Microfluidic ELISA Using Electrochemical Signal Transduction
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone of clinical diagnostics since its development in the 1970s. This immunoassay technique has evolved significantly over the decades, transitioning from traditional plate-based formats to more sophisticated microfluidic platforms. The integration of microfluidics with ELISA represents a pivotal advancement in diagnostic technology, offering enhanced sensitivity, reduced sample volumes, and accelerated analysis times.
The evolution of microfluidic ELISA has been driven by increasing demands for point-of-care testing capabilities, particularly in resource-limited settings where conventional laboratory infrastructure is unavailable. Historical development shows a clear trajectory from bulky laboratory equipment toward miniaturized, portable systems that maintain or exceed traditional performance metrics.
Recent technological convergence between microfluidics and electrochemical detection methods has created a particularly promising direction. Electrochemical signal transduction offers significant advantages over traditional optical detection methods, including reduced instrumentation complexity, enhanced sensitivity, and improved compatibility with miniaturized platforms.
The primary objective of microfluidic ELISA using electrochemical signal transduction is to develop highly sensitive, specific, and rapid diagnostic platforms that can be deployed in diverse settings with minimal infrastructure requirements. This technology aims to bridge the gap between sophisticated laboratory diagnostics and field-deployable testing solutions.
Current research focuses on several key technical goals: optimizing electrode materials and configurations for maximum sensitivity; developing stable and reproducible surface functionalization strategies; integrating sample preparation steps within the microfluidic architecture; and creating robust signal processing algorithms to enhance detection limits and dynamic range.
The field is witnessing accelerated development of multiplexed detection capabilities, allowing simultaneous analysis of multiple biomarkers from a single sample. This trend aligns with the growing emphasis on comprehensive diagnostic profiles rather than single-marker tests, particularly for complex conditions like cancer, autoimmune disorders, and infectious diseases.
Emerging trends indicate increasing integration of artificial intelligence and machine learning algorithms to enhance data interpretation and diagnostic accuracy. These computational approaches are particularly valuable for analyzing complex electrochemical signals and identifying subtle patterns that may indicate early disease states.
The ultimate technological objective is to create fully integrated, sample-to-answer diagnostic platforms that combine the specificity of immunoassays with the sensitivity of electrochemical detection and the efficiency of microfluidic sample handling. Such systems would represent a significant advancement in accessible healthcare diagnostics, potentially transforming disease management in both developed and developing regions.
The evolution of microfluidic ELISA has been driven by increasing demands for point-of-care testing capabilities, particularly in resource-limited settings where conventional laboratory infrastructure is unavailable. Historical development shows a clear trajectory from bulky laboratory equipment toward miniaturized, portable systems that maintain or exceed traditional performance metrics.
Recent technological convergence between microfluidics and electrochemical detection methods has created a particularly promising direction. Electrochemical signal transduction offers significant advantages over traditional optical detection methods, including reduced instrumentation complexity, enhanced sensitivity, and improved compatibility with miniaturized platforms.
The primary objective of microfluidic ELISA using electrochemical signal transduction is to develop highly sensitive, specific, and rapid diagnostic platforms that can be deployed in diverse settings with minimal infrastructure requirements. This technology aims to bridge the gap between sophisticated laboratory diagnostics and field-deployable testing solutions.
Current research focuses on several key technical goals: optimizing electrode materials and configurations for maximum sensitivity; developing stable and reproducible surface functionalization strategies; integrating sample preparation steps within the microfluidic architecture; and creating robust signal processing algorithms to enhance detection limits and dynamic range.
The field is witnessing accelerated development of multiplexed detection capabilities, allowing simultaneous analysis of multiple biomarkers from a single sample. This trend aligns with the growing emphasis on comprehensive diagnostic profiles rather than single-marker tests, particularly for complex conditions like cancer, autoimmune disorders, and infectious diseases.
Emerging trends indicate increasing integration of artificial intelligence and machine learning algorithms to enhance data interpretation and diagnostic accuracy. These computational approaches are particularly valuable for analyzing complex electrochemical signals and identifying subtle patterns that may indicate early disease states.
The ultimate technological objective is to create fully integrated, sample-to-answer diagnostic platforms that combine the specificity of immunoassays with the sensitivity of electrochemical detection and the efficiency of microfluidic sample handling. Such systems would represent a significant advancement in accessible healthcare diagnostics, potentially transforming disease management in both developed and developing regions.
Market Analysis for Microfluidic Immunoassay Technologies
The global microfluidic immunoassay market is experiencing robust growth, driven by increasing demand for point-of-care testing and personalized medicine. The market was valued at approximately $2.5 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 14.8%. This growth trajectory is particularly significant for microfluidic ELISA technologies utilizing electrochemical signal transduction, which are gaining traction due to their enhanced sensitivity and potential for miniaturization.
Healthcare facilities represent the largest end-user segment, accounting for 42% of the market share, followed by academic and research institutions at 28%. Pharmaceutical and biotechnology companies constitute 21% of the market, with the remaining 9% distributed among other end-users. Geographically, North America leads with 38% of the market share, followed by Europe (29%), Asia-Pacific (24%), and the rest of the world (9%). The Asia-Pacific region is expected to witness the highest growth rate of 17.2% during the forecast period, primarily due to increasing healthcare expenditure and growing awareness about early disease diagnosis.
