Microfluidic ELISA for Pandemic-Response Point-of-Care Testing
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Evolution and Objectives
Microfluidic ELISA technology has evolved significantly since its inception in the late 1990s, transforming from conventional laboratory-based immunoassays to miniaturized, integrated systems capable of rapid detection. The traditional ELISA technique, while highly sensitive and specific, has historically been limited by lengthy processing times, requirement for trained personnel, and bulky instrumentation—factors that severely restrict its utility during pandemic scenarios requiring immediate point-of-care (POC) testing.
The evolution trajectory began with simple microfluidic channels for sample transport, progressing to more sophisticated designs incorporating multiple functional elements such as mixing chambers, reaction zones, and detection areas on a single chip. By the early 2000s, researchers had successfully demonstrated proof-of-concept microfluidic ELISA platforms with improved reaction kinetics due to high surface-to-volume ratios and shorter diffusion distances.
A significant milestone occurred around 2010 with the integration of various detection methods including colorimetric, fluorescence, and electrochemical sensing into microfluidic platforms. This integration enabled quantitative analysis with sensitivity levels approaching traditional laboratory ELISA. The period between 2015-2020 witnessed remarkable advancements in materials science, fabrication techniques, and detection technologies, leading to more robust and cost-effective microfluidic ELISA systems.
The COVID-19 pandemic served as a catalyst for accelerated development, highlighting the critical need for rapid, accurate, and accessible diagnostic tools. This global health crisis exposed significant gaps in testing infrastructure and emphasized the importance of decentralized testing capabilities that could be deployed in resource-limited settings.
The primary objectives of current microfluidic ELISA development for pandemic response include achieving ultra-rapid detection (results in under 30 minutes), maintaining high sensitivity and specificity comparable to laboratory tests, ensuring user-friendly operation requiring minimal training, and developing systems that are portable, affordable, and suitable for deployment in diverse settings from urban hospitals to remote clinics.
Additional technical objectives include multiplexing capabilities to detect multiple biomarkers simultaneously, integration with digital health platforms for data management and epidemiological surveillance, and development of sustainable manufacturing processes that enable rapid scale-up during pandemic situations. Researchers are also focusing on creating systems with built-in quality control mechanisms and reduced dependence on cold-chain logistics—critical factors for global deployment.
The ultimate goal is to establish microfluidic ELISA as a cornerstone technology in pandemic preparedness infrastructure, enabling rapid containment through early detection and informed public health decision-making. This requires not only technical innovation but also consideration of regulatory pathways, integration with existing healthcare systems, and strategies for equitable global access.
The evolution trajectory began with simple microfluidic channels for sample transport, progressing to more sophisticated designs incorporating multiple functional elements such as mixing chambers, reaction zones, and detection areas on a single chip. By the early 2000s, researchers had successfully demonstrated proof-of-concept microfluidic ELISA platforms with improved reaction kinetics due to high surface-to-volume ratios and shorter diffusion distances.
A significant milestone occurred around 2010 with the integration of various detection methods including colorimetric, fluorescence, and electrochemical sensing into microfluidic platforms. This integration enabled quantitative analysis with sensitivity levels approaching traditional laboratory ELISA. The period between 2015-2020 witnessed remarkable advancements in materials science, fabrication techniques, and detection technologies, leading to more robust and cost-effective microfluidic ELISA systems.
The COVID-19 pandemic served as a catalyst for accelerated development, highlighting the critical need for rapid, accurate, and accessible diagnostic tools. This global health crisis exposed significant gaps in testing infrastructure and emphasized the importance of decentralized testing capabilities that could be deployed in resource-limited settings.
The primary objectives of current microfluidic ELISA development for pandemic response include achieving ultra-rapid detection (results in under 30 minutes), maintaining high sensitivity and specificity comparable to laboratory tests, ensuring user-friendly operation requiring minimal training, and developing systems that are portable, affordable, and suitable for deployment in diverse settings from urban hospitals to remote clinics.
Additional technical objectives include multiplexing capabilities to detect multiple biomarkers simultaneously, integration with digital health platforms for data management and epidemiological surveillance, and development of sustainable manufacturing processes that enable rapid scale-up during pandemic situations. Researchers are also focusing on creating systems with built-in quality control mechanisms and reduced dependence on cold-chain logistics—critical factors for global deployment.
The ultimate goal is to establish microfluidic ELISA as a cornerstone technology in pandemic preparedness infrastructure, enabling rapid containment through early detection and informed public health decision-making. This requires not only technical innovation but also consideration of regulatory pathways, integration with existing healthcare systems, and strategies for equitable global access.
Pandemic Response POC Testing Market Analysis
The global market for pandemic response point-of-care testing has experienced unprecedented growth following the COVID-19 outbreak, with microfluidic ELISA technologies emerging as a critical component. The market size for POC diagnostic testing reached $29.7 billion in 2022 and is projected to grow at a CAGR of 11.2% through 2030, driven primarily by the urgent need for rapid, accurate, and accessible testing solutions during public health emergencies.
Demand for microfluidic ELISA-based POC testing is particularly strong in regions with limited healthcare infrastructure, where centralized laboratory testing is not readily available. These technologies offer significant advantages in pandemic scenarios, including reduced sample-to-result time (typically under 30 minutes compared to hours or days for traditional laboratory tests), minimal sample volume requirements, and the ability to operate with limited technical expertise.
