Integration of Optical Sensors in Microfluidic ELISA Platforms
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Optical-Microfluidic ELISA Integration Background and Objectives
The integration of optical sensors into microfluidic ELISA platforms represents a convergence of multiple technological disciplines that has evolved significantly over the past two decades. Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone technique in clinical diagnostics since the 1970s, valued for its sensitivity and specificity in detecting antigens or antibodies. Traditional ELISA procedures, however, are labor-intensive, time-consuming, and require relatively large sample volumes.
The miniaturization trend in analytical systems led to the emergence of microfluidic technologies in the 1990s, offering advantages of reduced reagent consumption, faster analysis times, and potential for automation. By the early 2000s, researchers began exploring ways to integrate ELISA protocols onto microfluidic platforms, creating what became known as microfluidic ELISA systems.
Optical detection methods have historically been the predominant readout mechanism for ELISA, with colorimetric, fluorescence, and chemiluminescence approaches being most common. The challenge of integrating these optical detection systems with microfluidic platforms has driven significant innovation in sensor miniaturization and optical system design.
The evolution of integrated optical sensors has progressed from bulky external detection systems to fully integrated on-chip solutions. This transition has been enabled by advances in microfabrication techniques, novel materials development, and innovations in optical component miniaturization. Particularly significant has been the development of complementary metal-oxide-semiconductor (CMOS) technology and microelectromechanical systems (MEMS), which have facilitated the creation of compact, sensitive optical detection systems.
The primary objective of integrating optical sensors with microfluidic ELISA platforms is to develop fully automated, portable diagnostic systems capable of laboratory-quality analysis in point-of-care settings. This integration aims to maintain or enhance the analytical performance of traditional ELISA while dramatically reducing sample volume requirements, analysis time, and operational complexity.
Additional technical goals include achieving multiplexed detection capabilities, enhancing sensitivity through novel optical approaches, reducing manufacturing costs through scalable fabrication methods, and developing systems that can be operated by minimally trained personnel. The ultimate vision is to create integrated systems that can democratize access to sophisticated diagnostic capabilities in resource-limited settings.
Current research trends are focused on addressing challenges related to optical alignment precision, background signal reduction, integration of multiple sensing modalities, and development of robust calibration methods. The field is moving toward systems that combine multiple detection principles to enhance specificity and reliability, with particular emphasis on smartphone-based readout systems that leverage ubiquitous mobile technology.
The miniaturization trend in analytical systems led to the emergence of microfluidic technologies in the 1990s, offering advantages of reduced reagent consumption, faster analysis times, and potential for automation. By the early 2000s, researchers began exploring ways to integrate ELISA protocols onto microfluidic platforms, creating what became known as microfluidic ELISA systems.
Optical detection methods have historically been the predominant readout mechanism for ELISA, with colorimetric, fluorescence, and chemiluminescence approaches being most common. The challenge of integrating these optical detection systems with microfluidic platforms has driven significant innovation in sensor miniaturization and optical system design.
The evolution of integrated optical sensors has progressed from bulky external detection systems to fully integrated on-chip solutions. This transition has been enabled by advances in microfabrication techniques, novel materials development, and innovations in optical component miniaturization. Particularly significant has been the development of complementary metal-oxide-semiconductor (CMOS) technology and microelectromechanical systems (MEMS), which have facilitated the creation of compact, sensitive optical detection systems.
The primary objective of integrating optical sensors with microfluidic ELISA platforms is to develop fully automated, portable diagnostic systems capable of laboratory-quality analysis in point-of-care settings. This integration aims to maintain or enhance the analytical performance of traditional ELISA while dramatically reducing sample volume requirements, analysis time, and operational complexity.
Additional technical goals include achieving multiplexed detection capabilities, enhancing sensitivity through novel optical approaches, reducing manufacturing costs through scalable fabrication methods, and developing systems that can be operated by minimally trained personnel. The ultimate vision is to create integrated systems that can democratize access to sophisticated diagnostic capabilities in resource-limited settings.
Current research trends are focused on addressing challenges related to optical alignment precision, background signal reduction, integration of multiple sensing modalities, and development of robust calibration methods. The field is moving toward systems that combine multiple detection principles to enhance specificity and reliability, with particular emphasis on smartphone-based readout systems that leverage ubiquitous mobile technology.