The demand for microfluidic immunoassay technologies is being driven by several factors. First, the rising prevalence of chronic and infectious diseases necessitates rapid and accurate diagnostic solutions. Second, the growing elderly population worldwide requires more frequent monitoring and testing. Third, technological advancements in microfluidics and electrochemical detection methods are enhancing the performance and reducing the cost of these systems.
Market segmentation reveals that clinical diagnostics applications dominate with 45% market share, followed by research applications (30%), food safety testing (15%), and environmental monitoring (10%). Within clinical diagnostics, infectious disease testing represents the largest segment (38%), followed by cardiac markers (22%), hormone testing (18%), cancer biomarkers (14%), and others (8%).
Key customer needs identified include faster time-to-result, higher sensitivity and specificity, reduced sample volume requirements, ease of use, and cost-effectiveness. Electrochemical signal transduction in microfluidic ELISA addresses many of these needs by offering rapid analysis times (typically under 30 minutes), detection limits in the picogram range, sample volumes of less than 100 microliters, and potential for integration with portable devices.
The market is also witnessing a shift toward multiplexed assays that can detect multiple analytes simultaneously, integrated sample preparation capabilities, and connectivity features for data management and telemedicine applications. These trends align well with the capabilities of electrochemical microfluidic platforms, positioning them favorably for future market growth.
Healthcare facilities represent the largest end-user segment, accounting for 42% of the market share, followed by academic and research institutions at 28%. Pharmaceutical and biotechnology companies constitute 21% of the market, with the remaining 9% distributed among other end-users. Geographically, North America leads with 38% of the market share, followed by Europe (29%), Asia-Pacific (24%), and the rest of the world (9%). The Asia-Pacific region is expected to witness the highest growth rate of 17.2% during the forecast period, primarily due to increasing healthcare expenditure and growing awareness about early disease diagnosis.
The demand for microfluidic immunoassay technologies is being driven by several factors. First, the rising prevalence of chronic and infectious diseases necessitates rapid and accurate diagnostic solutions. Second, the growing elderly population worldwide requires more frequent monitoring and testing. Third, technological advancements in microfluidics and electrochemical detection methods are enhancing the performance and reducing the cost of these systems.
Market segmentation reveals that clinical diagnostics applications dominate with 45% market share, followed by research applications (30%), food safety testing (15%), and environmental monitoring (10%). Within clinical diagnostics, infectious disease testing represents the largest segment (38%), followed by cardiac markers (22%), hormone testing (18%), cancer biomarkers (14%), and others (8%).
Key customer needs identified include faster time-to-result, higher sensitivity and specificity, reduced sample volume requirements, ease of use, and cost-effectiveness. Electrochemical signal transduction in microfluidic ELISA addresses many of these needs by offering rapid analysis times (typically under 30 minutes), detection limits in the picogram range, sample volumes of less than 100 microliters, and potential for integration with portable devices.
The market is also witnessing a shift toward multiplexed assays that can detect multiple analytes simultaneously, integrated sample preparation capabilities, and connectivity features for data management and telemedicine applications. These trends align well with the capabilities of electrochemical microfluidic platforms, positioning them favorably for future market growth.
Electrochemical Signal Transduction: Current Status and Challenges
Electrochemical signal transduction has emerged as a powerful detection method in microfluidic ELISA systems, offering advantages in sensitivity, miniaturization, and quantification capabilities. Currently, the field employs various techniques including amperometry, voltammetry, impedance spectroscopy, and potentiometry, each with distinct operational principles and applications. Amperometric detection dominates commercial applications due to its relative simplicity and direct correlation between analyte concentration and measured current.
Despite significant progress, several technical challenges persist in this domain. Signal-to-noise ratio remains problematic, particularly in complex biological matrices where non-specific binding and interfering electroactive species can compromise detection accuracy. This issue becomes more pronounced at ultralow analyte concentrations, where distinguishing true signals from background noise requires sophisticated signal processing algorithms.
Electrode fouling represents another significant challenge, as biomolecules from samples can adsorb onto electrode surfaces, gradually degrading performance and limiting device longevity. Current solutions involving surface modifications with anti-fouling materials show promise but often introduce additional manufacturing complexity and cost considerations.
Standardization across different platforms presents a persistent obstacle, with variations in electrode materials, surface chemistries, and detection protocols making cross-platform result comparison difficult. This fragmentation impedes clinical translation and regulatory approval processes, as reproducibility and reliability standards remain inconsistent across research groups and commercial entities.
Miniaturization efforts face fundamental electrochemical constraints, particularly as electrode dimensions approach the micro and nanoscale. At these dimensions, mass transport limitations, double-layer capacitance effects, and increased electrode impedance can significantly alter electrochemical behavior, requiring specialized design considerations and signal processing techniques.
Geographically, electrochemical biosensor development shows distinct regional characteristics. North American institutions lead in fundamental research and intellectual property generation, while Asian manufacturers, particularly in China and South Korea, dominate production scaling and cost optimization. European research centers excel in developing novel electrode materials and surface modification strategies.