Healthcare systems worldwide are increasingly recognizing the value of decentralized testing networks that can be rapidly deployed during outbreaks. Government initiatives and funding for pandemic preparedness have created substantial market opportunities, with the WHO and various national health authorities establishing guidelines that favor POC solutions with high sensitivity and specificity profiles characteristic of microfluidic ELISA platforms.
The primary market segments include hospital emergency departments, urgent care centers, primary care settings, and field deployment scenarios such as airports, schools, and community testing sites. Each segment presents unique requirements regarding throughput, connectivity, and user interface design. Notably, there is growing demand for multiplexed testing capabilities that can simultaneously detect multiple pathogens or biomarkers from a single sample.
Consumer behavior has also shifted significantly, with increased acceptance and expectation of rapid diagnostic results. This trend is expected to persist beyond the current pandemic, creating sustained demand for advanced POC technologies. The willingness to pay for premium diagnostic solutions has increased, particularly among healthcare institutions seeking to enhance their emergency response capabilities.
Supply chain considerations remain critical to market success, with recent pandemic experiences highlighting vulnerabilities in global distribution networks. Consequently, there is growing emphasis on technologies that utilize shelf-stable reagents and require minimal cold chain infrastructure, features that microfluidic ELISA platforms can potentially address through innovative reagent formulations and device designs.
Reimbursement policies are evolving to accommodate POC testing models, with many insurers and national healthcare systems implementing specific codes and payment structures for pandemic-related diagnostics. This regulatory evolution represents both an opportunity and a challenge for new market entrants, as navigating approval pathways requires strategic planning and evidence generation.
Demand for microfluidic ELISA-based POC testing is particularly strong in regions with limited healthcare infrastructure, where centralized laboratory testing is not readily available. These technologies offer significant advantages in pandemic scenarios, including reduced sample-to-result time (typically under 30 minutes compared to hours or days for traditional laboratory tests), minimal sample volume requirements, and the ability to operate with limited technical expertise.
Healthcare systems worldwide are increasingly recognizing the value of decentralized testing networks that can be rapidly deployed during outbreaks. Government initiatives and funding for pandemic preparedness have created substantial market opportunities, with the WHO and various national health authorities establishing guidelines that favor POC solutions with high sensitivity and specificity profiles characteristic of microfluidic ELISA platforms.
The primary market segments include hospital emergency departments, urgent care centers, primary care settings, and field deployment scenarios such as airports, schools, and community testing sites. Each segment presents unique requirements regarding throughput, connectivity, and user interface design. Notably, there is growing demand for multiplexed testing capabilities that can simultaneously detect multiple pathogens or biomarkers from a single sample.
Consumer behavior has also shifted significantly, with increased acceptance and expectation of rapid diagnostic results. This trend is expected to persist beyond the current pandemic, creating sustained demand for advanced POC technologies. The willingness to pay for premium diagnostic solutions has increased, particularly among healthcare institutions seeking to enhance their emergency response capabilities.
Supply chain considerations remain critical to market success, with recent pandemic experiences highlighting vulnerabilities in global distribution networks. Consequently, there is growing emphasis on technologies that utilize shelf-stable reagents and require minimal cold chain infrastructure, features that microfluidic ELISA platforms can potentially address through innovative reagent formulations and device designs.
Reimbursement policies are evolving to accommodate POC testing models, with many insurers and national healthcare systems implementing specific codes and payment structures for pandemic-related diagnostics. This regulatory evolution represents both an opportunity and a challenge for new market entrants, as navigating approval pathways requires strategic planning and evidence generation.
Global Microfluidic ELISA Development Status and Barriers
Microfluidic ELISA technology has evolved significantly over the past decade, with global development efforts focused on miniaturizing conventional laboratory immunoassays for point-of-care applications. Currently, research institutions and companies across North America, Europe, and Asia are actively advancing this field, with notable progress in microfluidic chip design, reagent stability, and detection sensitivity. The United States and China lead in terms of research output and patent applications, while European countries excel in standardization efforts.
Despite these advancements, several critical technical barriers persist in global microfluidic ELISA development. Surface chemistry challenges remain prominent, as protein adsorption and biofouling issues continue to affect assay reliability and reproducibility. The miniaturization of detection systems presents another significant hurdle, with trade-offs between sensitivity, portability, and cost still unresolved for pandemic response applications.
Manufacturing scalability constitutes a major constraint, particularly for pandemic preparedness where rapid production scaling is essential. Current fabrication techniques for microfluidic chips often involve complex multi-step processes that are difficult to scale economically while maintaining precision. This manufacturing bottleneck has limited widespread adoption despite promising laboratory results.
Reagent stability under field conditions represents another universal challenge. Many microfluidic ELISA platforms demonstrate excellent performance in controlled laboratory environments but suffer significant degradation when exposed to variable temperatures and humidity levels typical in resource-limited settings where pandemic response is most critical.
Regulatory frameworks vary significantly across regions, creating inconsistent validation requirements that impede global deployment. While the FDA has established some guidance for microfluidic diagnostic devices, many countries lack specific regulatory pathways for these novel technologies, creating uncertainty for developers and slowing commercialization efforts.
