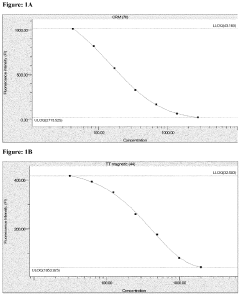
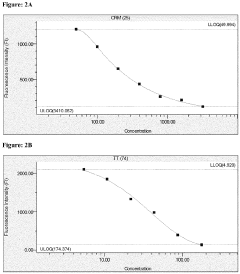
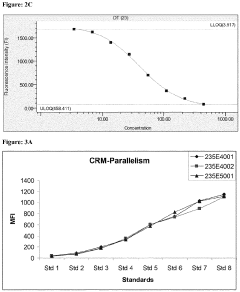
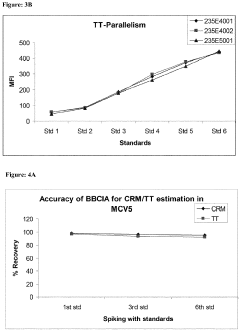
Microfluidic ELISA for Multiplexed Protein Quantification
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Microfluidic ELISA technology represents a significant advancement in the field of immunoassay diagnostics, evolving from traditional ELISA techniques first developed in the 1970s. The convergence of microfluidics and enzyme-linked immunosorbent assay (ELISA) methodologies has created a powerful platform for protein detection and quantification with enhanced sensitivity, reduced sample volumes, and multiplexing capabilities.
The historical trajectory of this technology began with conventional ELISA platforms requiring relatively large sample volumes and lengthy processing times. The integration of microfluidic principles into ELISA workflows emerged in the early 2000s, driven by the need for more efficient, portable, and sensitive diagnostic tools. This technological fusion has been accelerated by advances in microfabrication techniques, surface chemistry, and detection methodologies.
Current microfluidic ELISA systems leverage miniaturized channels, chambers, and detection zones to perform multiple immunoassay steps in an integrated fashion. The technology has progressed from simple single-channel devices to sophisticated multiplexed platforms capable of simultaneous quantification of numerous protein biomarkers from minimal sample volumes.
The primary objective of microfluidic ELISA development is to achieve high-throughput, multiplexed protein quantification with sensitivity comparable to or exceeding traditional methods while dramatically reducing reagent consumption, analysis time, and user intervention. This aligns with the broader goals of point-of-care diagnostics and personalized medicine.
Technical evolution trends indicate movement toward fully automated, integrated systems incorporating sample preparation, immunoreactions, washing steps, and detection within a single microfluidic chip. Recent innovations focus on enhancing multiplexing capabilities through spatial segregation of detection zones, spectral differentiation of detection signals, or temporal separation of reaction kinetics.
Another significant trend is the development of smartphone-compatible microfluidic ELISA platforms, enabling portable diagnostics with cloud connectivity for remote analysis and data management. This democratization of advanced protein quantification techniques has profound implications for global healthcare access.
The convergence with digital microfluidics, which manipulates discrete droplets rather than continuous flows, represents another frontier in this field, offering unprecedented flexibility in assay design and execution. Additionally, the integration of nanomaterials and novel detection schemes is pushing sensitivity limits toward single-molecule detection thresholds.
The ultimate technical goal is to develop robust, cost-effective microfluidic ELISA platforms capable of rapid, multiplexed protein quantification across diverse settings, from sophisticated research laboratories to resource-limited environments, thereby transforming both clinical diagnostics and fundamental proteomics research.
The historical trajectory of this technology began with conventional ELISA platforms requiring relatively large sample volumes and lengthy processing times. The integration of microfluidic principles into ELISA workflows emerged in the early 2000s, driven by the need for more efficient, portable, and sensitive diagnostic tools. This technological fusion has been accelerated by advances in microfabrication techniques, surface chemistry, and detection methodologies.