Integration with other detection modalities represents both a challenge and opportunity. Combining electrochemical detection with optical or mechanical sensing could provide complementary data streams and improved diagnostic accuracy, but requires sophisticated signal processing and system integration approaches that are still under development.
Power requirements for portable and point-of-care applications remain problematic, with sensitive electrochemical measurements often demanding stable power sources and precise control circuitry that conflict with miniaturization goals. Recent advances in low-power electronics and energy harvesting show promise but have yet to be widely implemented in commercial systems.
Despite significant progress, several technical challenges persist in this domain. Signal-to-noise ratio remains problematic, particularly in complex biological matrices where non-specific binding and interfering electroactive species can compromise detection accuracy. This issue becomes more pronounced at ultralow analyte concentrations, where distinguishing true signals from background noise requires sophisticated signal processing algorithms.
Electrode fouling represents another significant challenge, as biomolecules from samples can adsorb onto electrode surfaces, gradually degrading performance and limiting device longevity. Current solutions involving surface modifications with anti-fouling materials show promise but often introduce additional manufacturing complexity and cost considerations.
Standardization across different platforms presents a persistent obstacle, with variations in electrode materials, surface chemistries, and detection protocols making cross-platform result comparison difficult. This fragmentation impedes clinical translation and regulatory approval processes, as reproducibility and reliability standards remain inconsistent across research groups and commercial entities.
Miniaturization efforts face fundamental electrochemical constraints, particularly as electrode dimensions approach the micro and nanoscale. At these dimensions, mass transport limitations, double-layer capacitance effects, and increased electrode impedance can significantly alter electrochemical behavior, requiring specialized design considerations and signal processing techniques.
