Microfluidic ELISA with Integrated Photodetector Arrays
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Evolution and Objectives
Microfluidic ELISA technology represents a significant advancement in the field of immunoassay diagnostics, evolving from traditional ELISA methods that have been foundational in clinical diagnostics since the 1970s. The integration of microfluidic platforms with ELISA has progressively transformed this technology from macro-scale laboratory procedures to miniaturized, automated systems capable of rapid and sensitive detection with minimal sample volumes.
The evolution began in the late 1990s with early attempts to miniaturize ELISA procedures using simple microchannels. By the early 2000s, researchers had developed more sophisticated microfluidic chips incorporating multiple functional elements such as mixers, valves, and reaction chambers. The period between 2005-2010 saw significant advancements in materials science, with the introduction of polymers like PDMS (polydimethylsiloxane) that enabled more complex and cost-effective microfluidic designs.
A pivotal development occurred around 2010-2015 with the integration of detection systems directly onto microfluidic platforms. This period marked the first successful attempts to incorporate photodetector arrays with microfluidic ELISA, eliminating the need for external optical detection equipment and moving toward truly portable diagnostic devices.
Recent years (2015-2023) have witnessed remarkable progress in integrating advanced semiconductor technologies with microfluidics, including CMOS-based photodetector arrays that offer unprecedented sensitivity and multiplexing capabilities. Concurrently, smartphone-based detection systems have emerged, leveraging ubiquitous mobile technology to create accessible point-of-care diagnostic platforms.
The primary objective of current microfluidic ELISA with integrated photodetector arrays is to develop fully autonomous, highly sensitive diagnostic platforms capable of multiplex detection with minimal user intervention. These systems aim to achieve detection limits comparable to laboratory-based instruments while maintaining the advantages of portability, reduced sample volume, and rapid analysis time.
Additional technical goals include improving manufacturing scalability through standardized fabrication processes, enhancing system robustness for field deployment in resource-limited settings, and developing universal platforms adaptable to various biomarkers beyond traditional protein targets, including nucleic acids, cells, and small molecules.
The long-term vision encompasses the creation of integrated systems that combine sample preparation, multiple assay types, and data analysis in a single platform, potentially revolutionizing diagnostic approaches in clinical settings, environmental monitoring, and food safety. This evolution represents a convergence of multiple disciplines including microfluidics, immunoassay chemistry, optoelectronics, and data science, driving toward increasingly sophisticated yet accessible diagnostic technologies.
The evolution began in the late 1990s with early attempts to miniaturize ELISA procedures using simple microchannels. By the early 2000s, researchers had developed more sophisticated microfluidic chips incorporating multiple functional elements such as mixers, valves, and reaction chambers. The period between 2005-2010 saw significant advancements in materials science, with the introduction of polymers like PDMS (polydimethylsiloxane) that enabled more complex and cost-effective microfluidic designs.
A pivotal development occurred around 2010-2015 with the integration of detection systems directly onto microfluidic platforms. This period marked the first successful attempts to incorporate photodetector arrays with microfluidic ELISA, eliminating the need for external optical detection equipment and moving toward truly portable diagnostic devices.
Recent years (2015-2023) have witnessed remarkable progress in integrating advanced semiconductor technologies with microfluidics, including CMOS-based photodetector arrays that offer unprecedented sensitivity and multiplexing capabilities. Concurrently, smartphone-based detection systems have emerged, leveraging ubiquitous mobile technology to create accessible point-of-care diagnostic platforms.
The primary objective of current microfluidic ELISA with integrated photodetector arrays is to develop fully autonomous, highly sensitive diagnostic platforms capable of multiplex detection with minimal user intervention. These systems aim to achieve detection limits comparable to laboratory-based instruments while maintaining the advantages of portability, reduced sample volume, and rapid analysis time.
Additional technical goals include improving manufacturing scalability through standardized fabrication processes, enhancing system robustness for field deployment in resource-limited settings, and developing universal platforms adaptable to various biomarkers beyond traditional protein targets, including nucleic acids, cells, and small molecules.
The long-term vision encompasses the creation of integrated systems that combine sample preparation, multiple assay types, and data analysis in a single platform, potentially revolutionizing diagnostic approaches in clinical settings, environmental monitoring, and food safety. This evolution represents a convergence of multiple disciplines including microfluidics, immunoassay chemistry, optoelectronics, and data science, driving toward increasingly sophisticated yet accessible diagnostic technologies.
Market Analysis for Integrated Diagnostic Platforms
The global market for integrated diagnostic platforms, particularly those incorporating microfluidic ELISA with integrated photodetector arrays, is experiencing robust growth driven by increasing demand for point-of-care testing solutions. This market segment is projected to reach $7.8 billion by 2027, growing at a CAGR of 11.3% from 2022 to 2027, significantly outpacing traditional diagnostic equipment markets.
Healthcare systems worldwide are shifting toward decentralized testing models, creating substantial opportunities for compact, integrated diagnostic platforms. The COVID-19 pandemic has accelerated this trend, with healthcare providers seeking rapid, reliable testing solutions that can be deployed in various settings beyond central laboratories. This shift has expanded the addressable market for microfluidic ELISA technologies by approximately 35% since 2020.
