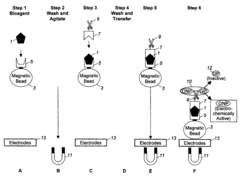
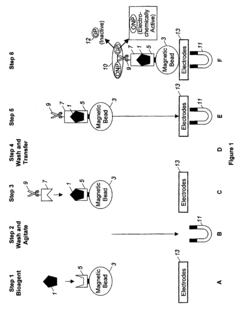
Automation of Washing Cycles in Microfluidic ELISA Systems
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Automation Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has evolved significantly since its inception in the 1970s, transforming from a manual laboratory technique to an increasingly automated process. The integration of microfluidic technologies with ELISA represents a pivotal advancement in diagnostic capabilities, offering enhanced sensitivity, reduced sample volumes, and accelerated analysis times. This technological convergence has been driven by growing demands for point-of-care testing solutions and the need for more efficient clinical diagnostics.
The washing cycle represents a critical yet challenging aspect of the ELISA workflow. Traditional ELISA protocols require multiple washing steps to remove unbound reagents, a process that is labor-intensive and prone to variability when performed manually. In microfluidic systems, these washing procedures become even more complex due to the confined geometries and laminar flow dynamics characteristic of microscale environments.
Recent technological developments have focused on addressing these challenges through various approaches, including pressure-driven flow systems, centrifugal microfluidics, and electrokinetic methods. The evolution of these solutions reflects a broader trend toward complete lab-on-a-chip systems that integrate all aspects of the ELISA workflow, from sample preparation to result analysis.
The primary objective of automating washing cycles in microfluidic ELISA systems is to enhance reproducibility while maintaining or improving assay sensitivity. This involves developing robust mechanisms for fluid handling that can consistently remove unbound reagents without disrupting the bound analyte-antibody complexes. Secondary objectives include reducing the overall assay time, minimizing reagent consumption, and simplifying the user interface to enable operation by non-specialists.
Market forces driving innovation in this field include the growing prevalence of chronic diseases requiring regular monitoring, the shift toward decentralized healthcare delivery models, and increasing pressure on healthcare systems to reduce costs while improving outcomes. These factors collectively create a compelling case for more efficient diagnostic tools that can be deployed in diverse settings.
The technological trajectory suggests a convergence toward fully integrated, automated microfluidic ELISA platforms capable of performing complex immunoassays with minimal user intervention. This vision aligns with broader trends in healthcare technology, including personalized medicine and remote patient monitoring, which require accessible and reliable diagnostic capabilities.
Achieving these objectives necessitates overcoming significant technical hurdles, including precise fluid control at microscale, prevention of non-specific binding, integration of detection systems, and development of user-friendly interfaces. The interdisciplinary nature of these challenges requires expertise spanning microfluidics, surface chemistry, optical sensing, and control systems engineering.
The washing cycle represents a critical yet challenging aspect of the ELISA workflow. Traditional ELISA protocols require multiple washing steps to remove unbound reagents, a process that is labor-intensive and prone to variability when performed manually. In microfluidic systems, these washing procedures become even more complex due to the confined geometries and laminar flow dynamics characteristic of microscale environments.
Recent technological developments have focused on addressing these challenges through various approaches, including pressure-driven flow systems, centrifugal microfluidics, and electrokinetic methods. The evolution of these solutions reflects a broader trend toward complete lab-on-a-chip systems that integrate all aspects of the ELISA workflow, from sample preparation to result analysis.
The primary objective of automating washing cycles in microfluidic ELISA systems is to enhance reproducibility while maintaining or improving assay sensitivity. This involves developing robust mechanisms for fluid handling that can consistently remove unbound reagents without disrupting the bound analyte-antibody complexes. Secondary objectives include reducing the overall assay time, minimizing reagent consumption, and simplifying the user interface to enable operation by non-specialists.
Market forces driving innovation in this field include the growing prevalence of chronic diseases requiring regular monitoring, the shift toward decentralized healthcare delivery models, and increasing pressure on healthcare systems to reduce costs while improving outcomes. These factors collectively create a compelling case for more efficient diagnostic tools that can be deployed in diverse settings.