Geographically, electrochemical biosensor development shows distinct regional characteristics. North American institutions lead in fundamental research and intellectual property generation, while Asian manufacturers, particularly in China and South Korea, dominate production scaling and cost optimization. European research centers excel in developing novel electrode materials and surface modification strategies.
Integration with other detection modalities represents both a challenge and opportunity. Combining electrochemical detection with optical or mechanical sensing could provide complementary data streams and improved diagnostic accuracy, but requires sophisticated signal processing and system integration approaches that are still under development.
Power requirements for portable and point-of-care applications remain problematic, with sensitive electrochemical measurements often demanding stable power sources and precise control circuitry that conflict with miniaturization goals. Recent advances in low-power electronics and energy harvesting show promise but have yet to be widely implemented in commercial systems.
Current Electrochemical Detection Methods in Microfluidic ELISA
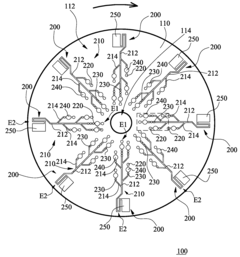
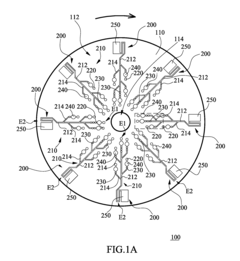
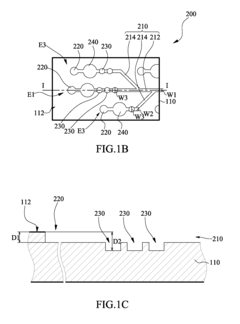
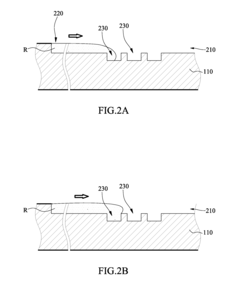
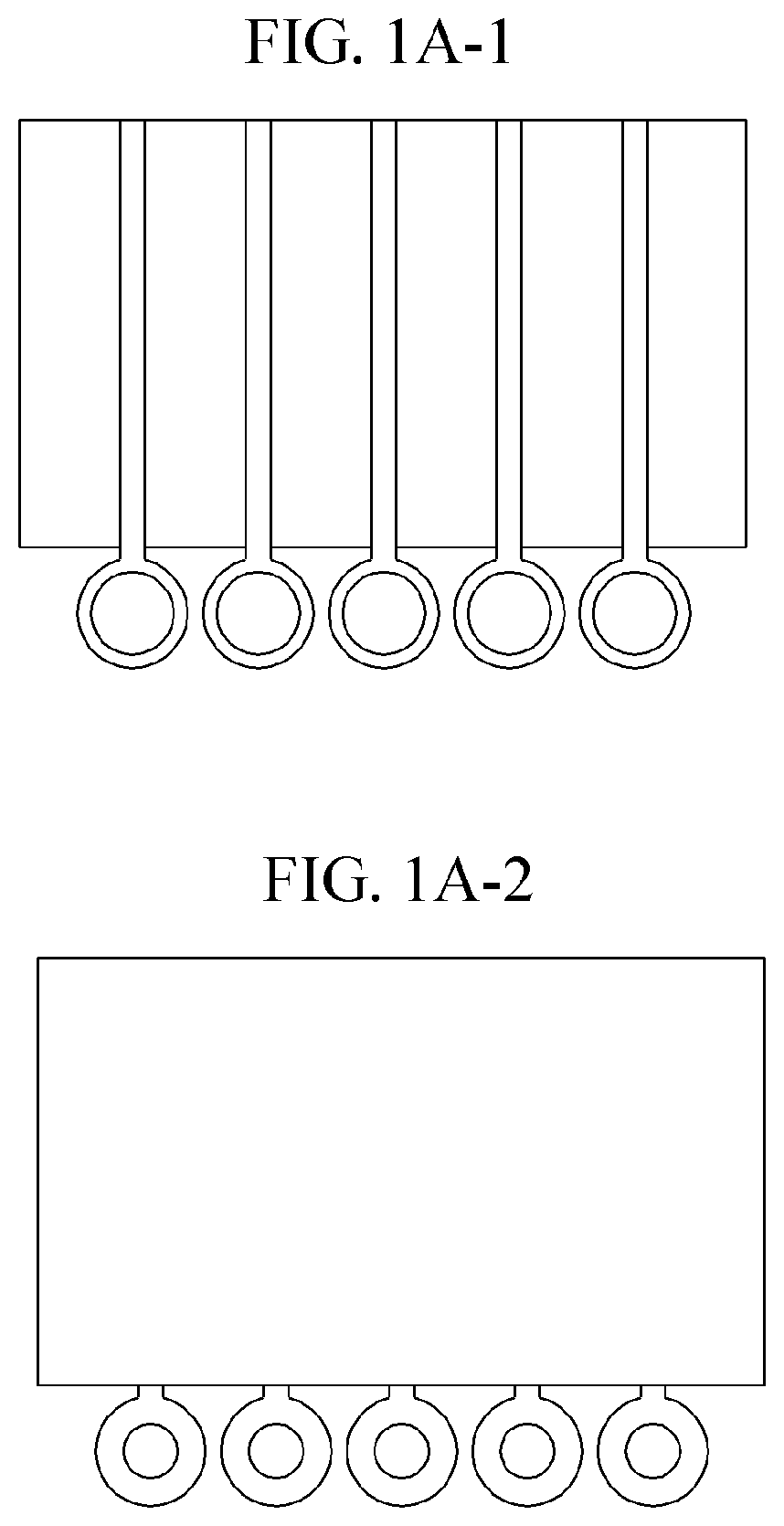
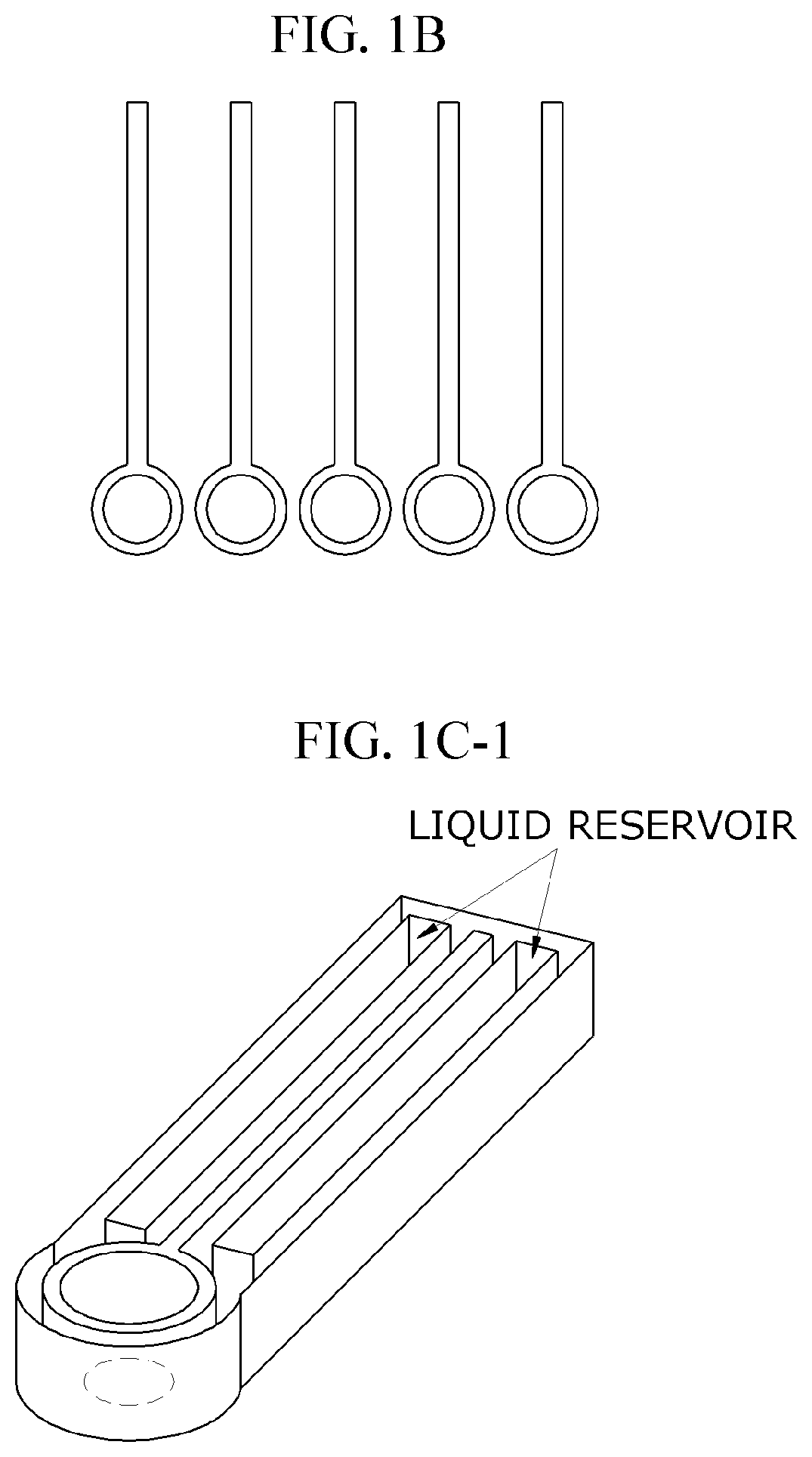
01 Microfluidic platforms for electrochemical ELISA detection
Microfluidic platforms integrate sample preparation, reagent handling, and electrochemical detection for ELISA assays. These systems utilize microchannels and chambers to control fluid flow, enabling precise manipulation of small sample volumes. The miniaturized format reduces reagent consumption, decreases analysis time, and improves sensitivity compared to traditional ELISA methods. These platforms often incorporate electrodes directly within the microfluidic channels for real-time electrochemical signal transduction.- Microfluidic platforms for electrochemical ELISA detection: Microfluidic platforms integrate sample preparation, reagent handling, and electrochemical detection for ELISA assays. These platforms utilize microchannels and chambers to control fluid flow, enabling precise manipulation of small sample volumes. The integration of electrodes within these microfluidic structures allows for direct electrochemical signal transduction, enhancing sensitivity and reducing analysis time compared to traditional ELISA methods. These systems often incorporate automated sample processing steps to improve reproducibility and throughput.