Market Analysis for Integrated Diagnostic Platforms
The integrated diagnostic platform market is experiencing robust growth, driven by increasing demand for point-of-care testing solutions and advancements in microfluidic technologies. The global market for integrated diagnostic platforms was valued at approximately $18.7 billion in 2022 and is projected to reach $32.5 billion by 2028, representing a compound annual growth rate (CAGR) of 9.6% during the forecast period.
The integration of optical sensors in microfluidic ELISA platforms represents a significant segment within this market, with particular strength in clinical diagnostics, pharmaceutical research, and academic research sectors. Clinical diagnostics accounts for the largest market share at 45%, followed by pharmaceutical research at 30% and academic research at 15%, with other applications comprising the remaining 10%.
Regionally, North America dominates the market with approximately 40% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with a CAGR of 12.3%, driven by increasing healthcare expenditure and expanding biotechnology sectors in China, Japan, and India.
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for rapid diagnostic solutions, and increasing adoption of personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic capabilities in healthcare systems worldwide.
Consumer demand trends indicate a strong preference for miniaturized, user-friendly diagnostic platforms that offer high sensitivity, specificity, and multiplexing capabilities. Healthcare providers increasingly seek integrated systems that can perform multiple assays simultaneously while reducing sample volume requirements and analysis time.
Market challenges include high development and manufacturing costs, regulatory hurdles for novel integrated diagnostic technologies, and interoperability issues with existing laboratory information systems. Additionally, reimbursement uncertainties in various healthcare systems pose adoption barriers for innovative diagnostic platforms.
The competitive landscape features established medical device manufacturers alongside emerging biotechnology companies specializing in microfluidic technologies. Strategic partnerships between technology developers and clinical laboratories are becoming increasingly common to accelerate commercialization pathways and expand market reach.
Future market opportunities lie in developing low-cost, portable integrated diagnostic platforms for resource-limited settings, enhancing connectivity features for remote monitoring applications, and expanding test menus to address emerging infectious diseases and biomarkers for chronic conditions.
The integration of optical sensors in microfluidic ELISA platforms represents a significant segment within this market, with particular strength in clinical diagnostics, pharmaceutical research, and academic research sectors. Clinical diagnostics accounts for the largest market share at 45%, followed by pharmaceutical research at 30% and academic research at 15%, with other applications comprising the remaining 10%.
Regionally, North America dominates the market with approximately 40% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows with 30% market share, while Asia-Pacific represents the fastest-growing region with a CAGR of 12.3%, driven by increasing healthcare expenditure and expanding biotechnology sectors in China, Japan, and India.
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for rapid diagnostic solutions, and increasing adoption of personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic capabilities in healthcare systems worldwide.
Consumer demand trends indicate a strong preference for miniaturized, user-friendly diagnostic platforms that offer high sensitivity, specificity, and multiplexing capabilities. Healthcare providers increasingly seek integrated systems that can perform multiple assays simultaneously while reducing sample volume requirements and analysis time.
Market challenges include high development and manufacturing costs, regulatory hurdles for novel integrated diagnostic technologies, and interoperability issues with existing laboratory information systems. Additionally, reimbursement uncertainties in various healthcare systems pose adoption barriers for innovative diagnostic platforms.
The competitive landscape features established medical device manufacturers alongside emerging biotechnology companies specializing in microfluidic technologies. Strategic partnerships between technology developers and clinical laboratories are becoming increasingly common to accelerate commercialization pathways and expand market reach.
Future market opportunities lie in developing low-cost, portable integrated diagnostic platforms for resource-limited settings, enhancing connectivity features for remote monitoring applications, and expanding test menus to address emerging infectious diseases and biomarkers for chronic conditions.
Current Challenges in Optical-Microfluidic Integration
The integration of optical sensors with microfluidic ELISA platforms represents a significant advancement in diagnostic technology, yet faces substantial technical challenges. Current microfluidic-optical integration struggles with alignment precision between optical components and microfluidic channels, often requiring complex fabrication techniques and specialized equipment. This misalignment can lead to significant signal loss and reduced detection sensitivity, compromising the overall performance of the diagnostic system.