The primary market segments for integrated diagnostic platforms include hospitals and clinics (42% market share), diagnostic laboratories (28%), research institutions (17%), and home healthcare settings (13%). Notably, the home healthcare segment is demonstrating the fastest growth rate at 16.8% annually, reflecting increasing consumer interest in self-monitoring and telehealth integration.
Regionally, North America currently dominates the market with 38% share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). However, the Asia-Pacific region is expected to witness the highest growth rate of 14.2% through 2027, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing adoption of advanced diagnostic technologies in countries like China, India, and South Korea.
Key market drivers include the growing prevalence of chronic diseases requiring regular monitoring, increasing geriatric population, technological advancements in microfluidics and sensor technologies, and rising healthcare expenditure. The integration of photodetector arrays with microfluidic ELISA specifically addresses market demands for higher sensitivity, multiplexed testing capabilities, and reduced sample volume requirements.
Reimbursement policies are evolving favorably for integrated diagnostic platforms, with several major insurance providers now covering point-of-care molecular tests. This trend is expected to continue, further expanding market accessibility. Additionally, regulatory pathways are becoming more streamlined for these technologies, with the FDA and European regulatory bodies establishing specific guidance for integrated diagnostic platforms.
Market challenges include high initial development costs, competition from established laboratory-based testing methods, and the need for clinical validation across diverse testing environments. Despite these challenges, the value proposition of faster results, reduced sample volumes, and decentralized testing capabilities positions microfluidic ELISA with integrated photodetector arrays as a high-growth segment within the broader diagnostic market.
Healthcare systems worldwide are shifting toward decentralized testing models, creating substantial opportunities for compact, integrated diagnostic platforms. The COVID-19 pandemic has accelerated this trend, with healthcare providers seeking rapid, reliable testing solutions that can be deployed in various settings beyond central laboratories. This shift has expanded the addressable market for microfluidic ELISA technologies by approximately 35% since 2020.
The primary market segments for integrated diagnostic platforms include hospitals and clinics (42% market share), diagnostic laboratories (28%), research institutions (17%), and home healthcare settings (13%). Notably, the home healthcare segment is demonstrating the fastest growth rate at 16.8% annually, reflecting increasing consumer interest in self-monitoring and telehealth integration.
Regionally, North America currently dominates the market with 38% share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). However, the Asia-Pacific region is expected to witness the highest growth rate of 14.2% through 2027, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing adoption of advanced diagnostic technologies in countries like China, India, and South Korea.
Key market drivers include the growing prevalence of chronic diseases requiring regular monitoring, increasing geriatric population, technological advancements in microfluidics and sensor technologies, and rising healthcare expenditure. The integration of photodetector arrays with microfluidic ELISA specifically addresses market demands for higher sensitivity, multiplexed testing capabilities, and reduced sample volume requirements.
Reimbursement policies are evolving favorably for integrated diagnostic platforms, with several major insurance providers now covering point-of-care molecular tests. This trend is expected to continue, further expanding market accessibility. Additionally, regulatory pathways are becoming more streamlined for these technologies, with the FDA and European regulatory bodies establishing specific guidance for integrated diagnostic platforms.
Market challenges include high initial development costs, competition from established laboratory-based testing methods, and the need for clinical validation across diverse testing environments. Despite these challenges, the value proposition of faster results, reduced sample volumes, and decentralized testing capabilities positions microfluidic ELISA with integrated photodetector arrays as a high-growth segment within the broader diagnostic market.
Current Challenges in Microfluidic-Photodetector Integration
The integration of microfluidic ELISA systems with photodetector arrays represents a significant advancement in point-of-care diagnostics, yet several technical challenges persist that impede widespread adoption. The miniaturization of traditional ELISA onto microfluidic platforms introduces complex fluid dynamics that affect reaction kinetics and detection sensitivity. Surface-to-volume ratios in microchannels significantly differ from conventional well plates, requiring recalibration of established protocols and potentially altering antibody-antigen binding efficiencies.
Material compatibility presents another substantial hurdle. Photodetector arrays typically require specific substrate materials that may not be compatible with common microfluidic fabrication processes. Silicon-based photodetectors often demand high-temperature processing steps that can damage polymer-based microfluidic components, while polymer-based photodetectors may suffer from chemical incompatibility with biological samples or cleaning agents used in ELISA protocols.
Optical alignment between microfluidic reaction chambers and photodetector elements remains exceptionally challenging at microscale dimensions. Even minor misalignments of a few micrometers can significantly reduce signal detection efficiency. Current manufacturing processes struggle to maintain consistent alignment tolerances across large arrays, resulting in performance variability between devices and limiting mass production capabilities.
Signal-to-noise ratio optimization represents a critical challenge in integrated systems. The limited sample volumes in microfluidic channels produce weaker optical signals compared to traditional ELISA. Simultaneously, proximity of electronic components to fluidic channels increases electrical noise. This combination necessitates sophisticated signal processing algorithms and shielding techniques that add complexity and cost to the overall system.
Thermal management issues arise from the integration of electronic components with fluid-handling systems. Photodetector arrays generate heat during operation, potentially creating temperature gradients across the microfluidic chip. These gradients can affect enzyme activity, reaction rates, and ultimately test reliability. Current passive cooling approaches prove insufficient for maintaining thermal uniformity across the integrated device.
