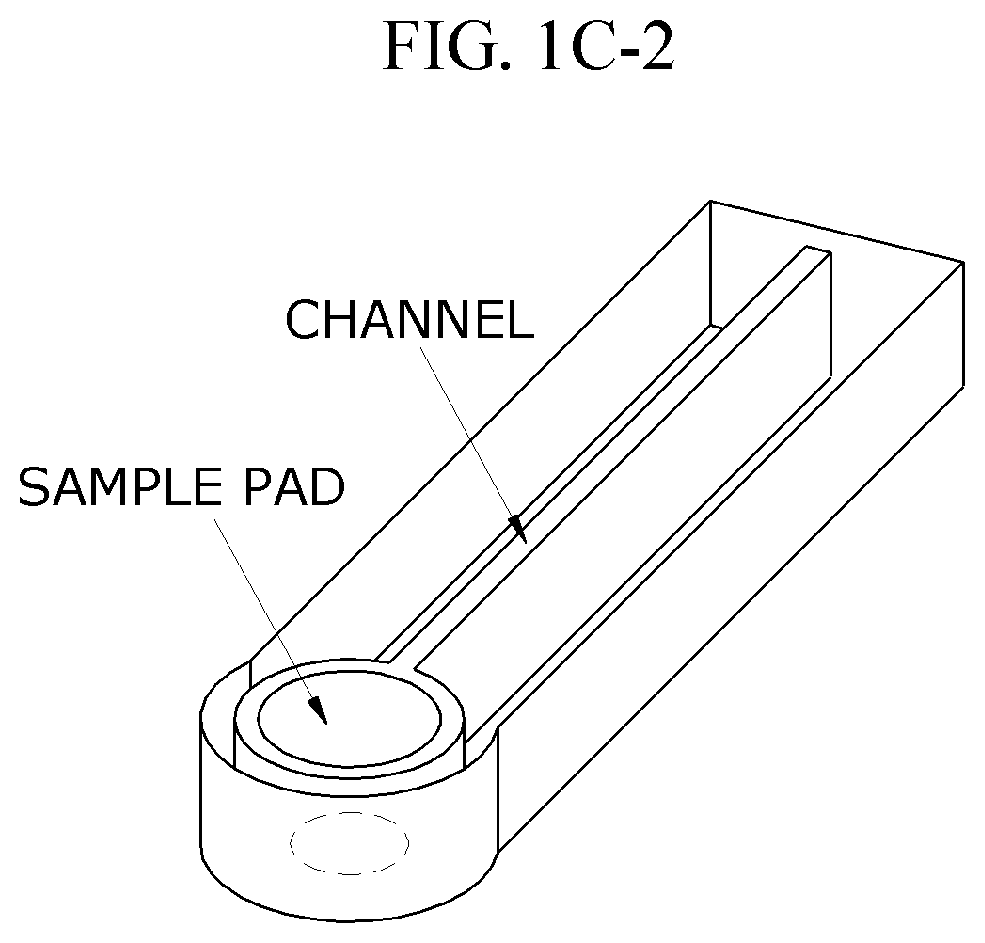
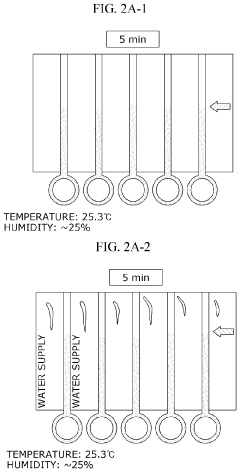
Lateral-flow microfluidic chip and flow velocity control method thereof
PatentInactiveUS20190374940A1
Innovation
- Increasing vapor pressure around specific channels in the microfluidic chip by using a separate vapor supply device or a liquid reservoir adjacent to the channel, which suppresses fluid evaporation and accelerates flow velocity, allowing for efficient sequential reactions without additional processes or equipment.
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Manufacturing Scalability of Optimized Microfluidic Chips
The scalability of manufacturing optimized microfluidic ELISA chips represents a critical factor in their commercial viability and widespread adoption. Current laboratory-scale production methods, primarily based on soft lithography using polydimethylsiloxane (PDMS), face significant challenges when transitioning to mass production. These challenges include maintaining precise channel geometries, ensuring consistent surface properties, and preserving the optimized mixing parameters that were established during research and development phases.
Industrial-scale manufacturing approaches for microfluidic ELISA chips include injection molding, hot embossing, and roll-to-roll processing. Injection molding offers high throughput and excellent reproducibility but requires substantial initial investment in molds and equipment. The technique struggles with ultra-fine features below 10 micrometers, which may be crucial for optimized reagent mixing. Hot embossing provides better resolution for smaller features but operates at lower production rates, creating a throughput bottleneck for large-scale deployment.
Material selection significantly impacts manufacturing scalability. While PDMS dominates laboratory prototyping due to its excellent optical properties and ease of fabrication, its absorption of small hydrophobic molecules and manufacturing limitations make it suboptimal for mass production. Thermoplastics such as polymethyl methacrylate (PMMA), cyclic olefin copolymer (COC), and polycarbonate (PC) offer better scalability but may require redesign of mixing geometries due to different surface properties affecting fluid dynamics.
Quality control represents another major challenge in scaling production. The optimized mixing performance in microfluidic ELISA depends on precise channel dimensions, surface chemistry, and bonding quality. Implementing inline quality control systems capable of detecting microscale defects without slowing production remains technically challenging. Advanced optical inspection systems and electrical impedance spectroscopy show promise but add complexity and cost to the manufacturing process.
Integration of functional components such as valves, pumps, and detection systems further complicates scalable manufacturing. While passive mixing structures can be relatively straightforward to mass-produce, active mixing components require additional assembly steps and increase production complexity. Recent advances in monolithic integration techniques and modular design approaches offer potential solutions by separating the manufacturing of complex functional elements from the main chip body.