Current microfluidic ELISA systems leverage miniaturized channels, chambers, and detection zones to perform multiple immunoassay steps in an integrated fashion. The technology has progressed from simple single-channel devices to sophisticated multiplexed platforms capable of simultaneous quantification of numerous protein biomarkers from minimal sample volumes.
The primary objective of microfluidic ELISA development is to achieve high-throughput, multiplexed protein quantification with sensitivity comparable to or exceeding traditional methods while dramatically reducing reagent consumption, analysis time, and user intervention. This aligns with the broader goals of point-of-care diagnostics and personalized medicine.
Technical evolution trends indicate movement toward fully automated, integrated systems incorporating sample preparation, immunoreactions, washing steps, and detection within a single microfluidic chip. Recent innovations focus on enhancing multiplexing capabilities through spatial segregation of detection zones, spectral differentiation of detection signals, or temporal separation of reaction kinetics.
Another significant trend is the development of smartphone-compatible microfluidic ELISA platforms, enabling portable diagnostics with cloud connectivity for remote analysis and data management. This democratization of advanced protein quantification techniques has profound implications for global healthcare access.
The convergence with digital microfluidics, which manipulates discrete droplets rather than continuous flows, represents another frontier in this field, offering unprecedented flexibility in assay design and execution. Additionally, the integration of nanomaterials and novel detection schemes is pushing sensitivity limits toward single-molecule detection thresholds.
The ultimate technical goal is to develop robust, cost-effective microfluidic ELISA platforms capable of rapid, multiplexed protein quantification across diverse settings, from sophisticated research laboratories to resource-limited environments, thereby transforming both clinical diagnostics and fundamental proteomics research.
Market Demand for Multiplexed Protein Quantification
The global market for multiplexed protein quantification technologies is experiencing robust growth, driven by increasing demand in biomedical research, clinical diagnostics, and pharmaceutical development. The market size for protein analysis technologies was valued at approximately $24 billion in 2022 and is projected to reach $45 billion by 2028, with multiplexed technologies representing a significant growth segment within this market.
Healthcare applications constitute the largest market segment, with clinical diagnostics increasingly adopting multiplexed protein quantification for disease biomarker detection. The ability to simultaneously measure multiple proteins from limited sample volumes provides substantial advantages in diagnosing complex conditions such as cancer, autoimmune disorders, and infectious diseases. This capability is particularly valuable in precision medicine initiatives, where comprehensive protein profiling enables personalized treatment approaches.
Pharmaceutical and biotechnology companies represent another major market driver, utilizing multiplexed protein quantification in drug discovery and development processes. The technology enables high-throughput screening of drug candidates, assessment of therapeutic efficacy, and monitoring of potential side effects through protein biomarker panels. This application segment is expected to grow at a CAGR of 12.5% through 2028, outpacing the overall market growth rate.
Academic and research institutions continue to be significant consumers of multiplexed protein quantification technologies, particularly for fundamental biological research and biomarker discovery. The demand for higher sensitivity, greater multiplexing capability, and reduced sample volume requirements is pushing technological innovation in this space.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is witnessing the fastest growth rate due to increasing research funding, expanding healthcare infrastructure, and growing biopharmaceutical manufacturing capabilities in countries like China, Japan, and South Korea.
The microfluidic ELISA approach to multiplexed protein quantification addresses several key market needs, including reduced sample consumption, increased throughput, improved sensitivity, and cost-effectiveness. End-users are increasingly demanding integrated systems that combine sample preparation, analysis, and data interpretation in automated workflows, presenting opportunities for comprehensive microfluidic solutions.
Market challenges include standardization issues, technical complexity requiring specialized expertise, and competition from alternative technologies such as mass spectrometry-based approaches. However, the inherent advantages of microfluidic ELISA platforms in terms of accessibility, portability, and compatibility with existing laboratory infrastructure position them favorably for continued market penetration, particularly in point-of-care and resource-limited settings.