Packaging and encapsulation technologies face significant limitations when combining electronic and fluidic components. Conventional electronic packaging methods often fail to accommodate fluidic interfaces, while microfluidic sealing techniques may not provide adequate protection for sensitive electronic components. This dichotomy necessitates novel hybrid packaging solutions that can simultaneously satisfy both requirements without compromising performance.
Scalable manufacturing remains perhaps the most significant barrier to commercialization. Current fabrication approaches typically involve multiple discrete manufacturing steps across different platforms, leading to high production costs and yield issues. The lack of standardized integration processes hampers industry adoption and limits the potential for mass production of these promising diagnostic platforms.
Material compatibility presents another substantial hurdle. Photodetector arrays typically require specific substrate materials that may not be compatible with common microfluidic fabrication processes. Silicon-based photodetectors often demand high-temperature processing steps that can damage polymer-based microfluidic components, while polymer-based photodetectors may suffer from chemical incompatibility with biological samples or cleaning agents used in ELISA protocols.
Optical alignment between microfluidic reaction chambers and photodetector elements remains exceptionally challenging at microscale dimensions. Even minor misalignments of a few micrometers can significantly reduce signal detection efficiency. Current manufacturing processes struggle to maintain consistent alignment tolerances across large arrays, resulting in performance variability between devices and limiting mass production capabilities.
Signal-to-noise ratio optimization represents a critical challenge in integrated systems. The limited sample volumes in microfluidic channels produce weaker optical signals compared to traditional ELISA. Simultaneously, proximity of electronic components to fluidic channels increases electrical noise. This combination necessitates sophisticated signal processing algorithms and shielding techniques that add complexity and cost to the overall system.
Thermal management issues arise from the integration of electronic components with fluid-handling systems. Photodetector arrays generate heat during operation, potentially creating temperature gradients across the microfluidic chip. These gradients can affect enzyme activity, reaction rates, and ultimately test reliability. Current passive cooling approaches prove insufficient for maintaining thermal uniformity across the integrated device.
Packaging and encapsulation technologies face significant limitations when combining electronic and fluidic components. Conventional electronic packaging methods often fail to accommodate fluidic interfaces, while microfluidic sealing techniques may not provide adequate protection for sensitive electronic components. This dichotomy necessitates novel hybrid packaging solutions that can simultaneously satisfy both requirements without compromising performance.
Scalable manufacturing remains perhaps the most significant barrier to commercialization. Current fabrication approaches typically involve multiple discrete manufacturing steps across different platforms, leading to high production costs and yield issues. The lack of standardized integration processes hampers industry adoption and limits the potential for mass production of these promising diagnostic platforms.
Current Integration Approaches for Microfluidic-Optical Detection
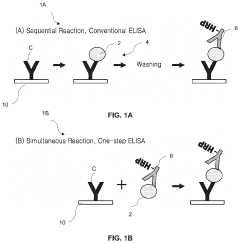
01 Microfluidic ELISA platforms with integrated photodetectors
Microfluidic platforms specifically designed for ELISA (Enzyme-Linked Immunosorbent Assay) that incorporate integrated photodetector arrays for signal detection. These systems combine microfluidic channels for sample handling and reagent delivery with on-chip photodetectors that can directly measure the optical signals generated during ELISA reactions. The integration of photodetectors eliminates the need for external optical equipment, making the systems more compact and portable while maintaining high sensitivity for biomarker detection.- Microfluidic ELISA platforms with integrated photodetectors: Microfluidic platforms designed specifically for ELISA applications that incorporate integrated photodetector arrays for signal detection. These systems combine the advantages of microfluidics (reduced sample volume, faster reaction times) with on-chip detection capabilities, eliminating the need for external optical equipment. The integration of photodetectors directly into the microfluidic chip allows for real-time monitoring of the ELISA reaction and improved sensitivity compared to traditional methods.
- Photodetector array configurations for enhanced sensitivity: Various configurations of photodetector arrays designed to enhance the sensitivity and specificity of microfluidic ELISA systems. These include arrangements of multiple photodiodes, CMOS sensors, or other light-sensing elements strategically positioned to capture signals from multiple reaction chambers simultaneously. Advanced designs incorporate filters to reduce background noise and improve signal-to-noise ratios, enabling detection of lower analyte concentrations and more reliable quantification of results.
- Integration of signal processing electronics with microfluidic ELISA: Systems that incorporate signal processing electronics directly with microfluidic ELISA platforms and photodetector arrays. These integrated circuits amplify, filter, and process the electrical signals generated by the photodetectors in response to the optical signals from the ELISA reaction. On-chip signal processing reduces noise, improves detection limits, and enables more compact and portable diagnostic devices. Some designs include wireless data transmission capabilities for remote monitoring and analysis.
- Fabrication methods for integrated microfluidic-photodetector systems: Novel fabrication techniques for creating integrated microfluidic ELISA platforms with photodetector arrays. These methods include microfabrication processes that allow for the seamless integration of fluidic channels, reaction chambers, and optical detection components on a single substrate. Advanced manufacturing approaches such as 3D printing, soft lithography, and semiconductor processing techniques are employed to create these complex integrated systems while maintaining cost-effectiveness and reproducibility.