Integration with existing healthcare infrastructure remains problematic worldwide. Many promising microfluidic ELISA technologies require specialized training or equipment that limits their utility in pandemic response scenarios, particularly in low-resource settings. The lack of standardized interfaces and data management systems further complicates deployment at scale.
Recent collaborative initiatives, such as the Microfluidic ELISA Standardization Consortium and the WHO's Pandemic Technology Access Pool, are attempting to address these barriers through coordinated international efforts. However, significant technical challenges persist in achieving the ideal combination of sensitivity, specificity, cost-effectiveness, and ease of use required for truly effective pandemic-response point-of-care testing solutions.
Despite these advancements, several critical technical barriers persist in global microfluidic ELISA development. Surface chemistry challenges remain prominent, as protein adsorption and biofouling issues continue to affect assay reliability and reproducibility. The miniaturization of detection systems presents another significant hurdle, with trade-offs between sensitivity, portability, and cost still unresolved for pandemic response applications.
Manufacturing scalability constitutes a major constraint, particularly for pandemic preparedness where rapid production scaling is essential. Current fabrication techniques for microfluidic chips often involve complex multi-step processes that are difficult to scale economically while maintaining precision. This manufacturing bottleneck has limited widespread adoption despite promising laboratory results.
Reagent stability under field conditions represents another universal challenge. Many microfluidic ELISA platforms demonstrate excellent performance in controlled laboratory environments but suffer significant degradation when exposed to variable temperatures and humidity levels typical in resource-limited settings where pandemic response is most critical.
Regulatory frameworks vary significantly across regions, creating inconsistent validation requirements that impede global deployment. While the FDA has established some guidance for microfluidic diagnostic devices, many countries lack specific regulatory pathways for these novel technologies, creating uncertainty for developers and slowing commercialization efforts.
Integration with existing healthcare infrastructure remains problematic worldwide. Many promising microfluidic ELISA technologies require specialized training or equipment that limits their utility in pandemic response scenarios, particularly in low-resource settings. The lack of standardized interfaces and data management systems further complicates deployment at scale.
Recent collaborative initiatives, such as the Microfluidic ELISA Standardization Consortium and the WHO's Pandemic Technology Access Pool, are attempting to address these barriers through coordinated international efforts. However, significant technical challenges persist in achieving the ideal combination of sensitivity, specificity, cost-effectiveness, and ease of use required for truly effective pandemic-response point-of-care testing solutions.
Current Microfluidic ELISA POC Testing Solutions
01 Microfluidic chip designs for ELISA
Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and reaction zones optimized for antibody-antigen interactions. The miniaturized platforms enable precise control of fluid flow, reduced sample volumes, and enhanced reaction kinetics. Some designs feature integrated detection systems for real-time monitoring of the ELISA reactions, while others incorporate multiple reaction chambers for parallel processing of samples.- Microfluidic chip designs for ELISA: Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and reaction zones optimized for the sequential steps of ELISA protocols. The miniaturized platforms enable precise control of fluid flow, reduced sample volumes, and enhanced sensitivity compared to conventional ELISA methods. These chip designs often include integrated detection zones for optical or electrochemical measurements of the assay results.
- Automated fluid handling systems for microfluidic ELISA: Automated fluid handling systems have been developed to control the precise movement of reagents and samples through microfluidic ELISA platforms. These systems incorporate micropumps, valves, and flow controllers to automate the sequential steps of ELISA protocols including sample introduction, antibody binding, washing, and detection. Automation reduces human error, improves reproducibility, and enables high-throughput screening applications while maintaining the sensitivity of the assay.
- Detection methods for microfluidic ELISA: Various detection methods have been integrated with microfluidic ELISA platforms to enhance sensitivity and quantification capabilities. These include optical detection systems using fluorescence, chemiluminescence, or colorimetric readouts, as well as electrochemical detection methods. The miniaturized detection systems are designed to work with the small sample volumes and reaction chambers of microfluidic devices, providing rapid and sensitive detection of analytes at low concentrations.
- Multiplexed microfluidic ELISA systems: Multiplexed microfluidic ELISA systems enable the simultaneous detection of multiple analytes in a single sample. These systems incorporate parallel reaction chambers or detection zones within a single microfluidic chip. By using different capture antibodies in each zone, multiple biomarkers can be detected simultaneously, increasing the information obtained from limited sample volumes. This approach is particularly valuable for diagnostic applications where multiple biomarkers need to be measured for accurate disease assessment.
- Point-of-care microfluidic ELISA devices: Point-of-care microfluidic ELISA devices have been developed for rapid diagnostic testing in resource-limited settings. These portable devices integrate sample preparation, ELISA reactions, and detection systems into a single platform that can be operated with minimal training. The devices are designed to be cost-effective, user-friendly, and capable of providing results within minutes rather than hours. Some designs incorporate smartphone-based detection systems for result analysis and data sharing.