Healthcare applications constitute the largest market segment, with clinical diagnostics increasingly adopting multiplexed protein quantification for disease biomarker detection. The ability to simultaneously measure multiple proteins from limited sample volumes provides substantial advantages in diagnosing complex conditions such as cancer, autoimmune disorders, and infectious diseases. This capability is particularly valuable in precision medicine initiatives, where comprehensive protein profiling enables personalized treatment approaches.
Pharmaceutical and biotechnology companies represent another major market driver, utilizing multiplexed protein quantification in drug discovery and development processes. The technology enables high-throughput screening of drug candidates, assessment of therapeutic efficacy, and monitoring of potential side effects through protein biomarker panels. This application segment is expected to grow at a CAGR of 12.5% through 2028, outpacing the overall market growth rate.
Academic and research institutions continue to be significant consumers of multiplexed protein quantification technologies, particularly for fundamental biological research and biomarker discovery. The demand for higher sensitivity, greater multiplexing capability, and reduced sample volume requirements is pushing technological innovation in this space.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is witnessing the fastest growth rate due to increasing research funding, expanding healthcare infrastructure, and growing biopharmaceutical manufacturing capabilities in countries like China, Japan, and South Korea.
The microfluidic ELISA approach to multiplexed protein quantification addresses several key market needs, including reduced sample consumption, increased throughput, improved sensitivity, and cost-effectiveness. End-users are increasingly demanding integrated systems that combine sample preparation, analysis, and data interpretation in automated workflows, presenting opportunities for comprehensive microfluidic solutions.
Market challenges include standardization issues, technical complexity requiring specialized expertise, and competition from alternative technologies such as mass spectrometry-based approaches. However, the inherent advantages of microfluidic ELISA platforms in terms of accessibility, portability, and compatibility with existing laboratory infrastructure position them favorably for continued market penetration, particularly in point-of-care and resource-limited settings.
Current Challenges in Microfluidic ELISA Development
Despite significant advancements in microfluidic ELISA technology for multiplexed protein quantification, several critical challenges continue to impede its widespread adoption and clinical implementation. These challenges span technical, operational, and commercial domains, requiring multidisciplinary approaches to overcome.
Sample preparation remains a fundamental bottleneck in microfluidic ELISA systems. Complex biological samples often contain interfering substances that can affect assay sensitivity and specificity. The miniaturized nature of microfluidic platforms limits the implementation of sophisticated sample preparation techniques, particularly when dealing with viscous samples like whole blood or tissue homogenates.
Surface chemistry optimization presents another significant hurdle. The high surface-to-volume ratio in microfluidic channels makes non-specific protein adsorption particularly problematic, leading to elevated background signals and reduced sensitivity. While various surface modification strategies exist, achieving consistent and stable surface properties across different batches of devices remains challenging.
Integration of multiple detection modalities poses considerable technical difficulties. Although multiplexed detection is a key advantage of microfluidic platforms, the simultaneous quantification of proteins with vastly different abundance levels requires sophisticated detection systems that can accommodate wide dynamic ranges without cross-reactivity or signal interference.
Automation and standardization deficiencies hinder reproducibility across laboratories. Many current microfluidic ELISA platforms still require significant manual intervention, introducing operator-dependent variability. The lack of standardized protocols and quality control measures further complicates inter-laboratory comparisons and clinical validation.
Manufacturing scalability represents a persistent challenge for commercial viability. Many innovative microfluidic designs demonstrate excellent performance in research settings but encounter significant hurdles in mass production. Materials compatibility, assembly complexity, and quality control during high-volume manufacturing often compromise the performance advantages demonstrated in prototype devices.
Regulatory pathways for microfluidic diagnostic devices remain complex and uncertain. The novel nature of these platforms often places them in uncharted regulatory territory, requiring extensive validation studies and sometimes pioneering new approval pathways, significantly extending time-to-market and development costs.