- Electrode materials and modifications for enhanced signal transduction: Various electrode materials and surface modifications are employed to enhance electrochemical signal transduction in microfluidic ELISA systems. Materials such as gold, carbon, and conductive polymers serve as electrode substrates, while surface modifications including nanoparticles, graphene, and conductive polymers improve electron transfer efficiency. These modifications increase the electroactive surface area and facilitate better immobilization of capture antibodies or enzymes, resulting in improved sensitivity and lower detection limits for target analytes.
- Enzyme-based signal amplification strategies: Enzyme-based signal amplification strategies enhance the sensitivity of electrochemical detection in microfluidic ELISA. Enzymes like horseradish peroxidase and alkaline phosphatase catalyze reactions that generate electroactive species, which are then detected at the electrode surface. Advanced approaches include enzyme cascades, where multiple enzymes work in sequence to amplify the signal, and the use of enzyme-loaded nanoparticles to increase the local concentration of catalytic molecules. These strategies significantly lower detection limits and improve assay performance in complex biological samples.
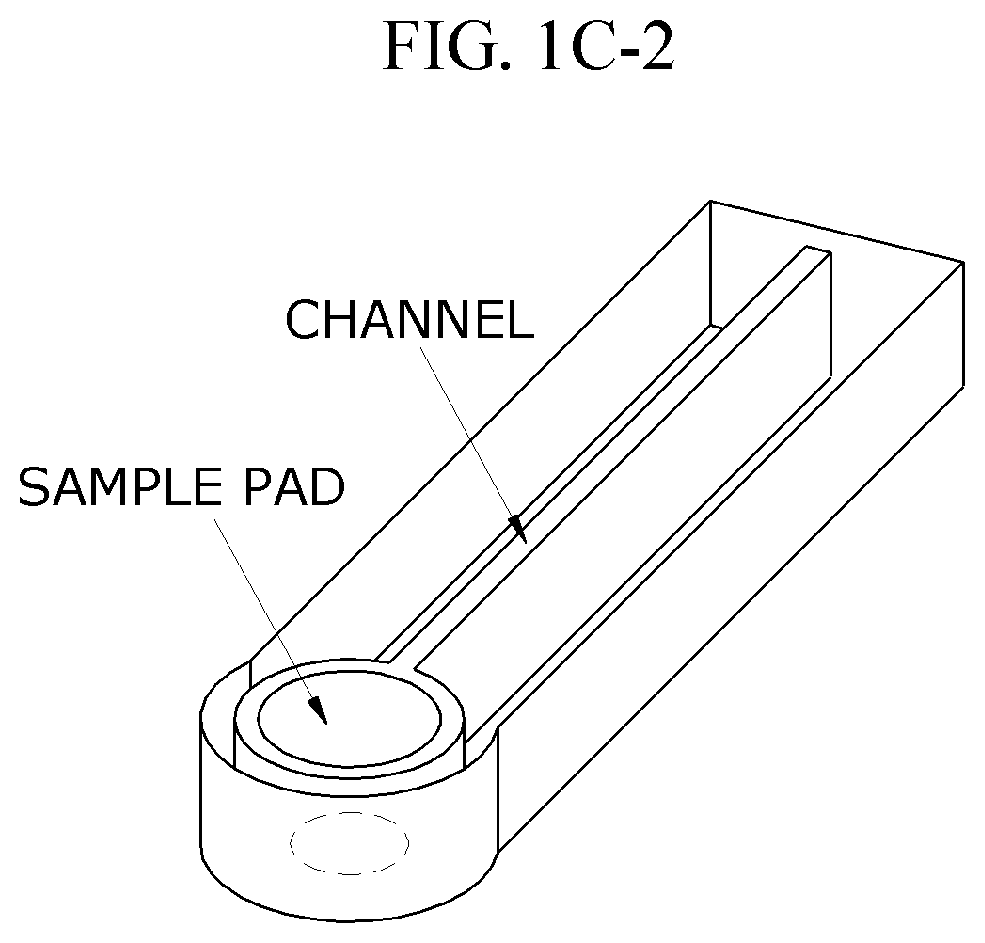
- Integration of sample preparation and electrochemical detection: Integrated microfluidic systems combine sample preparation steps with electrochemical detection for streamlined ELISA workflows. These systems incorporate modules for sample filtration, cell lysis, target enrichment, and reagent mixing prior to electrochemical detection. The integration minimizes sample loss, reduces contamination risks, and decreases analysis time. Advanced systems may include multiple detection chambers for multiplexed analysis or incorporate gradient generators for simultaneous testing under different conditions, enhancing the versatility and throughput of microfluidic electrochemical ELISA platforms.