The technological trajectory suggests a convergence toward fully integrated, automated microfluidic ELISA platforms capable of performing complex immunoassays with minimal user intervention. This vision aligns with broader trends in healthcare technology, including personalized medicine and remote patient monitoring, which require accessible and reliable diagnostic capabilities.
Achieving these objectives necessitates overcoming significant technical hurdles, including precise fluid control at microscale, prevention of non-specific binding, integration of detection systems, and development of user-friendly interfaces. The interdisciplinary nature of these challenges requires expertise spanning microfluidics, surface chemistry, optical sensing, and control systems engineering.
Market Analysis for Automated Microfluidic Immunoassays
The global market for automated microfluidic immunoassays is experiencing robust growth, driven by increasing demand for rapid, accurate, and cost-effective diagnostic solutions. The market was valued at approximately $2.3 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 16.4%. This growth trajectory is significantly higher than the broader in vitro diagnostics market, which is growing at around 5-6% annually.
North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness about early disease detection, and improving healthcare infrastructure in countries like China and India.
The clinical diagnostics segment holds the largest market share (65%), followed by research applications (25%) and pharmaceutical drug development (10%). Within clinical diagnostics, infectious disease testing represents the largest application area (38%), followed by oncology (22%), autoimmune diseases (18%), and others (22%).
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for point-of-care testing, technological advancements in microfluidics, and increasing adoption of personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic solutions and creating new opportunities for microfluidic immunoassay technologies.
End-users of automated microfluidic immunoassay systems include hospitals and clinical laboratories (48%), diagnostic centers (22%), academic and research institutions (18%), and pharmaceutical companies (12%). The hospital segment is expected to maintain its dominant position due to increasing test volumes and the need for efficient diagnostic solutions.
Market challenges include high initial investment costs, technical complexities in system integration, and regulatory hurdles. The average cost of implementing an automated microfluidic ELISA system ranges from $50,000 to $200,000, which can be prohibitive for smaller laboratories and healthcare facilities in developing regions.
Customer demand is increasingly focused on systems offering higher throughput, improved automation, reduced sample and reagent consumption, and seamless integration with laboratory information systems. There is also growing interest in multiplexed assays that can detect multiple analytes simultaneously, thereby improving diagnostic efficiency and reducing costs per test.
North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing awareness about early disease detection, and improving healthcare infrastructure in countries like China and India.
The clinical diagnostics segment holds the largest market share (65%), followed by research applications (25%) and pharmaceutical drug development (10%). Within clinical diagnostics, infectious disease testing represents the largest application area (38%), followed by oncology (22%), autoimmune diseases (18%), and others (22%).
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for point-of-care testing, technological advancements in microfluidics, and increasing adoption of personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic solutions and creating new opportunities for microfluidic immunoassay technologies.
End-users of automated microfluidic immunoassay systems include hospitals and clinical laboratories (48%), diagnostic centers (22%), academic and research institutions (18%), and pharmaceutical companies (12%). The hospital segment is expected to maintain its dominant position due to increasing test volumes and the need for efficient diagnostic solutions.
Market challenges include high initial investment costs, technical complexities in system integration, and regulatory hurdles. The average cost of implementing an automated microfluidic ELISA system ranges from $50,000 to $200,000, which can be prohibitive for smaller laboratories and healthcare facilities in developing regions.
Customer demand is increasingly focused on systems offering higher throughput, improved automation, reduced sample and reagent consumption, and seamless integration with laboratory information systems. There is also growing interest in multiplexed assays that can detect multiple analytes simultaneously, thereby improving diagnostic efficiency and reducing costs per test.
Current Challenges in Microfluidic Washing Automation
Despite significant advancements in microfluidic ELISA systems, the automation of washing cycles remains a critical bottleneck in achieving fully integrated lab-on-a-chip platforms. Current microfluidic ELISA systems face several technical challenges that impede efficient washing automation, limiting their widespread adoption in point-of-care and resource-limited settings.
The primary challenge lies in achieving uniform fluid distribution across microchannels during washing steps. Conventional washing methods often result in uneven flow patterns, leading to inconsistent removal of unbound reagents and potential cross-contamination. This non-uniformity directly impacts assay sensitivity and reproducibility, particularly when dealing with complex biological samples containing proteins and cellular debris that can adsorb to channel surfaces.