System integration with existing clinical workflows presents practical implementation barriers. Many healthcare settings lack the infrastructure, expertise, or resources to adopt novel microfluidic technologies, regardless of their technical merits. The requirement for specialized equipment or training creates significant adoption hurdles in resource-limited settings.
Sample preparation remains a fundamental bottleneck in microfluidic ELISA systems. Complex biological samples often contain interfering substances that can affect assay sensitivity and specificity. The miniaturized nature of microfluidic platforms limits the implementation of sophisticated sample preparation techniques, particularly when dealing with viscous samples like whole blood or tissue homogenates.
Surface chemistry optimization presents another significant hurdle. The high surface-to-volume ratio in microfluidic channels makes non-specific protein adsorption particularly problematic, leading to elevated background signals and reduced sensitivity. While various surface modification strategies exist, achieving consistent and stable surface properties across different batches of devices remains challenging.
Integration of multiple detection modalities poses considerable technical difficulties. Although multiplexed detection is a key advantage of microfluidic platforms, the simultaneous quantification of proteins with vastly different abundance levels requires sophisticated detection systems that can accommodate wide dynamic ranges without cross-reactivity or signal interference.
Automation and standardization deficiencies hinder reproducibility across laboratories. Many current microfluidic ELISA platforms still require significant manual intervention, introducing operator-dependent variability. The lack of standardized protocols and quality control measures further complicates inter-laboratory comparisons and clinical validation.
Manufacturing scalability represents a persistent challenge for commercial viability. Many innovative microfluidic designs demonstrate excellent performance in research settings but encounter significant hurdles in mass production. Materials compatibility, assembly complexity, and quality control during high-volume manufacturing often compromise the performance advantages demonstrated in prototype devices.
Regulatory pathways for microfluidic diagnostic devices remain complex and uncertain. The novel nature of these platforms often places them in uncharted regulatory territory, requiring extensive validation studies and sometimes pioneering new approval pathways, significantly extending time-to-market and development costs.
System integration with existing clinical workflows presents practical implementation barriers. Many healthcare settings lack the infrastructure, expertise, or resources to adopt novel microfluidic technologies, regardless of their technical merits. The requirement for specialized equipment or training creates significant adoption hurdles in resource-limited settings.
Current Microfluidic ELISA Implementation Approaches
01 Microfluidic chip designs for multiplexed ELISA
Various microfluidic chip designs have been developed specifically for multiplexed ELISA applications. These designs incorporate multiple reaction chambers, channels, and detection zones to enable simultaneous analysis of multiple protein targets. The chips are engineered with precise flow control mechanisms to ensure accurate sample delivery, reagent mixing, and washing steps critical for ELISA protocols. These designs optimize space utilization while maintaining sensitivity and specificity for protein quantification.- Microfluidic chip designs for multiplexed ELISA: Various microfluidic chip designs have been developed specifically for multiplexed ELISA applications. These designs incorporate multiple reaction chambers, channels, and detection zones to enable simultaneous analysis of multiple protein targets. The chips are often fabricated using materials such as PDMS, glass, or polymers, and may include features like integrated valves, pumps, and mixers to control fluid flow and enhance reaction efficiency. These designs aim to minimize sample volume requirements while maximizing detection sensitivity and throughput.
- Novel detection methods for microfluidic ELISA: Advanced detection methods have been integrated with microfluidic ELISA platforms to enhance sensitivity and quantification accuracy. These include fluorescence-based detection, chemiluminescence, electrochemical detection, and surface plasmon resonance. Some approaches incorporate nanomaterials such as quantum dots or gold nanoparticles as labels to amplify signals. Digital ELISA methods that count individual enzyme-labeled molecules have also been developed to achieve ultra-sensitive protein detection, enabling quantification down to femtomolar or even attomolar concentrations.