02 Automated microfluidic ELISA systems
Automated systems for microfluidic ELISA have been developed to streamline the testing process. These systems integrate sample preparation, reagent handling, incubation, washing steps, and detection into a single platform. Automation reduces human error, improves reproducibility, and increases throughput. Some systems incorporate programmable fluid handling components, temperature control modules, and detection systems that can be customized for different ELISA protocols.Expand Specific Solutions03 Detection methods for microfluidic ELISA
Various detection methods have been integrated into microfluidic ELISA platforms to enhance sensitivity and specificity. These include optical detection methods such as fluorescence, chemiluminescence, and colorimetric detection, as well as electrochemical detection methods. Some systems incorporate smartphone-based detection for point-of-care applications. Advanced signal amplification techniques have been developed to improve the detection limits of microfluidic ELISA, making it suitable for detecting low-abundance biomarkers.Expand Specific Solutions04 Microfluidic ELISA for point-of-care diagnostics
Microfluidic ELISA platforms have been adapted for point-of-care diagnostic applications. These platforms are designed to be portable, user-friendly, and capable of providing rapid results in resource-limited settings. Some designs incorporate sample preparation steps to enable testing of complex biological samples such as blood, saliva, or urine. Integration with digital health technologies allows for data storage, analysis, and transmission, facilitating remote diagnostics and monitoring.Expand Specific Solutions05 Fluid handling in microfluidic ELISA
Advanced fluid handling techniques have been developed for microfluidic ELISA to improve performance and reliability. These include passive and active fluid control methods such as capillary forces, pressure-driven flow, electrokinetic flow, and centrifugal forces. Some systems incorporate valves, pumps, and mixers to precisely control the movement and interaction of reagents. Specialized surface treatments and channel designs have been developed to prevent non-specific binding and ensure uniform flow distribution, which are critical for accurate ELISA results.Expand Specific Solutions
Leading Companies in Microfluidic Diagnostic Platforms
Microfluidic ELISA for pandemic response point-of-care testing is currently in an emerging growth phase, with the global market expected to reach significant expansion due to increasing demand for rapid diagnostic solutions. The technology has evolved from early research stages to commercial applications, with varying degrees of maturity across different platforms. Leading players include The Regents of the University of California and Cornell University pioneering fundamental research, while companies like Revvity Health Sciences and Zymo Research are commercializing advanced solutions. Chinese institutions including Tsinghua University and Fudan University are making significant contributions to technology development. The competitive landscape is diversifying with specialized startups like NanoIVD and Optofluidic Bioassay introducing innovative approaches, while established medical institutions such as Brigham & Women's Hospital provide clinical validation pathways.
The Regents of the University of California
Technical Solution: The University of California has developed an advanced microfluidic ELISA platform specifically designed for pandemic response point-of-care testing. Their system integrates digital microfluidics with electrochemical detection methods to create a portable, rapid diagnostic tool. The technology utilizes electrowetting-on-dielectric (EWOD) principles to manipulate nanoliter-sized droplets containing samples and reagents across a series of electrodes[1]. This approach eliminates the need for external pumps and complex fluidic controls typically required in traditional ELISA systems. The UC platform incorporates multiplexed detection capabilities, allowing simultaneous testing for multiple disease biomarkers from a single sample. Their system achieves detection limits in the picogram/mL range, comparable to laboratory-based ELISA tests, while delivering results in under 30 minutes compared to conventional ELISA's 3-5 hours[3]. The platform has been validated for COVID-19 antibody detection with clinical samples, demonstrating over 95% sensitivity and specificity.
Strengths: Exceptional portability and speed without sacrificing sensitivity; multiplexed detection capabilities allow testing for multiple pathogens simultaneously; minimal sample volume requirements (1-10 μL) make it suitable for finger-prick blood samples. Weaknesses: Higher manufacturing costs compared to lateral flow assays; requires specialized microfluidic chips that may present supply chain challenges during pandemic surges; limited shelf life of pre-loaded reagents may affect field deployment in resource-limited settings.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has engineered a comprehensive microfluidic ELISA solution called "FlexPoint™" specifically targeting pandemic response scenarios. Their technology combines proprietary microfluidic cartridge design with advanced immunoassay chemistry to deliver laboratory-quality results in point-of-care settings. The FlexPoint™ system utilizes a centrifugal microfluidic platform where fluid movement is controlled by rotational forces, eliminating the need for external pumps[2]. The cartridges incorporate pre-loaded, stabilized reagents with extended shelf-life, addressing key challenges in field deployment. Revvity's platform features a unique sample preparation module that automatically processes whole blood, saliva, or nasopharyngeal samples, reducing operator intervention and contamination risks. Their system achieves detection sensitivities in the sub-picogram/mL range with a total assay time of approximately 20 minutes[4]. The platform includes wireless connectivity for real-time data transmission to healthcare systems, enabling immediate epidemiological tracking during outbreaks. Revvity has validated their technology for multiple respiratory pathogens including SARS-CoV-2, influenza, and RSV with clinical performance exceeding 94% concordance with central laboratory testing.
Strengths: Exceptional reagent stability (12+ months shelf life) enables stockpiling for pandemic preparedness; automated sample preparation reduces user error and biohazard exposure; integrated connectivity facilitates real-time epidemiological surveillance. Weaknesses: Higher per-test cost compared to traditional lateral flow tests; instrument requires periodic calibration and maintenance which may be challenging in resource-limited settings; system depends on stable power supply which limits deployment in extremely remote locations.