- Multiplexed detection in microfluidic ELISA with photodetector arrays: Systems designed for multiplexed detection of multiple analytes simultaneously using microfluidic ELISA with integrated photodetector arrays. These platforms contain multiple reaction chambers or channels, each dedicated to a different biomarker, with corresponding photodetectors for signal readout. Multiplexing capabilities significantly increase throughput and efficiency while reducing sample volume requirements and analysis time. Advanced designs incorporate reference channels for calibration and quality control to ensure reliable results.
02 Photodetector array configurations for enhanced sensitivity
Various configurations of photodetector arrays designed to enhance the sensitivity and specificity of microfluidic ELISA systems. These include arrangements of multiple photodiodes, CMOS sensors, or other light-sensing elements positioned strategically to capture signals from multiple reaction chambers simultaneously. Some designs incorporate filter layers to select specific wavelengths relevant to the ELISA colorimetric or fluorescent reactions, while others use differential detection methods to improve signal-to-noise ratios and lower detection limits.Expand Specific Solutions03 Integration of signal processing electronics with microfluidic ELISA
Advanced microfluidic ELISA systems that incorporate not only photodetector arrays but also integrated signal processing electronics. These systems feature on-chip amplifiers, analog-to-digital converters, and sometimes microprocessors that can process the raw signals from photodetectors in real-time. The integration of electronics enables immediate data analysis, background subtraction, and calibration, resulting in more accurate and reliable test results without requiring external instrumentation for signal processing.Expand Specific Solutions04 Multiplexed detection systems using photodetector arrays
Microfluidic ELISA platforms designed for multiplexed detection of multiple analytes simultaneously using integrated photodetector arrays. These systems contain multiple reaction chambers or channels, each dedicated to detecting different biomarkers, with corresponding photodetector elements assigned to each detection site. The multiplexed approach significantly increases throughput and efficiency by enabling parallel processing of samples and detection of multiple disease markers or analytes in a single test run.Expand Specific Solutions05 Portable and point-of-care microfluidic ELISA devices
Compact, portable microfluidic ELISA devices with integrated photodetector arrays specifically designed for point-of-care applications. These devices are engineered to be battery-operated, user-friendly, and suitable for use outside traditional laboratory settings. The integration of microfluidics, photodetectors, and sometimes wireless communication capabilities enables rapid diagnostic testing in resource-limited settings, emergency situations, or at-home use, with results comparable to conventional laboratory-based ELISA methods.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
Microfluidic ELISA with integrated photodetector arrays is currently in an early growth phase, with the market expanding as this technology bridges traditional ELISA techniques with advanced microfluidics and detection systems. The global market is projected to grow significantly as point-of-care diagnostics demand increases. Technologically, the field shows varying maturity levels across companies. Academic institutions like Zhejiang University, University of Michigan, and Tsinghua University are driving fundamental research, while specialized firms like Optofluidic Bioassay LLC focus on commercialization. Established players including BOE Technology, Samsung Electronics, and Agilent Technologies are leveraging their manufacturing expertise to scale production. The integration of AI and IoT capabilities by companies such as Philips and ETRI represents the cutting edge of this evolving technology landscape.
BOE Technology Group Co., Ltd.
Technical Solution: BOE Technology has leveraged its expertise in display manufacturing to develop a microfluidic ELISA platform with integrated thin-film transistor (TFT) photodetector arrays. Their system utilizes the same production lines used for LCD/OLED displays to fabricate high-density photodetector arrays with over 1,000 sensing elements per cm². The microfluidic component features multiple parallel channels etched into glass substrates using processes adapted from display manufacturing. BOE's platform incorporates active matrix addressing to enable selective readout of individual detection sites, significantly reducing power consumption and increasing throughput. Their technology includes integrated temperature control elements for precise reaction conditions and on-chip reference standards for automatic calibration. The system achieves detection limits in the sub-picogram/mL range for protein biomarkers while maintaining a compact form factor suitable for desktop use in clinical settings. BOE has optimized their manufacturing process to achieve economies of scale, with projected costs competitive with centralized laboratory testing when produced at volume.
Strengths: Leverages established display manufacturing infrastructure for cost-effective production; high-density detection arrays enabling multiplexed testing; excellent reproducibility due to standardized manufacturing processes; potential for integration with display technologies for result visualization. Weaknesses: Limited flexibility in microfluidic design due to constraints of display manufacturing processes; higher initial investment costs; challenges in biological surface functionalization on display materials.
École Polytechnique Fédérale de Lausanne
Technical Solution: EPFL has pioneered a microfluidic ELISA platform that integrates organic photodetector arrays with paper-based microfluidics. Their innovative approach combines low-cost materials with high-performance detection capabilities. The system utilizes inkjet-printed organic photodiodes arranged in a matrix configuration directly on flexible substrates. These substrates are then bonded to paper-based microfluidic channels created through wax printing techniques. EPFL's technology employs capillary-driven flow, eliminating the need for external pumps while maintaining precise fluid control. Their detection scheme incorporates wavelength-selective organic semiconductors that enable multiplexed detection without optical filters. The platform achieves sub-nanogram/mL detection limits while using minimal reagent volumes (1-2 μL per test). The entire system is designed for extreme affordability, with production costs below $1 per test, making it particularly suitable for resource-limited settings in developing countries. The technology has been field-tested in rural clinics for infectious disease diagnostics including tuberculosis and malaria detection.