Material compatibility presents another major obstacle, as materials optimal for microfluidic functionality may not possess ideal optical properties. For instance, PDMS (polydimethylsiloxane), widely used in microfluidic fabrication due to its flexibility and biocompatibility, exhibits autofluorescence that can interfere with optical measurements, particularly at lower wavelengths. This interference creates background noise that reduces the signal-to-noise ratio in fluorescence-based ELISA detection.
Miniaturization constraints further complicate integration efforts. As devices become smaller to enhance portability and reduce sample volume requirements, the incorporation of optical components becomes increasingly challenging. Traditional optical elements like lenses, filters, and detectors often remain bulky relative to microfluidic channels, creating a fundamental scale mismatch that limits true system integration and miniaturization.
Surface chemistry at the interface between optical sensors and biological samples introduces additional complications. Protein adsorption and biofouling on optical surfaces can degrade signal quality over time, while surface modifications to prevent such fouling may alter optical properties or interfere with ELISA chemistry. This delicate balance between optical performance and biological compatibility remains difficult to optimize.
Manufacturing scalability represents a significant barrier to widespread adoption. Current integration approaches often rely on manual assembly or complex multi-step fabrication processes that are difficult to translate to mass production. The lack of standardized manufacturing protocols for integrated optical-microfluidic systems increases production costs and limits commercial viability.
Power requirements for optical components, particularly light sources and detectors, present challenges for creating truly portable point-of-care devices. High-sensitivity optical detection often demands substantial power, conflicting with the goal of developing battery-operated field-deployable systems. This power-sensitivity tradeoff forces compromises in system design that can limit diagnostic performance.
Cross-talk between adjacent detection channels in multiplexed systems remains problematic, particularly in highly integrated designs where multiple assays run simultaneously. Optical isolation techniques to prevent such interference often increase device complexity and size, counteracting miniaturization efforts and raising manufacturing costs.
Material compatibility presents another major obstacle, as materials optimal for microfluidic functionality may not possess ideal optical properties. For instance, PDMS (polydimethylsiloxane), widely used in microfluidic fabrication due to its flexibility and biocompatibility, exhibits autofluorescence that can interfere with optical measurements, particularly at lower wavelengths. This interference creates background noise that reduces the signal-to-noise ratio in fluorescence-based ELISA detection.
Miniaturization constraints further complicate integration efforts. As devices become smaller to enhance portability and reduce sample volume requirements, the incorporation of optical components becomes increasingly challenging. Traditional optical elements like lenses, filters, and detectors often remain bulky relative to microfluidic channels, creating a fundamental scale mismatch that limits true system integration and miniaturization.
Surface chemistry at the interface between optical sensors and biological samples introduces additional complications. Protein adsorption and biofouling on optical surfaces can degrade signal quality over time, while surface modifications to prevent such fouling may alter optical properties or interfere with ELISA chemistry. This delicate balance between optical performance and biological compatibility remains difficult to optimize.
Manufacturing scalability represents a significant barrier to widespread adoption. Current integration approaches often rely on manual assembly or complex multi-step fabrication processes that are difficult to translate to mass production. The lack of standardized manufacturing protocols for integrated optical-microfluidic systems increases production costs and limits commercial viability.
Power requirements for optical components, particularly light sources and detectors, present challenges for creating truly portable point-of-care devices. High-sensitivity optical detection often demands substantial power, conflicting with the goal of developing battery-operated field-deployable systems. This power-sensitivity tradeoff forces compromises in system design that can limit diagnostic performance.
Cross-talk between adjacent detection channels in multiplexed systems remains problematic, particularly in highly integrated designs where multiple assays run simultaneously. Optical isolation techniques to prevent such interference often increase device complexity and size, counteracting miniaturization efforts and raising manufacturing costs.
Existing Optical Detection Methods for Microfluidic ELISA
01 Integration of optical detection systems in microfluidic ELISA platforms
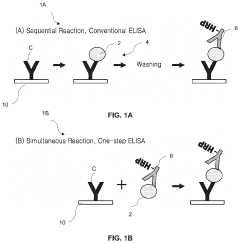
Optical detection systems can be integrated into microfluidic ELISA platforms to enable real-time monitoring of immunoassay reactions. These systems typically include light sources, photodetectors, and optical fibers that are strategically positioned within the microfluidic device. The integration allows for sensitive detection of analytes with minimal sample volumes and reduced analysis time compared to conventional ELISA methods.- Integration of optical detection systems in microfluidic ELISA platforms: Optical detection systems can be integrated into microfluidic ELISA platforms to enable real-time monitoring of immunoassay reactions. These systems typically include light sources, photodetectors, and optical fibers that are strategically positioned within the microfluidic device. The integration allows for sensitive detection of analytes with minimal sample volumes, making the platforms suitable for point-of-care diagnostics. Advanced optical configurations can enhance detection limits and improve the overall performance of microfluidic ELISA assays.