Key Patents and Innovations in Microfluidic Immunoassays
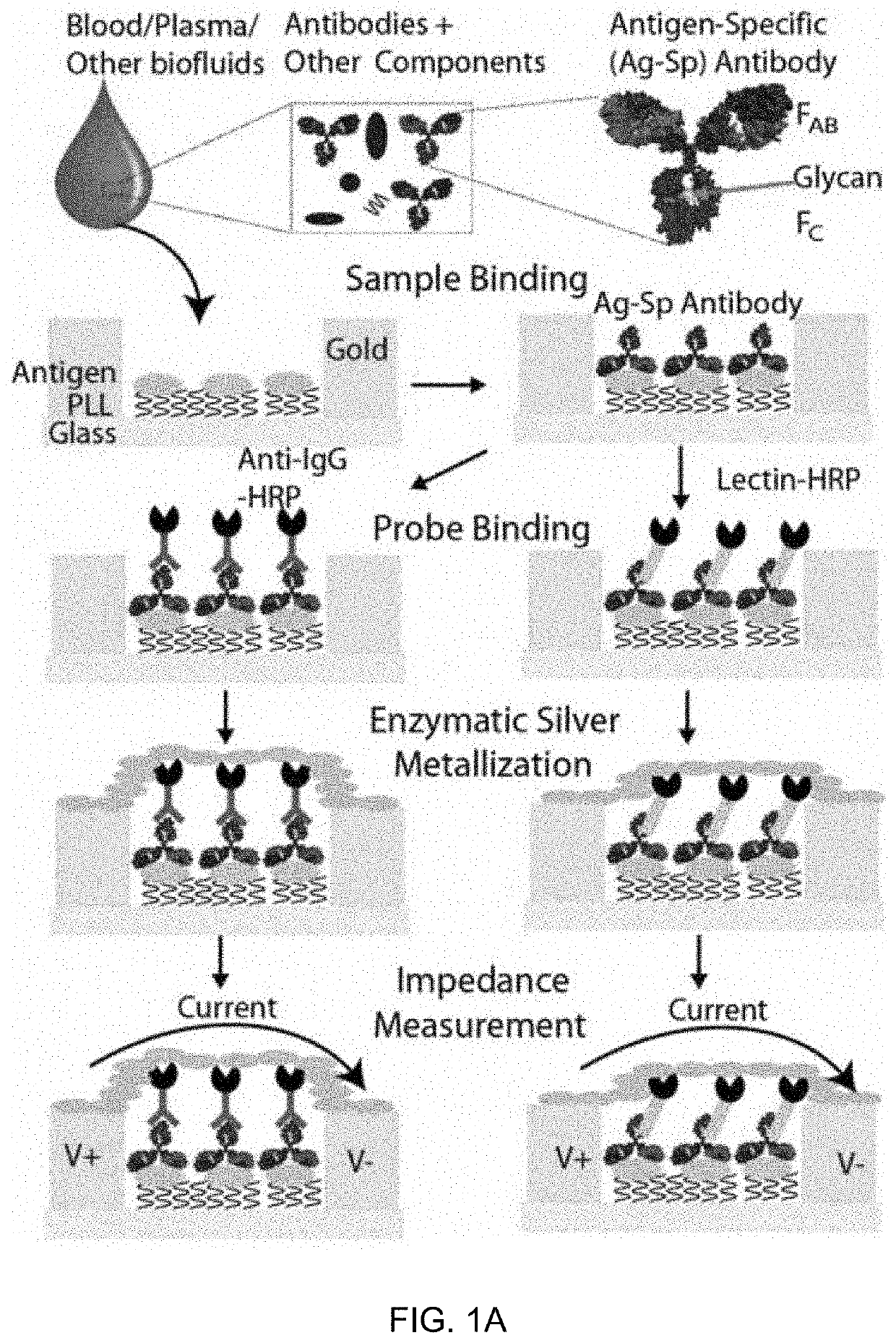
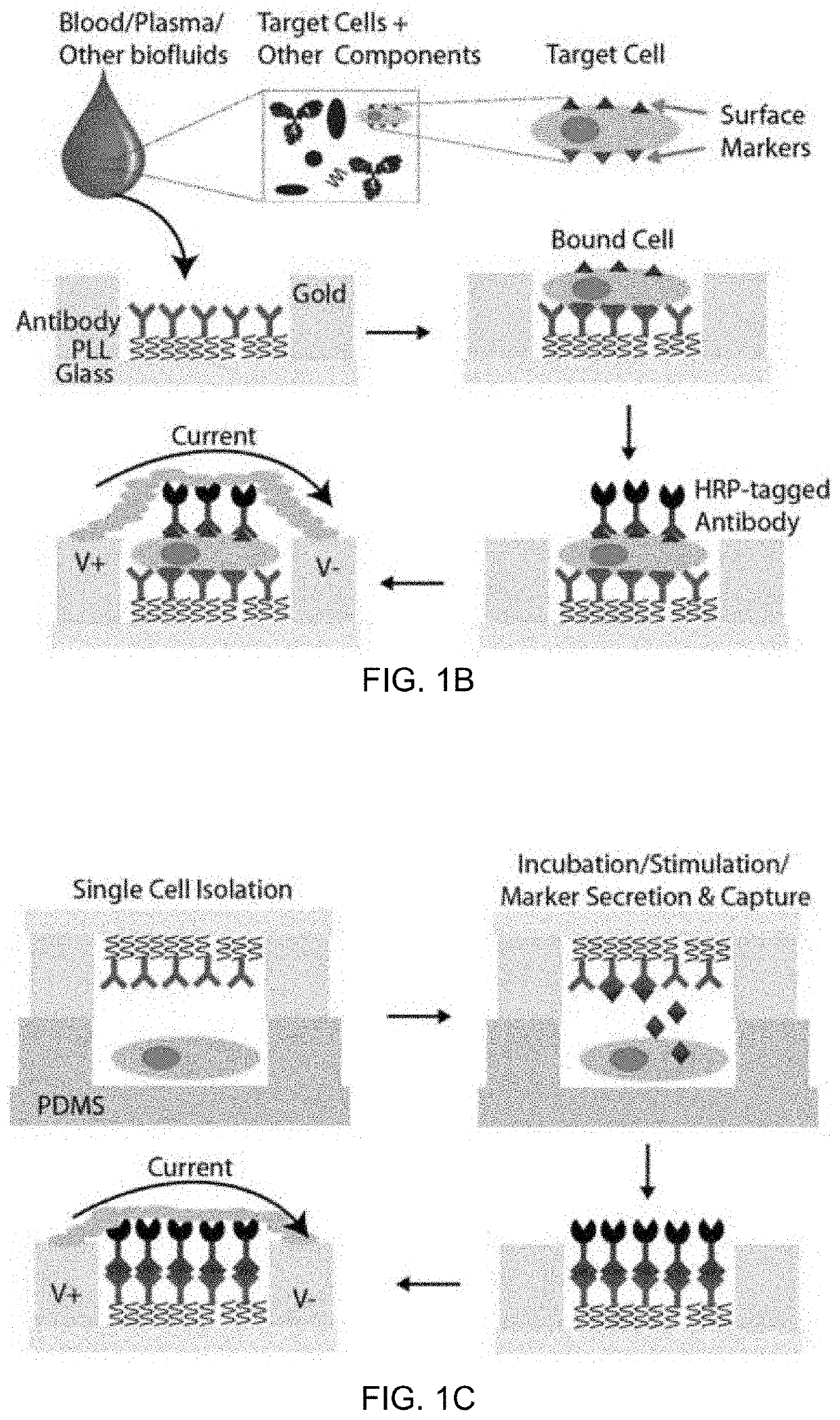
An integrated microfluidic electrode array system for enzyme-linked immuno-sorbent assay (easy-elisa) for point-of-care detection of molecular and cellular biomarkers
PatentInactiveUS20210373014A1
Innovation
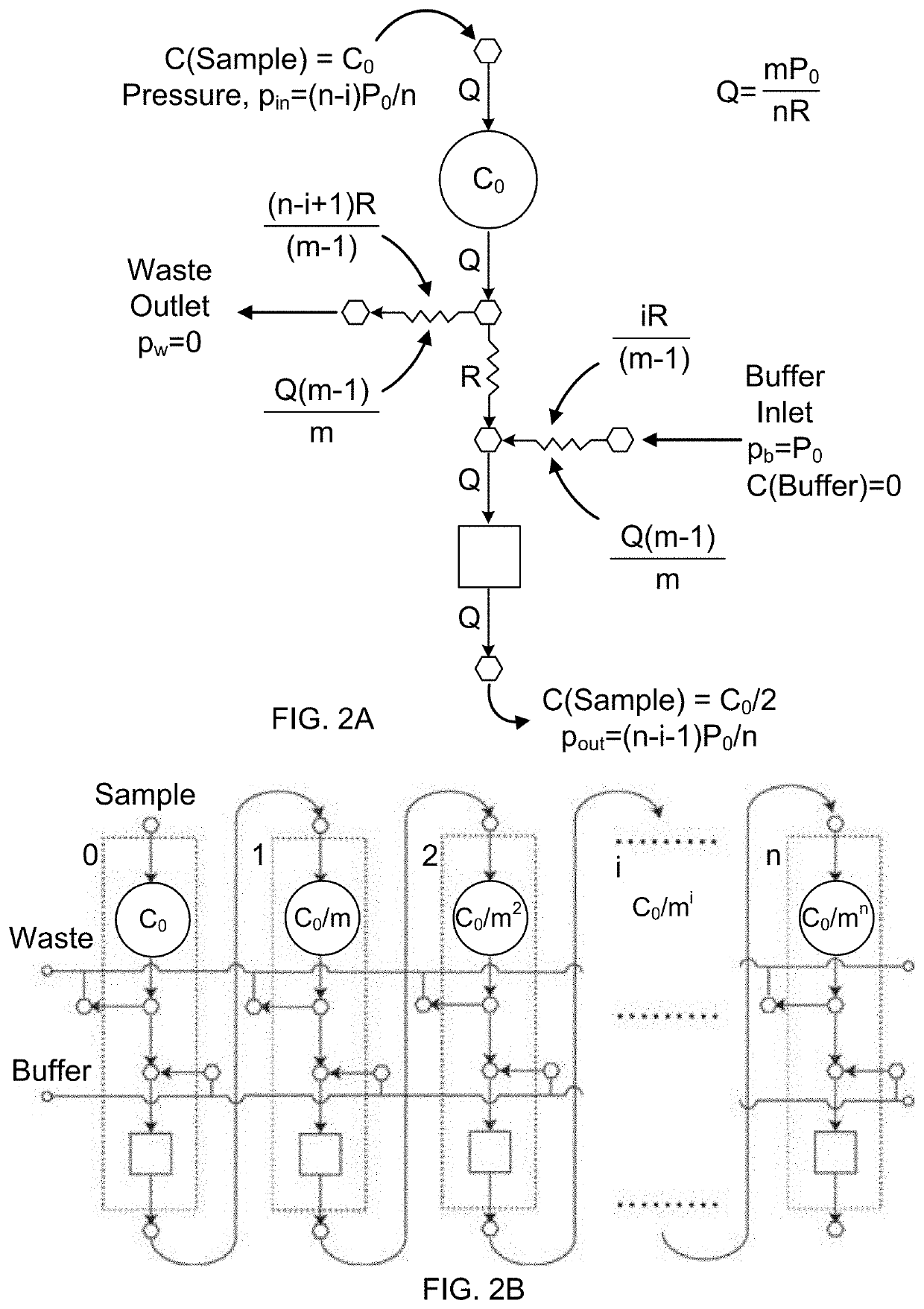
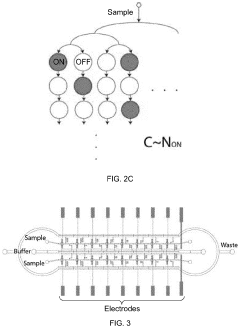
- A microfluidic Electrode Array System for Enzyme-Linked Immuno-Sorbent Assay (EASy-ELISA) that uses direct electrical impedance-based detection and quantitation, enabling sensitive and quantitative detection of molecular and cellular biomarkers without intermediate optics or optical detectors, integrated with a microfluidic serial dilution network for sample handling and distribution.
System and method for picoliter volume microfluidic diagnotics
PatentWO2014107698A1
Innovation
- A droplet microfluidics-based platform for picoliter volume bio-analyte detection and quantification using microspheres and fluorescently labeled antibodies, which reduces reagent volume by four orders of magnitude and enhances reaction rates, enabling rapid and low-cost analysis.
Supply Chain Resilience for Diagnostic Components
The global pandemic has exposed critical vulnerabilities in diagnostic supply chains, particularly for microfluidic ELISA-based point-of-care testing systems. These vulnerabilities stem from geographic concentration of manufacturing, just-in-time inventory practices, and reliance on single-source suppliers for critical components. When demand surged during COVID-19, shortages of reagents, microfluidic chips, and detection antibodies created significant bottlenecks in diagnostic testing capabilities worldwide.