Strengths: Extremely low cost of materials and manufacturing; minimal power requirements; environmentally friendly disposable components; suitable for deployment in resource-limited settings. Weaknesses: Lower sensitivity compared to silicon-based photodetector systems; limited long-term stability of organic materials; challenges in standardization across manufacturing batches.
Key Patents in Photodetector Array Integration for ELISA
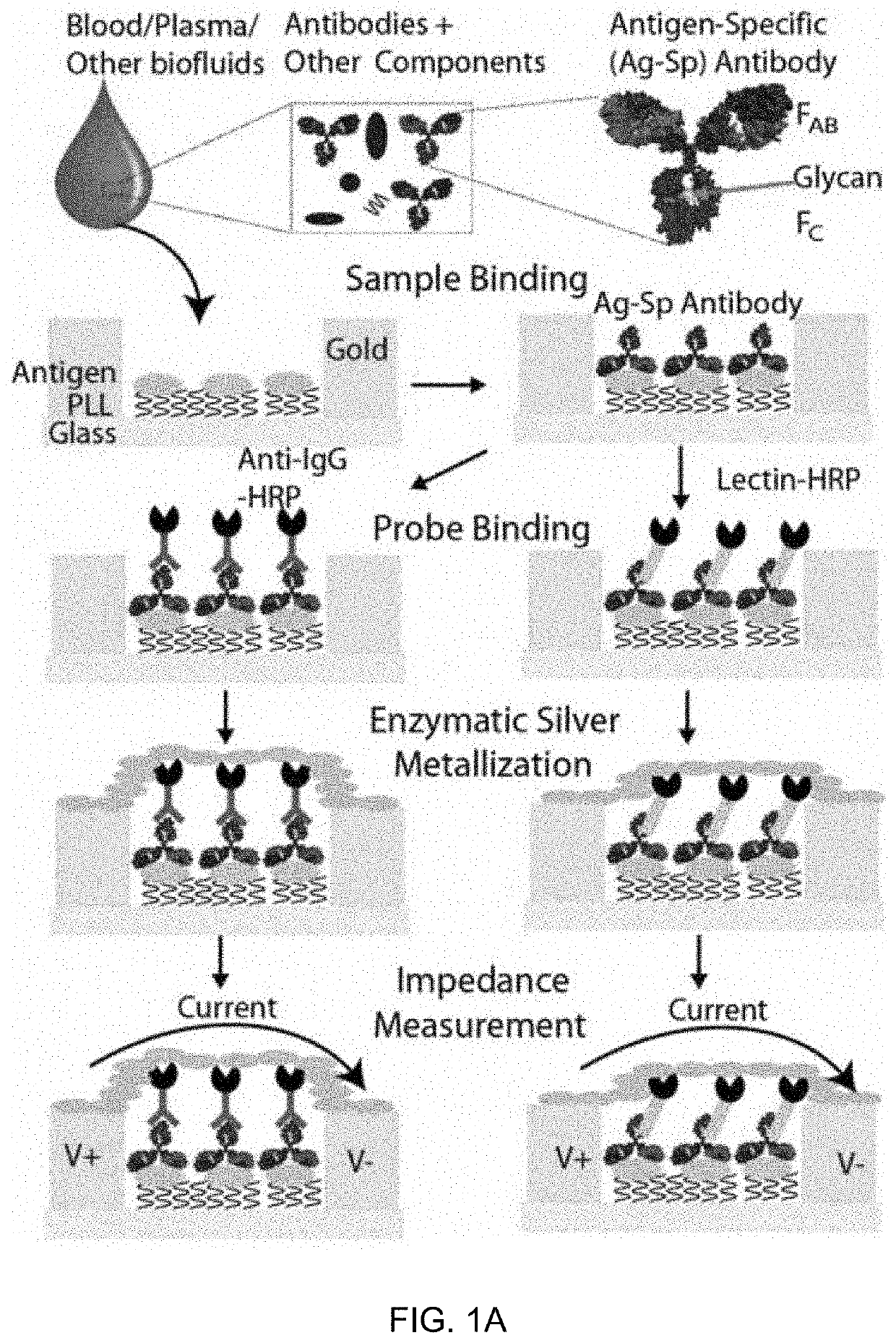
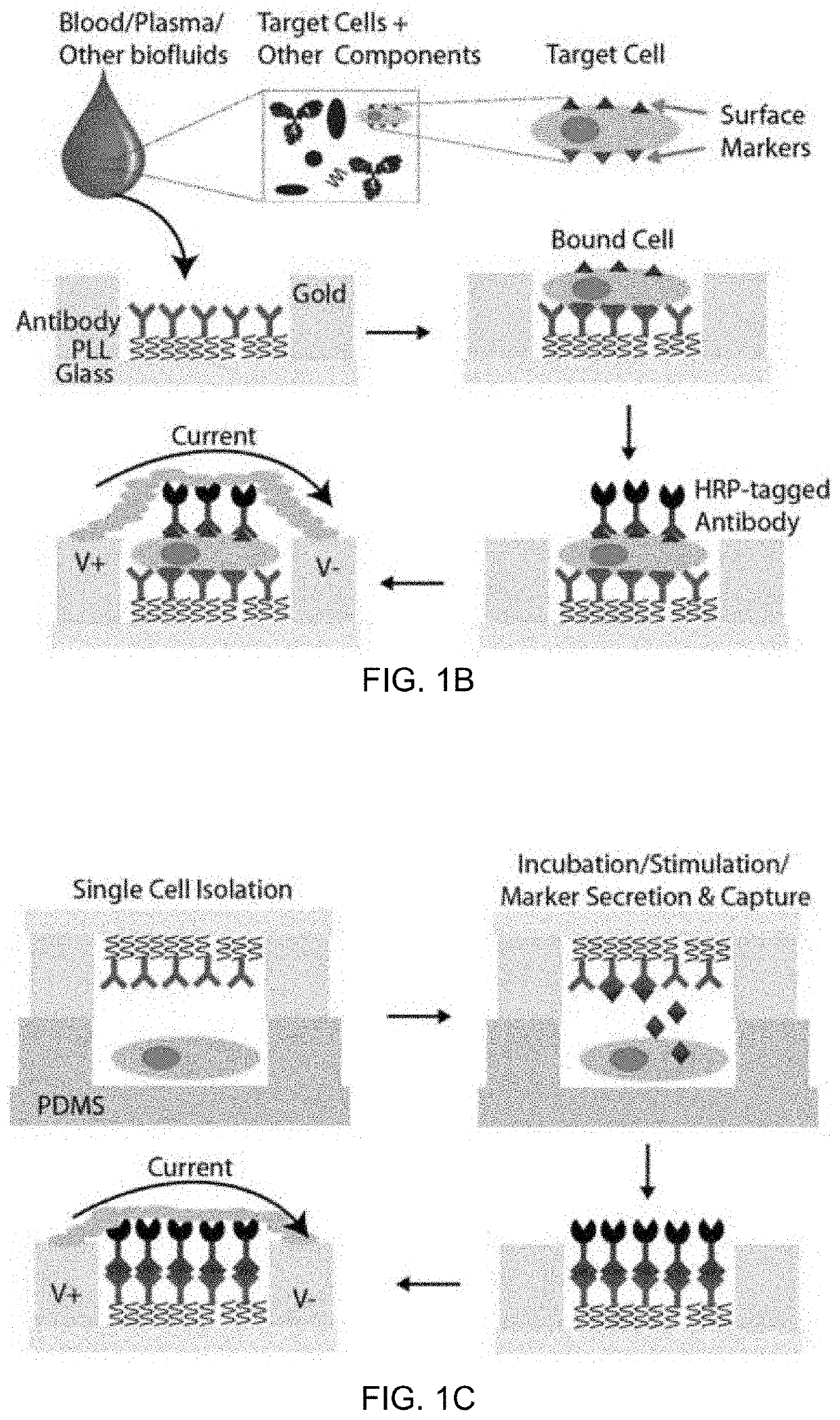
An integrated microfluidic electrode array system for enzyme-linked immuno-sorbent assay (easy-elisa) for point-of-care detection of molecular and cellular biomarkers
PatentInactiveUS20210373014A1
Innovation
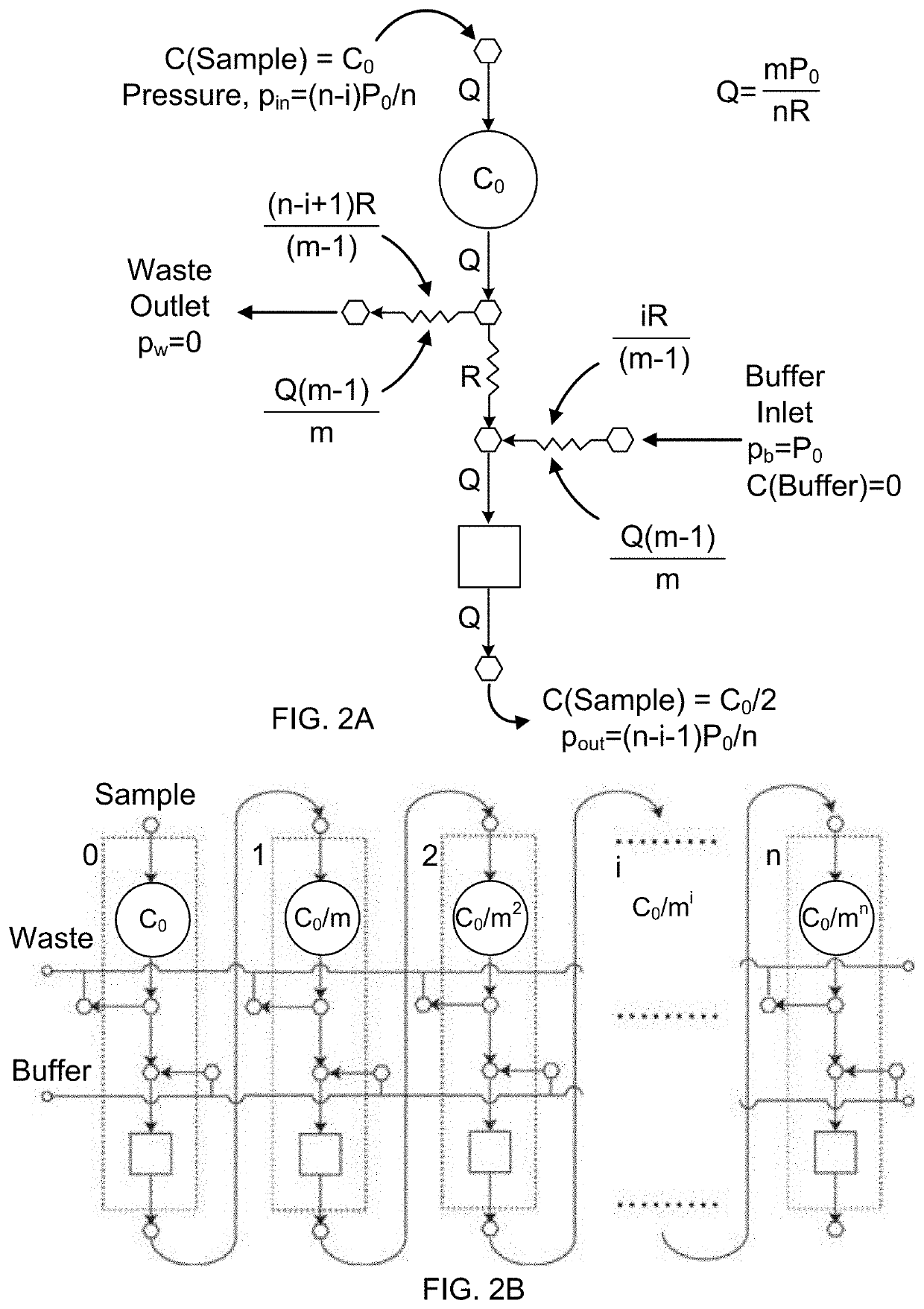
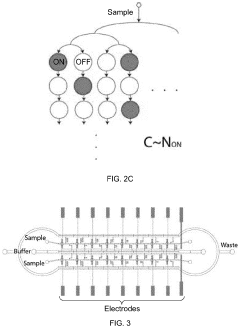
- A microfluidic Electrode Array System for Enzyme-Linked Immuno-Sorbent Assay (EASy-ELISA) that uses direct electrical impedance-based detection and quantitation, enabling sensitive and quantitative detection of molecular and cellular biomarkers without intermediate optics or optical detectors, integrated with a microfluidic serial dilution network for sample handling and distribution.
Surface and diffusion enhanced biosensor
PatentInactiveUS20190391142A1
Innovation
- The development of biosensor assemblies with enhanced surface areas, featuring microstructures or nanostructures on detection structures, which increase the concentration of immobilized biomolecules, thereby improving sensitivity and reducing analysis time by optimizing the surface-to-volume ratio and minimizing the hook effect.