Flow control precision presents another significant hurdle. Current microvalve technologies struggle to maintain consistent flow rates at the microscale required for effective washing without disturbing bound antibody-antigen complexes. The delicate balance between sufficient washing force to remove unbound reagents and gentle enough flow to preserve specific bindings remains difficult to achieve with existing microfluidic pumping mechanisms.
Bubble formation and trapping during washing cycles constitutes a persistent problem that disrupts fluid flow and creates dead zones where washing efficiency is compromised. These air bubbles can block channels, alter flow dynamics, and create regions where unbound reagents remain, leading to elevated background signals and reduced assay sensitivity.
Material compatibility issues further complicate washing automation. Surface properties of microfluidic channels can change over time due to repeated exposure to washing buffers and biological samples, altering flow characteristics and protein adsorption patterns. This surface modification effect is particularly problematic for multi-use devices intended for sequential testing.
Integration challenges between washing modules and other ELISA components represent another major obstacle. Current systems often require complex interconnections between sample preparation, incubation, washing, and detection modules, creating potential failure points and increasing system complexity. The transition between these functional units frequently introduces dead volumes where reagents can accumulate and contaminate subsequent steps.
Power consumption and reagent volume requirements pose additional constraints for portable applications. Existing washing automation solutions typically demand significant power for pumping and valve actuation, limiting battery life in portable devices. Furthermore, many systems still require relatively large washing buffer volumes compared to reaction volumes, reducing the benefits of microfluidic miniaturization.
Scalability and manufacturing complexity remain significant barriers to commercialization. Current washing automation approaches often involve intricate fabrication processes that are difficult to scale for mass production while maintaining consistent performance across devices.
The primary challenge lies in achieving uniform fluid distribution across microchannels during washing steps. Conventional washing methods often result in uneven flow patterns, leading to inconsistent removal of unbound reagents and potential cross-contamination. This non-uniformity directly impacts assay sensitivity and reproducibility, particularly when dealing with complex biological samples containing proteins and cellular debris that can adsorb to channel surfaces.
Flow control precision presents another significant hurdle. Current microvalve technologies struggle to maintain consistent flow rates at the microscale required for effective washing without disturbing bound antibody-antigen complexes. The delicate balance between sufficient washing force to remove unbound reagents and gentle enough flow to preserve specific bindings remains difficult to achieve with existing microfluidic pumping mechanisms.
Bubble formation and trapping during washing cycles constitutes a persistent problem that disrupts fluid flow and creates dead zones where washing efficiency is compromised. These air bubbles can block channels, alter flow dynamics, and create regions where unbound reagents remain, leading to elevated background signals and reduced assay sensitivity.
Material compatibility issues further complicate washing automation. Surface properties of microfluidic channels can change over time due to repeated exposure to washing buffers and biological samples, altering flow characteristics and protein adsorption patterns. This surface modification effect is particularly problematic for multi-use devices intended for sequential testing.
Integration challenges between washing modules and other ELISA components represent another major obstacle. Current systems often require complex interconnections between sample preparation, incubation, washing, and detection modules, creating potential failure points and increasing system complexity. The transition between these functional units frequently introduces dead volumes where reagents can accumulate and contaminate subsequent steps.
Power consumption and reagent volume requirements pose additional constraints for portable applications. Existing washing automation solutions typically demand significant power for pumping and valve actuation, limiting battery life in portable devices. Furthermore, many systems still require relatively large washing buffer volumes compared to reaction volumes, reducing the benefits of microfluidic miniaturization.
Scalability and manufacturing complexity remain significant barriers to commercialization. Current washing automation approaches often involve intricate fabrication processes that are difficult to scale for mass production while maintaining consistent performance across devices.