- Automated sample processing systems for multiplexed protein analysis: Automated systems have been developed to streamline the workflow of microfluidic ELISA for multiplexed protein quantification. These systems integrate sample preparation, reagent handling, incubation, washing steps, and detection into a single platform. They often incorporate robotics, precise fluid handling mechanisms, and software control to minimize human intervention and reduce variability. Some systems are designed for point-of-care applications, while others are optimized for high-throughput laboratory settings, enabling rapid and reliable protein quantification across multiple samples and targets simultaneously.
- Surface modification and immobilization strategies: Various surface modification and biomolecule immobilization strategies have been developed to enhance the performance of microfluidic ELISA systems. These include chemical functionalization of channel surfaces with reactive groups, application of capture antibody arrays, and use of specialized coatings to minimize non-specific binding. Some approaches utilize oriented antibody immobilization techniques to maximize antigen capture efficiency. Novel hydrogel-based matrices and 3D scaffolds have also been employed to increase the density of capture molecules and improve detection sensitivity for multiplexed protein quantification.
- Integration of data analysis and quantification algorithms: Advanced data analysis and quantification algorithms have been integrated with microfluidic ELISA platforms to improve accuracy and reliability of multiplexed protein measurements. These include machine learning approaches for signal processing, automated calibration methods, and statistical tools for handling complex datasets from multiple analytes. Some systems incorporate real-time data analysis capabilities to provide immediate results. Specialized software has been developed to account for cross-reactivity between antibodies in multiplexed assays and to normalize signals across different detection channels, ensuring accurate quantification of multiple proteins simultaneously.
02 Novel detection methods for microfluidic ELISA
Advanced detection methods have been integrated into microfluidic ELISA platforms to enhance sensitivity and quantification capabilities. These include fluorescence-based detection, electrochemical sensing, colorimetric analysis, and label-free detection techniques. These methods enable real-time monitoring of protein-antibody interactions and provide improved signal-to-noise ratios compared to traditional ELISA. The integration of these detection technologies with microfluidics allows for lower sample volume requirements while maintaining or improving detection limits.Expand Specific Solutions03 Automation and integration systems for microfluidic ELISA
Automated systems have been developed to integrate sample preparation, processing, and analysis steps in microfluidic ELISA platforms. These systems incorporate pumps, valves, mixers, and control software to automate fluid handling and assay protocols. The integration of these components enables high-throughput analysis with minimal user intervention, reducing human error and improving reproducibility. Complete lab-on-a-chip solutions provide sample-to-answer capabilities for multiplexed protein quantification in clinical and research settings.Expand Specific Solutions04 Surface modification and immobilization techniques
Various surface modification and biomolecule immobilization techniques have been developed to enhance the performance of microfluidic ELISA systems. These include chemical functionalization of channel surfaces, antibody patterning methods, and novel capture agent immobilization strategies. These techniques improve antibody orientation, reduce non-specific binding, and enhance capture efficiency. The optimized surfaces provide stable protein immobilization while maintaining biological activity, resulting in improved sensitivity and specificity for multiplexed protein quantification.Expand Specific Solutions05 Sample preparation and handling for microfluidic ELISA
Specialized sample preparation and handling methods have been developed for microfluidic ELISA platforms. These include on-chip sample filtration, dilution, concentration, and purification techniques to process complex biological samples. Innovations in sample introduction, metering, and distribution enable precise control over sample volumes and flow rates. These advancements address challenges associated with processing complex biological matrices like blood, serum, or cell lysates, improving the overall performance of multiplexed protein quantification assays.Expand Specific Solutions
Key Industry Players in Microfluidic Diagnostics