- Fluorescence-based detection methods in microfluidic immunoassays: Fluorescence-based detection methods are commonly employed in microfluidic ELISA platforms due to their high sensitivity and specificity. These methods involve the use of fluorescent labels or tags that emit light when excited at specific wavelengths. The integration of fluorescence detection systems into microfluidic devices enables quantitative analysis of biomarkers at low concentrations. Advanced fluorescence techniques, such as time-resolved fluorescence and fluorescence resonance energy transfer (FRET), can further enhance the detection capabilities of microfluidic ELISA platforms.
- Waveguide-based optical sensing in microfluidic devices: Waveguide-based optical sensing technologies can be incorporated into microfluidic ELISA platforms to improve detection sensitivity. These systems utilize optical waveguides to guide light through the microfluidic channels, allowing for efficient interaction between light and the sample. The evanescent field generated by the waveguide can be used to excite fluorophores or detect changes in refractive index associated with biomolecular interactions. This approach enables label-free detection and real-time monitoring of binding events in microfluidic immunoassays.
- Smartphone-based optical detection for portable microfluidic ELISA: Smartphone-based optical detection systems have emerged as cost-effective solutions for portable microfluidic ELISA platforms. These systems utilize the smartphone's camera and processing capabilities to capture and analyze optical signals from the microfluidic device. Additional components, such as external lenses, filters, and light sources, can be integrated to enhance the detection performance. This approach enables the development of affordable point-of-care diagnostic devices that can be used in resource-limited settings, with results that can be easily shared through wireless communication networks.
- Multiplexed optical sensing in microfluidic immunoassay arrays: Multiplexed optical sensing technologies enable the simultaneous detection of multiple analytes in microfluidic ELISA platforms. These systems typically incorporate array-based detection schemes, spectral multiplexing, or spatial multiplexing approaches. By integrating multiple optical sensors or detection channels within a single microfluidic device, these platforms can perform parallel immunoassays for different biomarkers. This capability is particularly valuable for comprehensive diagnostic testing and high-throughput screening applications, where the analysis of multiple biomarkers is required for accurate disease diagnosis or drug development.
02 Fluorescence-based detection in microfluidic immunoassays
Fluorescence-based detection methods are commonly employed in microfluidic ELISA platforms due to their high sensitivity and specificity. These methods involve the use of fluorescent labels or tags that emit light when excited at specific wavelengths. The integration of fluorescence detection components, such as filters and photomultiplier tubes, into microfluidic devices enables quantitative analysis of biomarkers at low concentrations, making it suitable for clinical diagnostics and point-of-care testing.Expand Specific Solutions03 Surface plasmon resonance sensors for label-free detection
Surface plasmon resonance (SPR) sensors offer label-free detection capabilities in microfluidic ELISA platforms. These optical sensors detect changes in the refractive index at the sensor surface when target analytes bind to immobilized antibodies. The integration of SPR sensors with microfluidic channels allows for real-time monitoring of biomolecular interactions without the need for fluorescent or enzymatic labels, simplifying the assay procedure while maintaining high sensitivity.Expand Specific Solutions04 Smartphone-based optical detection systems
Smartphone-based optical detection systems represent an innovative approach to creating portable and accessible microfluidic ELISA platforms. These systems utilize the smartphone's camera and processing capabilities to capture and analyze optical signals from immunoassays. By incorporating specialized attachments or adapters, smartphones can be transformed into powerful analytical tools for point-of-care diagnostics, enabling testing in resource-limited settings without the need for expensive laboratory equipment.Expand Specific Solutions05 Multiplexed optical sensing in microfluidic platforms
Multiplexed optical sensing enables the simultaneous detection of multiple analytes in a single microfluidic ELISA platform. This approach incorporates various optical sensing elements, such as waveguides, resonators, or array-based detectors, that can distinguish between different spectral signatures or spatial locations. Multiplexed systems increase throughput and efficiency while reducing sample volume requirements and analysis time, making them valuable for comprehensive biomarker profiling and diagnostic applications.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The integration of optical sensors in microfluidic ELISA platforms represents an emerging field in early-stage commercial development. The market is experiencing rapid growth with increasing applications in point-of-care diagnostics, estimated to reach $2-3 billion by 2025. The technology maturity varies significantly across key players: academic institutions like University of California, Johns Hopkins University, and Zhejiang University lead fundamental research, while companies such as Philips, Novartis, and BOE Technology are advancing commercial applications. Specialized firms like Optofluidic Bioassay LLC and Nicoya Lifesciences are developing targeted solutions bridging the gap between research and commercialization. The field is characterized by cross-sector collaboration between academic institutions and industry partners, with significant innovation occurring at this interface as the technology transitions from laboratory to market-ready applications.