- Portable and point-of-care electrochemical ELISA devices: Portable microfluidic electrochemical ELISA devices enable point-of-care testing in resource-limited settings. These devices integrate miniaturized pumps, valves, and electrochemical detection systems into compact, user-friendly formats. They often incorporate smartphone-based readout systems or dedicated portable potentiostats for signal processing and data analysis. Power requirements are minimized through efficient circuit design and low-power components, allowing operation with batteries or small power sources. These innovations make sophisticated immunoassay technology accessible for field use, remote diagnostics, and decentralized healthcare applications.
02 Electrode materials and modifications for enhanced signal transduction
Various electrode materials and surface modifications are employed to enhance electrochemical signal transduction in microfluidic ELISA. Materials such as gold, carbon, and platinum serve as base electrodes, while modifications with nanomaterials (carbon nanotubes, graphene, metal nanoparticles) significantly increase the electroactive surface area and electron transfer efficiency. Functionalization with biomolecules improves specificity and sensitivity of detection. These electrode enhancements enable lower detection limits and wider dynamic ranges for analyte quantification.Expand Specific Solutions03 Integrated signal processing and readout systems
Integrated signal processing systems convert electrochemical responses from microfluidic ELISA into quantifiable results. These systems incorporate potentiostats, signal amplifiers, and analog-to-digital converters to process the electrical signals generated during electrochemical reactions. Advanced systems feature wireless connectivity, cloud data storage, and smartphone interfaces for point-of-care applications. Machine learning algorithms may be employed to analyze complex signal patterns and improve diagnostic accuracy, enabling rapid and sensitive detection of biomarkers.Expand Specific Solutions04 Multiplexed detection strategies
Multiplexed detection strategies enable simultaneous analysis of multiple analytes within a single microfluidic ELISA platform. These approaches utilize electrode arrays, spatially separated detection zones, or frequency-based discrimination methods to distinguish between different target molecules. Multiplexing reduces sample volume requirements, analysis time, and cost while providing comprehensive diagnostic information. Advanced signal processing algorithms help differentiate overlapping electrochemical signals and minimize cross-reactivity between detection channels.Expand Specific Solutions05 Novel redox mediators and enzymatic labels
Novel redox mediators and enzymatic labels enhance the sensitivity and specificity of electrochemical signal transduction in microfluidic ELISA. Engineered enzymes like horseradish peroxidase and alkaline phosphatase generate electroactive species that amplify detection signals. Redox mediators facilitate electron transfer between enzymes and electrode surfaces, improving signal generation efficiency. Nanoparticle-based labels combine catalytic properties with large surface areas to further amplify signals. These innovations enable detection of biomarkers at femtomolar concentrations, suitable for early disease diagnosis.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidic Diagnostics
Microfluidic ELISA using electrochemical signal transduction is in an emerging growth phase, with the global market expanding due to increasing demand for point-of-care diagnostics. The technology combines microfluidics with electrochemical detection to enhance traditional ELISA sensitivity and portability. Leading institutions like California Institute of Technology, Johns Hopkins University, and Beijing University of Chemical Technology are advancing core innovations, while companies such as Mitsubishi Electric, TOPPAN, and Beijing Wantai Biological are commercializing applications. The field is approaching early maturity with established proof-of-concept demonstrations, but still requires further development for widespread clinical adoption. Industry-academic collaborations between entities like Industrial Technology Research Institute and universities are accelerating technological refinement and market readiness.
President & Fellows of Harvard College
Technical Solution: Harvard College has developed an advanced microfluidic ELISA platform that integrates electrochemical detection with digital microfluidics. Their system utilizes electrowetting-on-dielectric (EWOD) technology to manipulate nanoliter-scale droplets containing samples and reagents. The electrochemical detection is achieved through integrated microelectrodes that measure redox reactions from enzymatic products. Harvard's approach incorporates multiplexed detection capabilities, allowing simultaneous analysis of multiple biomarkers from a single sample. Their platform features automated sample processing with precise control over reaction conditions, significantly reducing reagent consumption by up to 100-fold compared to conventional ELISA methods. The system employs amperometric detection techniques with specialized electrode materials to enhance sensitivity, achieving detection limits in the picomolar range for various protein biomarkers.