Regulatory Pathway for Point-of-Care Diagnostic Devices
The regulatory landscape for microfluidic ELISA devices with integrated photodetector arrays presents significant complexity due to their classification as in vitro diagnostic (IVD) medical devices. In the United States, these point-of-care diagnostic systems fall under FDA oversight, typically requiring either 510(k) clearance or Premarket Approval (PMA) depending on risk classification. Most microfluidic ELISA systems would likely be classified as Class II devices, necessitating 510(k) submission demonstrating substantial equivalence to legally marketed predicate devices.
For novel integrated photodetector technologies without clear predicates, De Novo classification requests may be necessary, establishing a new regulatory pathway for subsequent similar devices. Clinical validation studies must demonstrate analytical performance metrics including sensitivity, specificity, accuracy, precision, and linearity across the intended measurement range.
The FDA's recent Digital Health Innovation Action Plan has implications for these integrated systems, particularly regarding software validation requirements when algorithms are incorporated for result interpretation. Manufacturers must comply with Quality System Regulation (21 CFR Part 820), including design controls, risk management, and post-market surveillance protocols.
In the European market, the transition from the IVD Directive (IVDD) to the more stringent IVD Regulation (IVDR) significantly impacts microfluidic diagnostic devices. Under IVDR, most ELISA-based systems are classified as Class C devices, requiring Notified Body assessment and comprehensive technical documentation including clinical evidence and performance evaluation reports.
For global market access, manufacturers should consider the MDSAP (Medical Device Single Audit Program) to streamline regulatory compliance across multiple jurisdictions including Australia, Brazil, Canada, Japan, and the United States. ISO 13485:2016 certification is virtually mandatory for quality management systems.
Emerging markets present varying regulatory frameworks. China's NMPA requires local clinical trials and testing for innovative diagnostic technologies, while India's CDSCO has implemented new Medical Device Rules with specific pathways for IVDs. These regional variations necessitate tailored regulatory strategies for global commercialization.
Reimbursement pathways represent another critical consideration, with manufacturers needing to demonstrate both clinical utility and cost-effectiveness to secure coverage from healthcare payers. The development of specific CPT codes for novel diagnostic technologies can significantly impact market adoption and commercial viability.