Microfluidic ELISA for multiplexed protein quantification is evolving rapidly in a growth market phase, with the global microfluidics market projected to reach significant expansion due to increasing demand for point-of-care diagnostics. The technology is approaching maturity with key players demonstrating varied levels of advancement. Companies like BioMerieux and Shimadzu lead with established commercial platforms, while Samsung Electronics leverages its manufacturing expertise to develop integrated systems. Research institutions including McGill University and Institut Curie contribute fundamental innovations. Emerging players such as Fertility Lab Sciences and SpinX are developing specialized applications, while pharmaceutical companies like Symphogen utilize the technology for antibody research. The competitive landscape shows a blend of established diagnostic companies, technology conglomerates, and specialized startups driving innovation through different approaches to multiplexed protein detection.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed an advanced microfluidic ELISA platform for multiplexed protein quantification that integrates with their established analytical instrumentation expertise. Their system employs a hybrid approach combining microfluidic sample handling with high-sensitivity detection technologies. The Shimadzu platform utilizes glass or polymer microchips with etched microchannels (typically 20-50 μm in width) arranged in a branching network that enables parallel processing of samples. Their technology incorporates precision fluid control through integrated micropumps and electrokinetic flow mechanisms that ensure accurate delivery of nanoliter-scale reagent volumes. For multiplexed detection, Shimadzu employs a combination of spatially-resolved detection zones and spectrally distinct fluorescent labels, enabling simultaneous quantification of up to 8 different protein targets from a single 20 μL sample. The system achieves exceptional sensitivity through integration with their mass spectrometry technology for label-free protein quantification or through advanced fluorescence detection with avalanche photodiode arrays. Shimadzu's microfluidic ELISA platform has been applied to pharmaceutical development for protein biomarker discovery, clinical diagnostics for cancer screening, and food safety testing, demonstrating detection limits in the sub-pg/mL range with analysis times of approximately 25-40 minutes.
Strengths: Exceptional analytical sensitivity through integration with advanced detection technologies; robust quality control and calibration systems; seamless data integration with existing laboratory information systems. Weaknesses: Higher initial equipment investment compared to conventional ELISA systems; requires specialized training for operation and maintenance; limited portability due to instrumentation requirements.
BIOMERIEUX SA
Technical Solution: bioMérieux has developed advanced microfluidic ELISA platforms for multiplexed protein quantification, integrating their patented VIDAS technology with microfluidic chips. Their system utilizes a network of microchannels (typically 10-100 μm in width) that enable simultaneous detection of multiple biomarkers from minimal sample volumes (1-10 μL). The technology incorporates automated sample preparation, reagent handling, and waste disposal within a single disposable cartridge. bioMérieux's approach employs fluorescence-based detection methods with specialized antibody arrays immobilized in discrete detection zones, allowing for quantification of up to 10 different protein targets simultaneously with sensitivity in the pg/mL range. The system includes integrated quality controls and calibration standards to ensure reliable results across different testing environments. Their microfluidic ELISA technology has been validated for clinical applications including sepsis biomarker panels, cardiac markers, and infectious disease diagnostics, demonstrating comparable or superior performance to traditional ELISA methods while significantly reducing analysis time from hours to under 30 minutes.
Strengths: Fully automated sample-to-result workflow reduces operator intervention and error; high multiplexing capability with maintained sensitivity; established global distribution network for clinical diagnostics. Weaknesses: Higher cost per test compared to traditional plate-based ELISA; proprietary system requires dedicated equipment; limited customization options for research applications.
Core Patents and Innovations in Multiplexed Protein Detection
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Multiplex bead based assay for quantification of multiple types of carrier protein in a multivalent conjugate vaccine composition
PatentActiveEP3296741A1
Innovation
- A multiplex bead-based competitive inhibition assay using fluorescently dyed beads and a suspension array system to measure individual carrier protein concentrations, allowing for rapid and cost-effective detection with high reproducibility.
Regulatory Considerations for Microfluidic Diagnostic Platforms
Microfluidic ELISA platforms for multiplexed protein quantification face a complex regulatory landscape that varies significantly across global markets. In the United States, the FDA regulates these devices primarily through the Medical Device Regulatory pathway, with most microfluidic diagnostic platforms classified as either Class II or Class III devices depending on their intended use and risk profile. Multiplexed protein quantification systems typically require premarket notification (510(k)) or premarket approval (PMA), with the latter involving more rigorous clinical validation requirements.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVD Directive in May 2022, significantly increasing regulatory requirements for microfluidic diagnostic platforms. Under IVDR, most multiplexed protein quantification systems fall into higher risk classes (Class C or D), requiring Notified Body involvement and more comprehensive technical documentation, including performance evaluation data and post-market surveillance plans.