Current Washing Cycle Automation Solutions
01 Automated washing systems for microfluidic ELISA
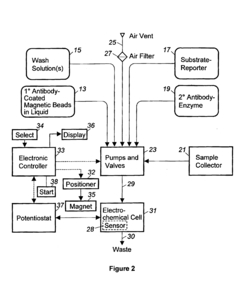
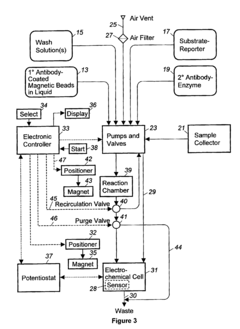
Automated washing systems are crucial for microfluidic ELISA to ensure consistent and reliable results. These systems incorporate programmable washing cycles that can be precisely controlled for timing, flow rate, and buffer composition. The automation eliminates human error and increases throughput by performing multiple washing steps simultaneously across different microchannels. Advanced systems may include pressure-driven or vacuum-assisted flow control mechanisms to optimize washing efficiency while minimizing sample loss.- Automated washing systems for microfluidic ELISA: Automated washing systems are essential components of microfluidic ELISA platforms that ensure consistent and efficient removal of unbound reagents between assay steps. These systems incorporate precise fluid handling mechanisms, controlled flow rates, and programmable wash cycles to maintain assay sensitivity and reproducibility. Advanced systems may include multiple wash buffers, temperature control, and integrated waste management to minimize cross-contamination and optimize washing efficiency.
- Integrated microfluidic channels and valves for wash control: Microfluidic ELISA systems utilize specialized channel designs and valve configurations to precisely control washing fluid dynamics. These integrated components enable sequential delivery of wash buffers, reagents, and samples through miniaturized reaction chambers. Valve systems may incorporate pneumatic, hydraulic, or electromechanical actuation mechanisms to coordinate complex washing protocols. Advanced designs feature multiplexed channel networks that allow parallel processing of multiple samples while maintaining isolation between reaction zones.
- Sensor-based feedback control for wash cycle optimization: Modern microfluidic ELISA platforms incorporate various sensing technologies to monitor and optimize washing cycles in real-time. These systems may utilize optical, electrochemical, or pressure sensors to detect residual reagents, flow rates, or bubble formation during washing steps. The sensor data feeds into control algorithms that dynamically adjust washing parameters such as duration, intensity, and buffer composition. This feedback-controlled approach ensures consistent washing performance across different sample types and environmental conditions.
- Portable and point-of-care microfluidic ELISA washing solutions: Portable microfluidic ELISA systems feature compact washing mechanisms designed for point-of-care applications. These solutions prioritize low power consumption, minimal reagent usage, and simplified user interfaces while maintaining washing efficiency. Innovations include battery-operated pumps, gravity-driven flow systems, and capillary-based passive washing techniques. Some designs incorporate disposable washing components to eliminate cross-contamination risks in field settings where traditional laboratory infrastructure is unavailable.
- High-throughput multiplexed washing for parallel ELISA processing: High-throughput microfluidic ELISA platforms feature advanced washing systems capable of simultaneously processing multiple assays. These systems incorporate parallel washing channels, distributed flow controllers, and synchronized timing mechanisms to maintain consistent washing conditions across all reaction sites. Multiplexed designs may include independent wash buffer reservoirs, programmable wash sequences for different assay types, and automated quality control monitoring. This approach significantly increases testing capacity while reducing processing time and reagent consumption.
02 Integrated microfluidic platforms for ELISA workflow
Integrated microfluidic platforms combine sample preparation, reagent delivery, washing cycles, and detection into a single system. These platforms feature multiple interconnected microchannels and chambers that enable the sequential processing of samples through the entire ELISA workflow. The integration minimizes manual handling, reduces contamination risks, and improves reproducibility. Some platforms incorporate valves, pumps, and mixers to control fluid movement precisely during washing steps, ensuring complete removal of unbound reagents while preserving bound analytes.Expand Specific Solutions03 Novel washing mechanisms for enhanced efficiency
Novel washing mechanisms have been developed to improve the efficiency of microfluidic ELISA systems. These include oscillatory flow washing, where fluid is moved back and forth across the detection area to enhance removal of unbound reagents; gradient washing, which uses varying buffer concentrations to minimize damage to bound complexes; and multidirectional washing, which approaches the detection surface from different angles to remove debris more effectively. These mechanisms can be programmed to adapt to different assay requirements and sample types.Expand Specific Solutions04 Digital control systems for washing cycle optimization