Cost considerations ultimately determine commercial viability. Current estimates place small-volume production costs between $10-50 per chip, which must decrease to $1-5 for widespread clinical adoption. Achieving this cost reduction requires not only optimized manufacturing processes but also design modifications that maintain mixing efficiency while simplifying fabrication requirements.
Industrial-scale manufacturing approaches for microfluidic ELISA chips include injection molding, hot embossing, and roll-to-roll processing. Injection molding offers high throughput and excellent reproducibility but requires substantial initial investment in molds and equipment. The technique struggles with ultra-fine features below 10 micrometers, which may be crucial for optimized reagent mixing. Hot embossing provides better resolution for smaller features but operates at lower production rates, creating a throughput bottleneck for large-scale deployment.
Material selection significantly impacts manufacturing scalability. While PDMS dominates laboratory prototyping due to its excellent optical properties and ease of fabrication, its absorption of small hydrophobic molecules and manufacturing limitations make it suboptimal for mass production. Thermoplastics such as polymethyl methacrylate (PMMA), cyclic olefin copolymer (COC), and polycarbonate (PC) offer better scalability but may require redesign of mixing geometries due to different surface properties affecting fluid dynamics.
Quality control represents another major challenge in scaling production. The optimized mixing performance in microfluidic ELISA depends on precise channel dimensions, surface chemistry, and bonding quality. Implementing inline quality control systems capable of detecting microscale defects without slowing production remains technically challenging. Advanced optical inspection systems and electrical impedance spectroscopy show promise but add complexity and cost to the manufacturing process.
Integration of functional components such as valves, pumps, and detection systems further complicates scalable manufacturing. While passive mixing structures can be relatively straightforward to mass-produce, active mixing components require additional assembly steps and increase production complexity. Recent advances in monolithic integration techniques and modular design approaches offer potential solutions by separating the manufacturing of complex functional elements from the main chip body.
Cost considerations ultimately determine commercial viability. Current estimates place small-volume production costs between $10-50 per chip, which must decrease to $1-5 for widespread clinical adoption. Achieving this cost reduction requires not only optimized manufacturing processes but also design modifications that maintain mixing efficiency while simplifying fabrication requirements.
Regulatory Considerations for Diagnostic Microfluidic Devices
The regulatory landscape for microfluidic ELISA chips presents significant challenges for developers seeking to optimize reagent mixing technologies. In the United States, the FDA classifies most microfluidic diagnostic devices under the in vitro diagnostic (IVD) regulatory framework, with ELISA-based systems typically falling under Class II medical devices requiring 510(k) clearance. This classification demands extensive validation of mixing efficiency and reproducibility, as inconsistent reagent mixing can directly impact diagnostic accuracy and reliability.
European regulatory frameworks under the In Vitro Diagnostic Regulation (IVDR) impose additional requirements, particularly regarding risk classification and performance evaluation. Microfluidic ELISA devices with optimized mixing protocols must demonstrate consistent performance across their intended operational parameters, with special attention to the uniformity of reagent distribution throughout the microchannels.
Quality control standards such as ISO 13485 specifically address manufacturing processes critical to microfluidic devices, including material selection and surface treatment protocols that may affect reagent mixing behavior. Developers must implement robust quality management systems that can track and validate the performance of mixing mechanisms across production batches.
Regulatory bodies increasingly require developers to provide comprehensive validation data demonstrating that optimized mixing protocols maintain consistent performance across various environmental conditions and user scenarios. This includes temperature variations, handling errors, and shelf-life considerations that might affect fluid dynamics within the microchannels.
Clinical Laboratory Improvement Amendments (CLIA) regulations in the US further impact the deployment of microfluidic ELISA technologies in clinical settings, with requirements for ongoing quality assessment programs that monitor the consistency of reagent mixing performance over time.
Emerging global harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are creating pathways for streamlined regulatory approval across multiple jurisdictions, though these still require robust evidence of mixing optimization and reproducibility. Developers must navigate these complex regulatory frameworks while designing mixing protocols that balance performance with compliance requirements.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, introduce additional compliance considerations for connected microfluidic devices that may collect and transmit diagnostic data. These regulations necessitate secure data handling protocols alongside the physical device optimization.
European regulatory frameworks under the In Vitro Diagnostic Regulation (IVDR) impose additional requirements, particularly regarding risk classification and performance evaluation. Microfluidic ELISA devices with optimized mixing protocols must demonstrate consistent performance across their intended operational parameters, with special attention to the uniformity of reagent distribution throughout the microchannels.
Quality control standards such as ISO 13485 specifically address manufacturing processes critical to microfluidic devices, including material selection and surface treatment protocols that may affect reagent mixing behavior. Developers must implement robust quality management systems that can track and validate the performance of mixing mechanisms across production batches.
Regulatory bodies increasingly require developers to provide comprehensive validation data demonstrating that optimized mixing protocols maintain consistent performance across various environmental conditions and user scenarios. This includes temperature variations, handling errors, and shelf-life considerations that might affect fluid dynamics within the microchannels.
Clinical Laboratory Improvement Amendments (CLIA) regulations in the US further impact the deployment of microfluidic ELISA technologies in clinical settings, with requirements for ongoing quality assessment programs that monitor the consistency of reagent mixing performance over time.
Emerging global harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are creating pathways for streamlined regulatory approval across multiple jurisdictions, though these still require robust evidence of mixing optimization and reproducibility. Developers must navigate these complex regulatory frameworks while designing mixing protocols that balance performance with compliance requirements.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, introduce additional compliance considerations for connected microfluidic devices that may collect and transmit diagnostic data. These regulations necessitate secure data handling protocols alongside the physical device optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!