Strengths: Exceptional sensitivity (picomolar detection limits), significant reagent reduction, multiplexed analysis capabilities, and automated operation. Weaknesses: Requires specialized fabrication techniques, potential challenges in mass production, and higher initial investment compared to traditional ELISA platforms.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has pioneered a microfluidic ELISA system with electrochemical detection that focuses on point-of-care diagnostics. Their technology employs a paper-based microfluidic platform integrated with screen-printed carbon electrodes for cost-effective manufacturing. The system utilizes a novel flow control mechanism that enables sequential delivery of reagents without external pumping equipment. Their electrochemical detection strategy incorporates nanomaterial-modified electrodes (including gold nanoparticles and carbon nanotubes) to amplify signals and improve sensitivity. The platform features smartphone integration for signal readout and data analysis, making it suitable for resource-limited settings. Johns Hopkins researchers have demonstrated clinical utility through detection of infectious disease biomarkers with performance comparable to laboratory-based methods but at a fraction of the cost and time.
Strengths: Low-cost materials, portable design suitable for field use, smartphone integration for easy readout, and minimal external equipment requirements. Weaknesses: Potentially lower sensitivity compared to more sophisticated lab-based systems and limited multiplexing capabilities.
Key Patents and Innovations in Electrochemical Signal Transduction
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Lateral-flow microfluidic chip and flow velocity control method thereof
PatentInactiveUS20190374940A1
Innovation
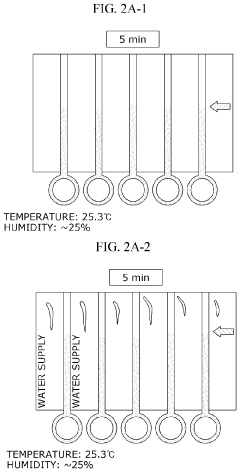
- Increasing vapor pressure around specific channels in the microfluidic chip by using a separate vapor supply device or a liquid reservoir adjacent to the channel, which suppresses fluid evaporation and accelerates flow velocity, allowing for efficient sequential reactions without additional processes or equipment.
Regulatory Framework for Point-of-Care Diagnostic Devices
The regulatory landscape for point-of-care diagnostic devices incorporating microfluidic ELISA with electrochemical signal transduction is complex and multifaceted. In the United States, the Food and Drug Administration (FDA) classifies these devices primarily under the in vitro diagnostic (IVD) regulatory framework, with most microfluidic ELISA systems falling into Class II or Class III categories depending on their intended use and risk profile. The 510(k) clearance pathway is commonly applicable for devices similar to predicate technologies, while novel implementations may require the more rigorous Premarket Approval (PMA) process.
European regulations have undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive. This regulation imposes stricter requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic electrochemical ELISA systems are typically classified as Class B or C devices under the IVDR, necessitating conformity assessment involving notified bodies.
In emerging markets, particularly China, the National Medical Products Administration (NMPA) has established specific pathways for innovative diagnostic technologies. Their "Special Review Procedure for Innovative Medical Devices" can expedite approval for novel microfluidic platforms that demonstrate significant advantages over conventional methods.
Clinical Laboratory Improvement Amendments (CLIA) regulations in the US further impact the deployment of these technologies, with complexity categorization determining where and by whom tests can be performed. Most electrochemical microfluidic systems aim for CLIA-waived status to enable point-of-care implementation, though this requires demonstrating exceptional ease of use and minimal risk of erroneous results.
International standards play a crucial harmonizing role, with ISO 13485 for quality management systems and IEC 61010 for safety requirements being particularly relevant. The specific standard ISO 18113 addresses labeling requirements for in vitro diagnostic reagents, while IEC 62304 covers software lifecycle processes for medical devices—increasingly important as electrochemical signal processing often involves sophisticated algorithms.
Regulatory considerations for data security and privacy have gained prominence, especially when diagnostic results are transmitted or stored electronically. Compliance with regulations such as HIPAA in the US or GDPR in Europe becomes essential when implementing connected microfluidic ELISA platforms with electrochemical detection.
Navigating these regulatory frameworks requires strategic planning from early development stages, with consideration for verification and validation protocols that will satisfy multiple jurisdictions simultaneously. Manufacturers increasingly adopt a global regulatory strategy to streamline approvals across major markets while maintaining compliance with region-specific requirements.
European regulations have undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive. This regulation imposes stricter requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic electrochemical ELISA systems are typically classified as Class B or C devices under the IVDR, necessitating conformity assessment involving notified bodies.
In emerging markets, particularly China, the National Medical Products Administration (NMPA) has established specific pathways for innovative diagnostic technologies. Their "Special Review Procedure for Innovative Medical Devices" can expedite approval for novel microfluidic platforms that demonstrate significant advantages over conventional methods.