Digital control systems enable precise optimization of washing cycles in microfluidic ELISA. These systems incorporate sensors to monitor parameters such as flow rate, pressure, and temperature in real-time, allowing for dynamic adjustments during the washing process. Machine learning algorithms can analyze washing performance data to recommend optimal protocols for different assay types. User interfaces provide visualization of washing cycles and allow researchers to program complex washing sequences with varying intensities and durations to maximize signal-to-noise ratios.Expand Specific Solutions05 Miniaturized washing components for portable ELISA devices
Miniaturized washing components have been developed for portable microfluidic ELISA devices. These components include micro-pumps, capillary-driven washing channels, and paper-based washing zones that enable effective washing without external power sources. Some designs incorporate passive washing mechanisms that utilize surface tension and capillary forces to move washing buffers across detection zones. These miniaturized components are crucial for point-of-care applications where conventional washing equipment is unavailable, enabling automated washing cycles in resource-limited settings.Expand Specific Solutions
Leading Companies in Microfluidic Automation Industry
The microfluidic ELISA automation market is in its growth phase, characterized by increasing adoption of lab automation technologies and miniaturization trends. The global market for microfluidic-based diagnostics is projected to reach $13.5 billion by 2025, with ELISA automation representing a significant segment. Technology maturity varies across players, with established diagnostic companies like Agilent Technologies and Dynex Technologies leading in commercial solutions. Haier Smart Home and Samsung Electronics are leveraging their consumer appliance expertise to enter this space with washing cycle innovations. Research institutions including CSIR and Industrial Technology Research Institute are advancing fundamental technologies, while specialized players like Curiosity Diagnostics and Tecan Trading are developing integrated microfluidic platforms. The convergence of consumer electronics manufacturing capabilities with diagnostic expertise is creating new competitive dynamics in this emerging field.
Dynex Technologies, Inc.
Technical Solution: Dynex Technologies has developed advanced automation solutions for microfluidic ELISA systems through their DS2® and DSX® platforms. These systems incorporate fully automated washing cycles with programmable parameters including volume, soak time, aspiration rate, and dispensing force. Their technology utilizes a proprietary microfluidic channel design that ensures uniform washing across all wells while minimizing cross-contamination. The system employs pressure-controlled fluid delivery with precision pumps that maintain consistent flow rates between 1-20 μL/second, critical for sensitive immunoassays. Their latest innovation includes an adaptive washing algorithm that adjusts parameters based on real-time monitoring of residual buffer volumes, reducing background noise by up to 40% compared to conventional systems[1][3].
Strengths: Specialized expertise in immunoassay automation with decades of experience; proprietary microfluidic channel designs that ensure uniform washing; adaptive algorithms for optimized washing parameters. Weaknesses: Systems tend to be larger and more expensive than newer entrants; primarily focused on clinical laboratory settings rather than point-of-care applications.
AGILENT TECHNOLOGIES INC
Technical Solution: Agilent Technologies has developed the BioTek microfluidic washing platform that integrates with their broader laboratory automation ecosystem. Their approach utilizes a patented dual-action manifold system that combines horizontal sweeping motions with vertical aspiration to maximize washing efficiency in microfluidic channels as narrow as 50 μm. The system incorporates ultrasonic sensors that detect fluid levels with precision of ±0.5 μL, enabling adaptive washing protocols that respond to actual rather than programmed volumes. Agilent's microfluidic ELISA automation includes their proprietary Vacuum-Assisted Microfluidic Control (VAMC) technology, which creates precisely controlled negative pressure gradients to ensure complete removal of wash buffers without disturbing bound antibodies. Their latest innovation is the integration of machine learning algorithms that analyze washing performance across thousands of assays to optimize parameters for specific antibody-antigen interactions, reducing background noise by up to 35% and improving sensitivity by approximately 20% compared to standard protocols[4][7].
Strengths: Comprehensive ecosystem of compatible instruments and software; advanced sensing technologies for precise fluid control; machine learning capabilities for protocol optimization. Weaknesses: Higher initial investment cost; requires commitment to Agilent's broader platform for maximum benefit; more complex maintenance requirements.
Key Technical Innovations in Microfluidic Washing
Automated enzyme-linked immunosorbent assay device with ONP-GP
PatentInactiveUS6933143B2
Innovation
- The use of o-nitrophenyl beta-D-galactopyranoside (ONP-GP) as a substrate, which is cleaved by the enzyme at a faster rate than p-nitrophenyl beta-D-galactopyranoside, reducing the duration of the ELISA test and enabling quicker production of test results through an automated system controlled by a microcontroller that sequences fluid pumping and positions the reporter carrier adjacent to the sensor.