Building resilient supply chains for microfluidic ELISA diagnostic components requires a multi-faceted approach. Diversification of supplier networks across different geographic regions can mitigate risks associated with regional disruptions. The establishment of strategic component reserves for critical materials like specialized antibodies, enzymes, and microfluidic substrates provides buffer capacity during demand spikes. These reserves should be managed with appropriate shelf-life considerations and rotation protocols.
Advanced manufacturing technologies offer promising solutions for supply chain resilience. Additive manufacturing (3D printing) of microfluidic components enables on-demand production capabilities closer to points of use. This localized manufacturing approach reduces dependence on global shipping networks and allows for rapid scaling during emergencies. Similarly, automated assembly systems for diagnostic kits can increase production flexibility and reduce labor dependencies.
Regulatory frameworks significantly impact supply chain resilience. Harmonized international standards for diagnostic components would facilitate qualification of alternative suppliers during shortages. Expedited approval pathways for manufacturing changes during public health emergencies, while maintaining quality standards, could enable faster adaptation to supply disruptions. Regulatory bodies should consider supply chain risk assessments as part of approval processes for pandemic-response diagnostics.
Material innovations also contribute to supply chain resilience. Development of shelf-stable reagents that eliminate cold-chain requirements reduces logistical complexities. Standardized microfluidic interfaces and modular design approaches enable component interchangeability across different diagnostic platforms. Research into synthetic alternatives for biologically-derived reagents, such as aptamers replacing antibodies, could reduce dependence on biological production systems with inherent supply constraints.
Digital supply chain tools incorporating artificial intelligence for demand forecasting and inventory optimization enhance visibility across the supply network. Blockchain-based tracking systems for component provenance improve transparency and help identify counterfeit materials. These technologies, combined with collaborative industry-government partnerships for coordinated stockpiling and emergency production capacity, form the foundation of a resilient diagnostic supply chain ecosystem capable of responding effectively to future pandemic challenges.
Building resilient supply chains for microfluidic ELISA diagnostic components requires a multi-faceted approach. Diversification of supplier networks across different geographic regions can mitigate risks associated with regional disruptions. The establishment of strategic component reserves for critical materials like specialized antibodies, enzymes, and microfluidic substrates provides buffer capacity during demand spikes. These reserves should be managed with appropriate shelf-life considerations and rotation protocols.
Advanced manufacturing technologies offer promising solutions for supply chain resilience. Additive manufacturing (3D printing) of microfluidic components enables on-demand production capabilities closer to points of use. This localized manufacturing approach reduces dependence on global shipping networks and allows for rapid scaling during emergencies. Similarly, automated assembly systems for diagnostic kits can increase production flexibility and reduce labor dependencies.