The Regents of the University of California
Technical Solution: The University of California has developed a sophisticated microfluidic ELISA platform with integrated optical sensing capabilities based on photonic crystal technology. Their system incorporates one-dimensional photonic crystal structures directly into microfluidic channels, creating highly sensitive label-free detection zones. The platform utilizes a proprietary nanofabrication process that enables mass production of these integrated devices with exceptional reproducibility. Their technology employs a specialized optical setup with angle-resolved spectroscopy to detect minute changes in the photonic crystal resonance conditions upon biomolecular binding events. The system features automated microvalve control for precise reagent delivery and washing steps, significantly improving assay reproducibility. UC researchers have implemented advanced surface chemistry modifications that enhance protein immobilization while minimizing non-specific binding, resulting in improved signal-to-noise ratios. The platform includes custom software for real-time data analysis and visualization, enabling rapid interpretation of complex binding kinetics[9][11].
Strengths: Exceptional sensitivity through photonic crystal enhancement without requiring fluorescent labels; highly reproducible manufacturing process suitable for commercial scale-up; versatile platform adaptable to various biomarker detection applications. Weaknesses: More complex optical alignment requirements compared to conventional systems; higher initial development costs; requires specialized expertise for optimal performance tuning.
École Polytechnique Fédérale de Lausanne
Technical Solution: EPFL has developed an advanced integrated optical sensing platform for microfluidic ELISA that utilizes photonic integrated circuits (PICs) fabricated on silicon nitride substrates. Their approach incorporates ring resonator arrays and Mach-Zehnder interferometers directly into microfluidic channels, enabling multiplexed, label-free detection with exceptional sensitivity. The system features proprietary surface functionalization techniques that enhance biomolecule capture efficiency while minimizing non-specific binding. EPFL's platform employs a tunable laser source coupled with an integrated photodetector array for precise optical measurements across multiple wavelengths. Their technology includes innovative microfluidic channel designs that optimize sample delivery to sensing regions while minimizing reagent consumption. The platform incorporates automated temperature control systems to maintain optimal reaction conditions and compensate for thermal drift effects on optical measurements[4][7].
Strengths: Exceptional multiplexing capability through integrated photonic circuit design; extremely high sensitivity approaching single-molecule detection; compatibility with standard semiconductor manufacturing processes enables scalable production. Weaknesses: Complex optical coupling requirements between external light sources and integrated waveguides; higher technical expertise required for operation compared to conventional systems; limited field deployment options due to precise alignment requirements.
Key Patents and Innovations in Optical-Microfluidic Systems
An integrated microfluidic electrode array system for enzyme-linked immuno-sorbent assay for point- of-care detection of biomarkers
PatentWO2019084051A1
Innovation
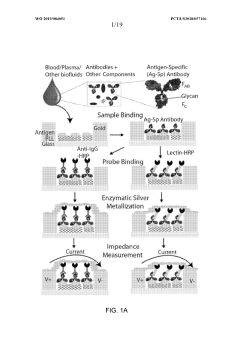
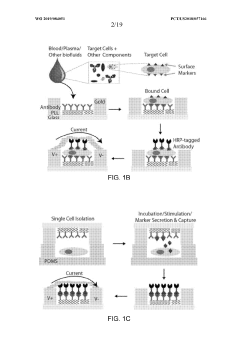
- A microfluidic electrode array system that uses direct electrical impedance-based detection and enzymatically-amplified metal nanoparticle deposition for sensitive and quantitative detection of molecular and cellular biomarkers without intermediate optics or complex fluidics, enabling a portable, inexpensive platform for point-of-care diagnostics.