Quality management systems compliance represents another critical regulatory consideration, with ISO 13485 serving as the international standard specifically for medical devices. Manufacturers must implement robust quality systems covering design controls, risk management, and validation protocols tailored to microfluidic technologies' unique characteristics, including channel dimensions, flow dynamics, and surface chemistry interactions that affect assay performance.
Clinical validation requirements present particular challenges for multiplexed assays, as regulatory bodies increasingly demand evidence of clinical utility beyond analytical performance. This includes demonstrating that the simultaneous measurement of multiple protein biomarkers provides actionable clinical information with established reference ranges and decision thresholds for each analyte in the panel.
Data management and privacy regulations add another layer of complexity, especially as many microfluidic platforms incorporate digital components for data analysis and interpretation. Compliance with regulations such as HIPAA in the US and GDPR in Europe becomes essential when patient data is processed, stored, or transmitted as part of the diagnostic workflow.
Manufacturing process validation presents unique challenges for microfluidic ELISA platforms due to their microscale features and complex fabrication requirements. Regulatory bodies expect robust process validation data demonstrating consistent production of devices that maintain critical quality attributes affecting assay performance, such as channel dimensions, surface properties, and reagent deposition precision.
Regulatory pathways for novel microfluidic technologies can be accelerated through programs like the FDA's Breakthrough Devices Program or equivalent schemes in other jurisdictions, which may provide priority review for innovative diagnostics addressing unmet clinical needs. However, these pathways still require substantial evidence of safety and effectiveness, albeit with more flexible approaches to clinical evidence generation.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVD Directive in May 2022, significantly increasing regulatory requirements for microfluidic diagnostic platforms. Under IVDR, most multiplexed protein quantification systems fall into higher risk classes (Class C or D), requiring Notified Body involvement and more comprehensive technical documentation, including performance evaluation data and post-market surveillance plans.
Quality management systems compliance represents another critical regulatory consideration, with ISO 13485 serving as the international standard specifically for medical devices. Manufacturers must implement robust quality systems covering design controls, risk management, and validation protocols tailored to microfluidic technologies' unique characteristics, including channel dimensions, flow dynamics, and surface chemistry interactions that affect assay performance.
Clinical validation requirements present particular challenges for multiplexed assays, as regulatory bodies increasingly demand evidence of clinical utility beyond analytical performance. This includes demonstrating that the simultaneous measurement of multiple protein biomarkers provides actionable clinical information with established reference ranges and decision thresholds for each analyte in the panel.
Data management and privacy regulations add another layer of complexity, especially as many microfluidic platforms incorporate digital components for data analysis and interpretation. Compliance with regulations such as HIPAA in the US and GDPR in Europe becomes essential when patient data is processed, stored, or transmitted as part of the diagnostic workflow.
Manufacturing process validation presents unique challenges for microfluidic ELISA platforms due to their microscale features and complex fabrication requirements. Regulatory bodies expect robust process validation data demonstrating consistent production of devices that maintain critical quality attributes affecting assay performance, such as channel dimensions, surface properties, and reagent deposition precision.
Regulatory pathways for novel microfluidic technologies can be accelerated through programs like the FDA's Breakthrough Devices Program or equivalent schemes in other jurisdictions, which may provide priority review for innovative diagnostics addressing unmet clinical needs. However, these pathways still require substantial evidence of safety and effectiveness, albeit with more flexible approaches to clinical evidence generation.