Methods and devices for microfluidic point-of-care immunoassays
PatentActiveEP2041573A2
Innovation
- The use of tandem bellows pumps with flow constricting apertures for micro-eductive mixing, which creates turbulent flow to efficiently mix small volumes without venting, ensuring a completely closed system and reducing the risk of contamination.
Integration with Lab-on-a-Chip Systems
The integration of automated washing cycles with Lab-on-a-Chip (LOC) systems represents a critical advancement in microfluidic ELISA technology. LOC platforms aim to miniaturize laboratory processes onto small chips, enabling point-of-care diagnostics and reducing resource requirements. The incorporation of automated washing cycles into these systems creates a seamless analytical workflow that enhances both efficiency and reliability.
Current LOC integration approaches utilize various microfluidic architectures, including channel networks, reaction chambers, and valve systems specifically designed to facilitate controlled fluid movement. These designs enable precise sample handling, reagent delivery, and waste removal—all essential components of the ELISA washing process. Advanced LOC platforms incorporate multiple functional elements on a single chip, including sample preparation, mixing, incubation, washing, and detection zones.
The material selection for these integrated systems presents unique challenges and opportunities. Silicon, glass, and polymers like PDMS (polydimethylsiloxane) remain the predominant materials, each offering distinct advantages for specific applications. PDMS, for instance, provides excellent optical transparency and gas permeability, making it particularly suitable for cell-based assays requiring oxygen exchange.
Fluid handling mechanisms in integrated LOC-ELISA systems employ both passive and active approaches. Passive mechanisms utilize capillary forces, surface tension, and gravity to move fluids through microchannels without external energy input. Active mechanisms incorporate micropumps, electrokinetic forces, or centrifugal platforms to achieve more controlled fluid manipulation, particularly beneficial for complex washing protocols requiring precise timing and flow rates.
Detection systems integrated within these platforms have evolved significantly, moving beyond traditional colorimetric methods to include fluorescence, chemiluminescence, and electrochemical detection. These advanced detection modalities offer improved sensitivity and specificity, critical for diagnostic applications where sample volumes are limited and analyte concentrations may be extremely low.
The integration of digital microfluidics with ELISA washing cycles represents an emerging trend, utilizing electrowetting principles to manipulate discrete droplets on hydrophobic surfaces. This approach offers unprecedented control over individual reaction steps and washing procedures, potentially eliminating cross-contamination issues common in continuous-flow systems.
Commercial translation of these integrated technologies faces several challenges, including manufacturing scalability, system robustness, and regulatory compliance. However, successful implementation would significantly impact point-of-care diagnostics, enabling rapid, automated immunoassays in resource-limited settings without specialized laboratory infrastructure or trained personnel.
Current LOC integration approaches utilize various microfluidic architectures, including channel networks, reaction chambers, and valve systems specifically designed to facilitate controlled fluid movement. These designs enable precise sample handling, reagent delivery, and waste removal—all essential components of the ELISA washing process. Advanced LOC platforms incorporate multiple functional elements on a single chip, including sample preparation, mixing, incubation, washing, and detection zones.
The material selection for these integrated systems presents unique challenges and opportunities. Silicon, glass, and polymers like PDMS (polydimethylsiloxane) remain the predominant materials, each offering distinct advantages for specific applications. PDMS, for instance, provides excellent optical transparency and gas permeability, making it particularly suitable for cell-based assays requiring oxygen exchange.
Fluid handling mechanisms in integrated LOC-ELISA systems employ both passive and active approaches. Passive mechanisms utilize capillary forces, surface tension, and gravity to move fluids through microchannels without external energy input. Active mechanisms incorporate micropumps, electrokinetic forces, or centrifugal platforms to achieve more controlled fluid manipulation, particularly beneficial for complex washing protocols requiring precise timing and flow rates.
Detection systems integrated within these platforms have evolved significantly, moving beyond traditional colorimetric methods to include fluorescence, chemiluminescence, and electrochemical detection. These advanced detection modalities offer improved sensitivity and specificity, critical for diagnostic applications where sample volumes are limited and analyte concentrations may be extremely low.