Surface and diffusion enhanced biosensor
PatentInactiveUS20190391142A1
Innovation
- The development of biosensor assemblies with enhanced surface areas, featuring microstructures or nanostructures on detection structures, which increase the concentration of immobilized biomolecules, thereby improving sensitivity and reducing analysis time by optimizing the surface-to-volume ratio and minimizing the hook effect.
Manufacturing Scalability and Cost Considerations
The scalability of manufacturing processes for integrating optical sensors into microfluidic ELISA platforms represents a critical consideration for commercial viability. Current fabrication methods predominantly rely on cleanroom facilities and specialized equipment, creating significant barriers to mass production. Photolithography techniques, while precise, incur substantial costs estimated at $50-100 per device at laboratory scales, making them prohibitive for widespread clinical adoption. Alternative approaches such as injection molding offer potential cost reductions to $1-5 per device at high volumes, but require substantial initial investment in molds and equipment, typically exceeding $50,000.
Material selection significantly impacts both manufacturing scalability and operational costs. Traditional glass and silicon substrates provide excellent optical properties but present challenges in mass production due to brittle nature and complex processing requirements. Polymeric materials like PMMA and PDMS offer improved manufacturability but may compromise optical performance through autofluorescence or limited chemical compatibility, necessitating careful optimization of material selection against performance requirements.
Integration of optical components presents particular scaling challenges. Current approaches often involve manual alignment and assembly of discrete components, resulting in device-to-device variability and increased labor costs. Automated assembly solutions exist but require significant capital investment, with industrial-grade optical alignment systems costing $100,000-500,000. Emerging integrated approaches, such as waveguides fabricated directly within microfluidic channels, show promise for reducing assembly complexity but remain technologically immature.
Supply chain considerations further impact scalability, with specialized optical components often sourced from limited suppliers with extended lead times. Establishing robust supply networks and potentially developing standardized optical modules could significantly enhance manufacturing reliability and reduce costs. Industry partnerships between microfluidic manufacturers and optical component suppliers have demonstrated 30-40% cost reductions through collaborative design optimization.
Economic viability ultimately depends on production volume thresholds. Analysis indicates that integrated optical-microfluidic ELISA platforms typically require production volumes exceeding 10,000 units annually to achieve competitive per-unit costs below $20. Modular design approaches that separate optical and fluidic components may offer intermediate solutions, allowing selective outsourcing of components to specialized manufacturers while maintaining critical integration expertise in-house. This hybrid approach has demonstrated 25-35% cost reductions in pilot manufacturing studies while maintaining necessary performance specifications.
Material selection significantly impacts both manufacturing scalability and operational costs. Traditional glass and silicon substrates provide excellent optical properties but present challenges in mass production due to brittle nature and complex processing requirements. Polymeric materials like PMMA and PDMS offer improved manufacturability but may compromise optical performance through autofluorescence or limited chemical compatibility, necessitating careful optimization of material selection against performance requirements.
Integration of optical components presents particular scaling challenges. Current approaches often involve manual alignment and assembly of discrete components, resulting in device-to-device variability and increased labor costs. Automated assembly solutions exist but require significant capital investment, with industrial-grade optical alignment systems costing $100,000-500,000. Emerging integrated approaches, such as waveguides fabricated directly within microfluidic channels, show promise for reducing assembly complexity but remain technologically immature.
Supply chain considerations further impact scalability, with specialized optical components often sourced from limited suppliers with extended lead times. Establishing robust supply networks and potentially developing standardized optical modules could significantly enhance manufacturing reliability and reduce costs. Industry partnerships between microfluidic manufacturers and optical component suppliers have demonstrated 30-40% cost reductions through collaborative design optimization.
Economic viability ultimately depends on production volume thresholds. Analysis indicates that integrated optical-microfluidic ELISA platforms typically require production volumes exceeding 10,000 units annually to achieve competitive per-unit costs below $20. Modular design approaches that separate optical and fluidic components may offer intermediate solutions, allowing selective outsourcing of components to specialized manufacturers while maintaining critical integration expertise in-house. This hybrid approach has demonstrated 25-35% cost reductions in pilot manufacturing studies while maintaining necessary performance specifications.