Clinical Laboratory Improvement Amendments (CLIA) regulations in the US further impact the deployment of these technologies, with complexity categorization determining where and by whom tests can be performed. Most electrochemical microfluidic systems aim for CLIA-waived status to enable point-of-care implementation, though this requires demonstrating exceptional ease of use and minimal risk of erroneous results.
International standards play a crucial harmonizing role, with ISO 13485 for quality management systems and IEC 61010 for safety requirements being particularly relevant. The specific standard ISO 18113 addresses labeling requirements for in vitro diagnostic reagents, while IEC 62304 covers software lifecycle processes for medical devices—increasingly important as electrochemical signal processing often involves sophisticated algorithms.
Regulatory considerations for data security and privacy have gained prominence, especially when diagnostic results are transmitted or stored electronically. Compliance with regulations such as HIPAA in the US or GDPR in Europe becomes essential when implementing connected microfluidic ELISA platforms with electrochemical detection.
Navigating these regulatory frameworks requires strategic planning from early development stages, with consideration for verification and validation protocols that will satisfy multiple jurisdictions simultaneously. Manufacturers increasingly adopt a global regulatory strategy to streamline approvals across major markets while maintaining compliance with region-specific requirements.
Cost-Benefit Analysis of Microfluidic ELISA Implementation
Implementing microfluidic ELISA systems with electrochemical signal transduction requires careful evaluation of financial implications against potential benefits. Initial capital expenditure represents a significant consideration, with specialized equipment costs ranging from $50,000 to $200,000 depending on automation level and throughput capacity. This includes microfluidic chip fabrication systems, electrochemical detection instruments, and supporting analytical equipment.
Operational expenses must be factored into long-term planning, with reagent costs typically 30-50% lower than conventional ELISA due to reduced sample volumes. Microfluidic platforms require as little as 1-10 μL of sample and reagents compared to 50-200 μL in traditional systems, translating to substantial savings for high-throughput operations. Maintenance costs average 5-10% of initial equipment investment annually, primarily for electrode replacement and microfluidic channel maintenance.
Labor efficiency presents a compelling advantage, with studies demonstrating 60-70% reduction in hands-on technician time. Automated microfluidic systems can process samples in parallel with minimal human intervention, allowing reallocation of skilled personnel to higher-value activities. This efficiency translates to approximately $15,000-25,000 annual savings per full-time equivalent in medium-sized diagnostic laboratories.
Time-to-result improvements deliver both direct and indirect financial benefits. Conventional ELISA typically requires 2-4 hours for completion, while microfluidic electrochemical systems can deliver results in 15-30 minutes. This acceleration enables faster clinical decision-making, reduced hospital stays, and improved laboratory throughput—factors particularly valuable in emergency medicine and infectious disease management.
Quality improvements and error reduction represent less quantifiable but significant benefits. Automated microfluidic systems demonstrate 25-40% lower coefficient of variation in results compared to manual ELISA, reducing costly repeat testing and improving diagnostic confidence. Enhanced sensitivity—often 10-100 times greater than conventional colorimetric detection—enables earlier disease detection and intervention, potentially reducing downstream healthcare costs.
Return on investment calculations indicate break-even periods of 2-3 years for high-volume testing environments, with accelerated returns for specialized applications requiring ultrasensitive detection. Organizations should consider phased implementation strategies, beginning with high-value diagnostic applications where improved sensitivity and reduced turnaround time deliver immediate clinical and financial benefits.
Operational expenses must be factored into long-term planning, with reagent costs typically 30-50% lower than conventional ELISA due to reduced sample volumes. Microfluidic platforms require as little as 1-10 μL of sample and reagents compared to 50-200 μL in traditional systems, translating to substantial savings for high-throughput operations. Maintenance costs average 5-10% of initial equipment investment annually, primarily for electrode replacement and microfluidic channel maintenance.
Labor efficiency presents a compelling advantage, with studies demonstrating 60-70% reduction in hands-on technician time. Automated microfluidic systems can process samples in parallel with minimal human intervention, allowing reallocation of skilled personnel to higher-value activities. This efficiency translates to approximately $15,000-25,000 annual savings per full-time equivalent in medium-sized diagnostic laboratories.
Time-to-result improvements deliver both direct and indirect financial benefits. Conventional ELISA typically requires 2-4 hours for completion, while microfluidic electrochemical systems can deliver results in 15-30 minutes. This acceleration enables faster clinical decision-making, reduced hospital stays, and improved laboratory throughput—factors particularly valuable in emergency medicine and infectious disease management.
Quality improvements and error reduction represent less quantifiable but significant benefits. Automated microfluidic systems demonstrate 25-40% lower coefficient of variation in results compared to manual ELISA, reducing costly repeat testing and improving diagnostic confidence. Enhanced sensitivity—often 10-100 times greater than conventional colorimetric detection—enables earlier disease detection and intervention, potentially reducing downstream healthcare costs.
Return on investment calculations indicate break-even periods of 2-3 years for high-volume testing environments, with accelerated returns for specialized applications requiring ultrasensitive detection. Organizations should consider phased implementation strategies, beginning with high-value diagnostic applications where improved sensitivity and reduced turnaround time deliver immediate clinical and financial benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!