Regulatory frameworks significantly impact supply chain resilience. Harmonized international standards for diagnostic components would facilitate qualification of alternative suppliers during shortages. Expedited approval pathways for manufacturing changes during public health emergencies, while maintaining quality standards, could enable faster adaptation to supply disruptions. Regulatory bodies should consider supply chain risk assessments as part of approval processes for pandemic-response diagnostics.
Material innovations also contribute to supply chain resilience. Development of shelf-stable reagents that eliminate cold-chain requirements reduces logistical complexities. Standardized microfluidic interfaces and modular design approaches enable component interchangeability across different diagnostic platforms. Research into synthetic alternatives for biologically-derived reagents, such as aptamers replacing antibodies, could reduce dependence on biological production systems with inherent supply constraints.
Digital supply chain tools incorporating artificial intelligence for demand forecasting and inventory optimization enhance visibility across the supply network. Blockchain-based tracking systems for component provenance improve transparency and help identify counterfeit materials. These technologies, combined with collaborative industry-government partnerships for coordinated stockpiling and emergency production capacity, form the foundation of a resilient diagnostic supply chain ecosystem capable of responding effectively to future pandemic challenges.
Regulatory Pathways for Emergency Diagnostic Authorization
The regulatory landscape for emergency diagnostic authorization has evolved significantly in response to global health crises, particularly during the COVID-19 pandemic. For microfluidic ELISA point-of-care testing systems, understanding these pathways is crucial for rapid deployment during pandemic situations.
The U.S. Food and Drug Administration (FDA) established the Emergency Use Authorization (EUA) framework under Section 564 of the Federal Food, Drug, and Cosmetic Act. This pathway allows for expedited review and authorization of medical devices during public health emergencies when no adequate approved alternatives exist. For microfluidic ELISA technologies, the EUA process typically requires demonstration of analytical validity, clinical performance data, and benefit-risk assessment, but with streamlined documentation requirements compared to traditional approval pathways.
The European Union implemented similar emergency provisions through the European Medicines Agency (EMA) and national competent authorities. The EU's Medical Device Regulation (MDR) contains specific clauses for emergency situations that allow for temporary derogations from standard conformity assessment procedures. Manufacturers of microfluidic ELISA systems can leverage these provisions to obtain CE marking through expedited reviews during declared emergencies.
In the Asia-Pacific region, regulatory frameworks vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) established the Priority Review System for pandemic diagnostics, while China's National Medical Products Administration (NMPA) implemented the Special Approval Procedure for emergency situations. These pathways reduce review timelines from years to weeks or months.
The World Health Organization's Emergency Use Listing (EUL) procedure serves as a global mechanism to expedite availability of diagnostics during public health emergencies. For microfluidic ELISA developers targeting global deployment, WHO EUL can facilitate multi-country recognition and adoption, particularly in resource-limited settings.
Post-authorization requirements remain critical components of these emergency pathways. Manufacturers must commit to ongoing data collection, adverse event reporting, and quality management system maintenance. For microfluidic ELISA platforms, this typically includes real-world performance monitoring across diverse populations and settings to ensure continued safety and effectiveness.
Navigating these regulatory pathways requires strategic planning and early engagement with regulatory authorities. Successful microfluidic ELISA developers typically establish regulatory intelligence functions to monitor evolving requirements and maintain communication channels with key agencies. This approach enables rapid adaptation to changing regulatory expectations during dynamic pandemic situations.
The U.S. Food and Drug Administration (FDA) established the Emergency Use Authorization (EUA) framework under Section 564 of the Federal Food, Drug, and Cosmetic Act. This pathway allows for expedited review and authorization of medical devices during public health emergencies when no adequate approved alternatives exist. For microfluidic ELISA technologies, the EUA process typically requires demonstration of analytical validity, clinical performance data, and benefit-risk assessment, but with streamlined documentation requirements compared to traditional approval pathways.
The European Union implemented similar emergency provisions through the European Medicines Agency (EMA) and national competent authorities. The EU's Medical Device Regulation (MDR) contains specific clauses for emergency situations that allow for temporary derogations from standard conformity assessment procedures. Manufacturers of microfluidic ELISA systems can leverage these provisions to obtain CE marking through expedited reviews during declared emergencies.
In the Asia-Pacific region, regulatory frameworks vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) established the Priority Review System for pandemic diagnostics, while China's National Medical Products Administration (NMPA) implemented the Special Approval Procedure for emergency situations. These pathways reduce review timelines from years to weeks or months.
The World Health Organization's Emergency Use Listing (EUL) procedure serves as a global mechanism to expedite availability of diagnostics during public health emergencies. For microfluidic ELISA developers targeting global deployment, WHO EUL can facilitate multi-country recognition and adoption, particularly in resource-limited settings.
Post-authorization requirements remain critical components of these emergency pathways. Manufacturers must commit to ongoing data collection, adverse event reporting, and quality management system maintenance. For microfluidic ELISA platforms, this typically includes real-world performance monitoring across diverse populations and settings to ensure continued safety and effectiveness.
Navigating these regulatory pathways requires strategic planning and early engagement with regulatory authorities. Successful microfluidic ELISA developers typically establish regulatory intelligence functions to monitor evolving requirements and maintain communication channels with key agencies. This approach enables rapid adaptation to changing regulatory expectations during dynamic pandemic situations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!