Regulatory Pathway for Integrated Diagnostic Devices
The regulatory landscape for integrated diagnostic devices combining optical sensors with microfluidic ELISA platforms presents significant complexity due to their hybrid nature. These devices fall under the jurisdiction of multiple regulatory frameworks, primarily the FDA in the United States through its medical device pathways. Specifically, such integrated diagnostic platforms typically require evaluation through the 510(k) clearance process if substantially equivalent predecessors exist, or through the more rigorous Premarket Approval (PMA) pathway for novel technologies.
The European regulatory framework presents additional considerations with the implementation of the In Vitro Diagnostic Regulation (IVDR), which has replaced the previous IVDD directive. Under IVDR, microfluidic ELISA platforms with integrated optical sensors are generally classified as Class C devices due to their diagnostic significance, requiring conformity assessment involving notified bodies and more comprehensive technical documentation.
Regulatory submissions for these integrated devices must address both the analytical and clinical validation aspects. This includes demonstrating sensitivity, specificity, precision, and accuracy of the optical sensing mechanisms within the microfluidic environment. Particular attention must be paid to calibration stability, as optical sensors may drift over time or under varying environmental conditions, potentially affecting diagnostic reliability.
Quality system requirements represent another critical regulatory component. Manufacturers must implement comprehensive quality management systems compliant with ISO 13485 standards, with specific provisions for design controls, risk management, and post-market surveillance tailored to the unique characteristics of integrated optical-microfluidic systems.
Software validation emerges as an increasingly important regulatory consideration, especially as many modern optical sensor systems incorporate sophisticated algorithms for signal processing and data analysis. Regulatory bodies now require thorough validation of these software components, including verification of algorithm performance across diverse patient populations and clinical scenarios.
Global harmonization efforts, particularly through the International Medical Device Regulators Forum (IMDRF), are gradually establishing more consistent approaches to regulating such integrated diagnostic technologies. However, significant regional variations persist, necessitating tailored regulatory strategies for different markets. Manufacturers must navigate these differences while maintaining core documentation that addresses fundamental safety and performance requirements.
The regulatory pathway typically requires 12-36 months from initial submission to market approval, depending on the novelty of the technology and the chosen regulatory route. Strategic planning of the regulatory approach early in the development process can significantly impact time-to-market and overall development costs for these integrated diagnostic platforms.
The European regulatory framework presents additional considerations with the implementation of the In Vitro Diagnostic Regulation (IVDR), which has replaced the previous IVDD directive. Under IVDR, microfluidic ELISA platforms with integrated optical sensors are generally classified as Class C devices due to their diagnostic significance, requiring conformity assessment involving notified bodies and more comprehensive technical documentation.
Regulatory submissions for these integrated devices must address both the analytical and clinical validation aspects. This includes demonstrating sensitivity, specificity, precision, and accuracy of the optical sensing mechanisms within the microfluidic environment. Particular attention must be paid to calibration stability, as optical sensors may drift over time or under varying environmental conditions, potentially affecting diagnostic reliability.
Quality system requirements represent another critical regulatory component. Manufacturers must implement comprehensive quality management systems compliant with ISO 13485 standards, with specific provisions for design controls, risk management, and post-market surveillance tailored to the unique characteristics of integrated optical-microfluidic systems.
Software validation emerges as an increasingly important regulatory consideration, especially as many modern optical sensor systems incorporate sophisticated algorithms for signal processing and data analysis. Regulatory bodies now require thorough validation of these software components, including verification of algorithm performance across diverse patient populations and clinical scenarios.
Global harmonization efforts, particularly through the International Medical Device Regulators Forum (IMDRF), are gradually establishing more consistent approaches to regulating such integrated diagnostic technologies. However, significant regional variations persist, necessitating tailored regulatory strategies for different markets. Manufacturers must navigate these differences while maintaining core documentation that addresses fundamental safety and performance requirements.
The regulatory pathway typically requires 12-36 months from initial submission to market approval, depending on the novelty of the technology and the chosen regulatory route. Strategic planning of the regulatory approach early in the development process can significantly impact time-to-market and overall development costs for these integrated diagnostic platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!