Cost-Benefit Analysis of Microfluidic ELISA Systems
The implementation of microfluidic ELISA systems for multiplexed protein quantification presents a complex economic proposition that requires thorough cost-benefit analysis. When evaluating these systems from a financial perspective, initial capital expenditure represents a significant consideration. The equipment costs for microfluidic platforms typically range from $10,000 to $100,000 depending on automation level, detection sensitivity, and throughput capacity. This substantial upfront investment must be weighed against the long-term operational advantages.
Operational cost reductions constitute a primary benefit of microfluidic ELISA systems. These platforms dramatically decrease reagent consumption, with typical reductions of 80-95% compared to conventional plate-based ELISA. For high-value antibodies and specialized reagents costing hundreds of dollars per milliliter, this translates to thousands of dollars saved annually in medium-throughput laboratories.
Labor efficiency gains provide another significant economic advantage. Automated microfluidic systems can reduce hands-on technician time by 60-75%, allowing skilled personnel to focus on data analysis and interpretation rather than repetitive manual procedures. This efficiency translates to approximately $15,000-$30,000 in annual labor cost savings for a typical clinical laboratory.
Multiplexing capabilities deliver substantial economic benefits through increased analytical throughput. The ability to simultaneously quantify multiple proteins from a single sample accelerates testing workflows by 3-5 fold compared to running separate conventional ELISAs. This throughput enhancement improves laboratory economics while reducing per-test costs by approximately 40-60% when analyzing multiple biomarkers.
Sample conservation represents another valuable economic consideration, particularly in clinical settings where patient specimens may be limited. Microfluidic systems typically require 5-20 μL of sample compared to 50-100 μL for conventional methods, enabling more comprehensive testing from limited specimens and reducing the need for repeat sampling.
Return on investment calculations indicate that most laboratories can recoup microfluidic ELISA system costs within 1.5-3 years, depending on testing volume and reagent costs. Facilities processing more than 5,000 multiplex assays annually typically achieve faster payback periods, making these systems particularly attractive for high-throughput environments.
Maintenance expenses must be factored into long-term cost projections. Annual maintenance costs typically range from 8-15% of the initial system price, including calibration, software updates, and replacement of consumable components. These ongoing expenses partially offset operational savings but generally remain favorable in the overall economic analysis.
Operational cost reductions constitute a primary benefit of microfluidic ELISA systems. These platforms dramatically decrease reagent consumption, with typical reductions of 80-95% compared to conventional plate-based ELISA. For high-value antibodies and specialized reagents costing hundreds of dollars per milliliter, this translates to thousands of dollars saved annually in medium-throughput laboratories.
Labor efficiency gains provide another significant economic advantage. Automated microfluidic systems can reduce hands-on technician time by 60-75%, allowing skilled personnel to focus on data analysis and interpretation rather than repetitive manual procedures. This efficiency translates to approximately $15,000-$30,000 in annual labor cost savings for a typical clinical laboratory.
Multiplexing capabilities deliver substantial economic benefits through increased analytical throughput. The ability to simultaneously quantify multiple proteins from a single sample accelerates testing workflows by 3-5 fold compared to running separate conventional ELISAs. This throughput enhancement improves laboratory economics while reducing per-test costs by approximately 40-60% when analyzing multiple biomarkers.
Sample conservation represents another valuable economic consideration, particularly in clinical settings where patient specimens may be limited. Microfluidic systems typically require 5-20 μL of sample compared to 50-100 μL for conventional methods, enabling more comprehensive testing from limited specimens and reducing the need for repeat sampling.
Return on investment calculations indicate that most laboratories can recoup microfluidic ELISA system costs within 1.5-3 years, depending on testing volume and reagent costs. Facilities processing more than 5,000 multiplex assays annually typically achieve faster payback periods, making these systems particularly attractive for high-throughput environments.
Maintenance expenses must be factored into long-term cost projections. Annual maintenance costs typically range from 8-15% of the initial system price, including calibration, software updates, and replacement of consumable components. These ongoing expenses partially offset operational savings but generally remain favorable in the overall economic analysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!