For novel integrated photodetector technologies without clear predicates, De Novo classification requests may be necessary, establishing a new regulatory pathway for subsequent similar devices. Clinical validation studies must demonstrate analytical performance metrics including sensitivity, specificity, accuracy, precision, and linearity across the intended measurement range.
The FDA's recent Digital Health Innovation Action Plan has implications for these integrated systems, particularly regarding software validation requirements when algorithms are incorporated for result interpretation. Manufacturers must comply with Quality System Regulation (21 CFR Part 820), including design controls, risk management, and post-market surveillance protocols.
In the European market, the transition from the IVD Directive (IVDD) to the more stringent IVD Regulation (IVDR) significantly impacts microfluidic diagnostic devices. Under IVDR, most ELISA-based systems are classified as Class C devices, requiring Notified Body assessment and comprehensive technical documentation including clinical evidence and performance evaluation reports.
For global market access, manufacturers should consider the MDSAP (Medical Device Single Audit Program) to streamline regulatory compliance across multiple jurisdictions including Australia, Brazil, Canada, Japan, and the United States. ISO 13485:2016 certification is virtually mandatory for quality management systems.
Emerging markets present varying regulatory frameworks. China's NMPA requires local clinical trials and testing for innovative diagnostic technologies, while India's CDSCO has implemented new Medical Device Rules with specific pathways for IVDs. These regional variations necessitate tailored regulatory strategies for global commercialization.
Reimbursement pathways represent another critical consideration, with manufacturers needing to demonstrate both clinical utility and cost-effectiveness to secure coverage from healthcare payers. The development of specific CPT codes for novel diagnostic technologies can significantly impact market adoption and commercial viability.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of microfluidic ELISA systems with integrated photodetector arrays presents significant challenges and opportunities for commercial deployment. Current production methods primarily rely on cleanroom-based microfabrication techniques, which entail substantial capital investment and operational costs. The integration of photodetector arrays with microfluidic channels requires precise alignment and bonding processes that are difficult to automate at scale, resulting in low throughput and high unit costs.
Material selection significantly impacts both manufacturing complexity and overall system cost. Traditional materials like glass and silicon offer excellent optical properties and chemical resistance but are expensive to process. Polymeric materials such as PDMS (polydimethylsiloxane) provide cost advantages for prototyping but present challenges for mass production due to their absorption characteristics and potential for biomolecule adsorption. Alternative materials like cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) show promise for balancing performance requirements with manufacturing scalability.
Cost analysis reveals that integrated photodetector arrays constitute approximately 40-50% of the total device cost, with microfluidic channel fabrication accounting for 25-30%, and assembly/packaging representing 20-25%. The current per-unit cost ranges from $50-200 depending on complexity and production volume, significantly higher than traditional ELISA platforms. However, economies of scale could potentially reduce costs by 60-70% at volumes exceeding 100,000 units annually.
Emerging manufacturing approaches such as roll-to-roll processing and injection molding offer pathways to dramatically improve production throughput. These techniques could reduce fabrication costs by an estimated 80% compared to traditional methods. Additionally, advances in printed electronics may enable direct integration of photodetector components onto flexible substrates, further streamlining the manufacturing process.
Supply chain considerations also impact scalability, particularly regarding specialized materials and components. The photodetector arrays often require custom specifications that limit supplier options and increase procurement costs. Establishing reliable supply chains for these critical components remains a significant challenge for large-scale production.
Quality control represents another manufacturing hurdle, as the integration of optical and fluidic components demands stringent testing protocols. Current yield rates for fully functional devices typically range from 70-85%, with defects at the interface between microfluidic channels and photodetectors being the primary failure mode. Implementing automated inspection systems could improve yields while reducing quality control costs.
Material selection significantly impacts both manufacturing complexity and overall system cost. Traditional materials like glass and silicon offer excellent optical properties and chemical resistance but are expensive to process. Polymeric materials such as PDMS (polydimethylsiloxane) provide cost advantages for prototyping but present challenges for mass production due to their absorption characteristics and potential for biomolecule adsorption. Alternative materials like cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) show promise for balancing performance requirements with manufacturing scalability.
Cost analysis reveals that integrated photodetector arrays constitute approximately 40-50% of the total device cost, with microfluidic channel fabrication accounting for 25-30%, and assembly/packaging representing 20-25%. The current per-unit cost ranges from $50-200 depending on complexity and production volume, significantly higher than traditional ELISA platforms. However, economies of scale could potentially reduce costs by 60-70% at volumes exceeding 100,000 units annually.
Emerging manufacturing approaches such as roll-to-roll processing and injection molding offer pathways to dramatically improve production throughput. These techniques could reduce fabrication costs by an estimated 80% compared to traditional methods. Additionally, advances in printed electronics may enable direct integration of photodetector components onto flexible substrates, further streamlining the manufacturing process.
Supply chain considerations also impact scalability, particularly regarding specialized materials and components. The photodetector arrays often require custom specifications that limit supplier options and increase procurement costs. Establishing reliable supply chains for these critical components remains a significant challenge for large-scale production.
Quality control represents another manufacturing hurdle, as the integration of optical and fluidic components demands stringent testing protocols. Current yield rates for fully functional devices typically range from 70-85%, with defects at the interface between microfluidic channels and photodetectors being the primary failure mode. Implementing automated inspection systems could improve yields while reducing quality control costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!