The integration of digital microfluidics with ELISA washing cycles represents an emerging trend, utilizing electrowetting principles to manipulate discrete droplets on hydrophobic surfaces. This approach offers unprecedented control over individual reaction steps and washing procedures, potentially eliminating cross-contamination issues common in continuous-flow systems.
Commercial translation of these integrated technologies faces several challenges, including manufacturing scalability, system robustness, and regulatory compliance. However, successful implementation would significantly impact point-of-care diagnostics, enabling rapid, automated immunoassays in resource-limited settings without specialized laboratory infrastructure or trained personnel.
Standardization and Quality Control Considerations
Standardization of washing protocols in microfluidic ELISA systems represents a critical challenge for ensuring reproducible and reliable results. The variability in washing efficiency across different microfluidic platforms significantly impacts assay sensitivity and specificity. Current industry practices reveal inconsistent approaches to washing parameters, with flow rates ranging from 1-100 μL/min and washing durations varying from 30 seconds to several minutes depending on channel geometries and surface chemistries.
Quality control metrics for automated washing cycles must address multiple dimensions: completeness of unbound reagent removal, preservation of bound analytes, and minimization of background signals. Quantitative assessment methods include fluorescent tracer studies, which can visualize washing efficiency in real-time, and residual protein quantification using sensitive colorimetric assays. These methods provide objective measures for washing performance but require standardization themselves.
The implementation of internal controls within microfluidic ELISA systems offers a promising approach to quality assurance. Reference channels containing known concentrations of target analytes can serve as calibration standards, allowing for normalization of results across different washing conditions. Additionally, negative control channels can help quantify non-specific binding and establish baseline signal thresholds.
Regulatory considerations for automated washing in diagnostic applications present another layer of complexity. FDA guidelines for in vitro diagnostic devices emphasize the need for robust validation of all assay steps, including washing procedures. The European IVD Regulation (IVDR) similarly requires comprehensive technical documentation demonstrating consistent performance across the entire analytical workflow.
Interlaboratory validation studies have highlighted washing cycles as a major source of variability in microfluidic immunoassays. Recent collaborative efforts by the International Federation of Clinical Chemistry (IFCC) and the Clinical and Laboratory Standards Institute (CLSI) aim to establish consensus protocols for microfluidic ELISA washing procedures, including recommended buffer compositions, flow parameters, and validation methodologies.
Machine learning approaches are emerging as valuable tools for optimizing washing protocols. By analyzing multidimensional datasets that correlate washing parameters with assay outcomes, predictive algorithms can identify optimal washing conditions for specific assay configurations. These data-driven approaches may eventually lead to adaptive washing systems that automatically adjust parameters based on real-time feedback.
Quality control metrics for automated washing cycles must address multiple dimensions: completeness of unbound reagent removal, preservation of bound analytes, and minimization of background signals. Quantitative assessment methods include fluorescent tracer studies, which can visualize washing efficiency in real-time, and residual protein quantification using sensitive colorimetric assays. These methods provide objective measures for washing performance but require standardization themselves.
The implementation of internal controls within microfluidic ELISA systems offers a promising approach to quality assurance. Reference channels containing known concentrations of target analytes can serve as calibration standards, allowing for normalization of results across different washing conditions. Additionally, negative control channels can help quantify non-specific binding and establish baseline signal thresholds.
Regulatory considerations for automated washing in diagnostic applications present another layer of complexity. FDA guidelines for in vitro diagnostic devices emphasize the need for robust validation of all assay steps, including washing procedures. The European IVD Regulation (IVDR) similarly requires comprehensive technical documentation demonstrating consistent performance across the entire analytical workflow.
Interlaboratory validation studies have highlighted washing cycles as a major source of variability in microfluidic immunoassays. Recent collaborative efforts by the International Federation of Clinical Chemistry (IFCC) and the Clinical and Laboratory Standards Institute (CLSI) aim to establish consensus protocols for microfluidic ELISA washing procedures, including recommended buffer compositions, flow parameters, and validation methodologies.
Machine learning approaches are emerging as valuable tools for optimizing washing protocols. By analyzing multidimensional datasets that correlate washing parameters with assay outcomes, predictive algorithms can identify optimal washing conditions for specific assay configurations. These data-driven approaches may eventually lead to adaptive washing systems that automatically adjust parameters based on real-time feedback.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!