Microfluidic ELISA for Portable Immunodiagnostic Testing
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone of clinical diagnostics since its development in the 1970s, providing a reliable method for detecting antigens or antibodies in biological samples. Traditional ELISA procedures, however, require specialized laboratory equipment, trained personnel, and considerable time for analysis, limiting their application in resource-constrained settings or point-of-care scenarios.
Microfluidic technology emerged in the 1990s as a revolutionary approach to miniaturize laboratory processes. By manipulating fluids at the microscale level, microfluidic systems enable precise control over small volumes of reagents, reduced sample consumption, faster reaction kinetics, and integration of multiple analytical steps on a single platform. The convergence of microfluidics with ELISA represents a significant technological advancement with the potential to transform immunodiagnostic testing.
The evolution of Microfluidic ELISA has been driven by increasing demands for decentralized healthcare solutions, particularly in remote areas lacking sophisticated laboratory infrastructure. Early developments focused on simple channel designs, while recent innovations have incorporated advanced features such as automated sample preparation, multiplexed detection capabilities, and smartphone-based readout systems, reflecting the technology's maturation over time.
Current technological trends in Microfluidic ELISA include the integration of nanomaterials for signal amplification, development of paper-based microfluidic devices for ultra-low-cost applications, and incorporation of artificial intelligence for result interpretation. These advancements are progressively enhancing the sensitivity, specificity, and user-friendliness of portable immunodiagnostic platforms.
The primary objective of Microfluidic ELISA development is to create fully integrated, user-friendly diagnostic devices that maintain the analytical performance of laboratory-based ELISA while eliminating the need for specialized equipment or technical expertise. These portable systems aim to provide rapid, accurate results at the point of care, enabling timely clinical decisions and improving patient outcomes.
Additional technical goals include achieving detection limits comparable to conventional ELISA, ensuring reproducibility across different manufacturing batches, extending shelf-life stability for field deployment, and developing standardized protocols for quality control. The ultimate vision is to create a platform technology adaptable to various biomarkers, from infectious disease agents to cancer markers and environmental contaminants.
From a broader perspective, Microfluidic ELISA technology seeks to democratize access to sophisticated immunodiagnostic testing, supporting global health initiatives and addressing healthcare disparities. By enabling rapid, on-site detection of disease biomarkers, this technology could revolutionize disease surveillance, epidemic control, and personalized medicine approaches in both developed and developing regions.
Microfluidic technology emerged in the 1990s as a revolutionary approach to miniaturize laboratory processes. By manipulating fluids at the microscale level, microfluidic systems enable precise control over small volumes of reagents, reduced sample consumption, faster reaction kinetics, and integration of multiple analytical steps on a single platform. The convergence of microfluidics with ELISA represents a significant technological advancement with the potential to transform immunodiagnostic testing.
The evolution of Microfluidic ELISA has been driven by increasing demands for decentralized healthcare solutions, particularly in remote areas lacking sophisticated laboratory infrastructure. Early developments focused on simple channel designs, while recent innovations have incorporated advanced features such as automated sample preparation, multiplexed detection capabilities, and smartphone-based readout systems, reflecting the technology's maturation over time.
Current technological trends in Microfluidic ELISA include the integration of nanomaterials for signal amplification, development of paper-based microfluidic devices for ultra-low-cost applications, and incorporation of artificial intelligence for result interpretation. These advancements are progressively enhancing the sensitivity, specificity, and user-friendliness of portable immunodiagnostic platforms.
The primary objective of Microfluidic ELISA development is to create fully integrated, user-friendly diagnostic devices that maintain the analytical performance of laboratory-based ELISA while eliminating the need for specialized equipment or technical expertise. These portable systems aim to provide rapid, accurate results at the point of care, enabling timely clinical decisions and improving patient outcomes.
Additional technical goals include achieving detection limits comparable to conventional ELISA, ensuring reproducibility across different manufacturing batches, extending shelf-life stability for field deployment, and developing standardized protocols for quality control. The ultimate vision is to create a platform technology adaptable to various biomarkers, from infectious disease agents to cancer markers and environmental contaminants.
From a broader perspective, Microfluidic ELISA technology seeks to democratize access to sophisticated immunodiagnostic testing, supporting global health initiatives and addressing healthcare disparities. By enabling rapid, on-site detection of disease biomarkers, this technology could revolutionize disease surveillance, epidemic control, and personalized medicine approaches in both developed and developing regions.
Market Demand Analysis for Portable Immunodiagnostic Solutions
The global immunodiagnostic testing market has witnessed substantial growth in recent years, driven by increasing prevalence of infectious diseases, rising chronic disease burden, and growing awareness about early disease detection. The portable immunodiagnostic solutions segment, particularly microfluidic ELISA-based technologies, represents one of the fastest-growing sectors within this market, with a compound annual growth rate exceeding traditional laboratory-based diagnostics.
Healthcare systems worldwide are experiencing mounting pressure to reduce costs while improving accessibility, creating significant demand for decentralized testing solutions. Point-of-care testing (POCT) market research indicates that portable immunodiagnostic devices address critical needs in resource-limited settings, emergency response scenarios, and home healthcare environments where traditional laboratory infrastructure is unavailable or impractical.
Consumer behavior analysis reveals growing preference for self-monitoring health solutions, with patients increasingly seeking greater control over their healthcare management. This trend has accelerated following the COVID-19 pandemic, which demonstrated the critical importance of rapid, accessible diagnostic capabilities during public health emergencies and normalized the concept of at-home testing for many consumers.
The primary market segments for portable microfluidic ELISA technology include clinical diagnostics (hospitals, clinics, physician offices), home healthcare, research institutions, and pharmaceutical companies. Geographic market analysis shows particularly strong demand growth in developing regions where healthcare infrastructure remains limited but smartphone penetration is high, enabling integration with mobile health platforms.
Regulatory landscapes are evolving to accommodate innovative diagnostic technologies, with many countries establishing expedited approval pathways for point-of-care solutions that demonstrate significant public health benefits. This regulatory shift has created more favorable market conditions for novel portable immunodiagnostic technologies.
Key unmet needs identified through market research include improved sensitivity comparable to laboratory-based tests, reduced cost per test, simplified user interfaces for non-technical operators, expanded test menus on single platforms, and seamless data integration with electronic health records. Stakeholder interviews consistently highlight the importance of balancing analytical performance with practical considerations like shelf stability, minimal sample preparation, and intuitive result interpretation.
Market forecasts project continued strong growth for portable immunodiagnostic solutions, with microfluidic ELISA technologies positioned to capture significant market share due to their potential for multiplexed testing, reduced reagent consumption, and compatibility with various biomarkers. The convergence of microfluidics with smartphone-based detection systems represents a particularly promising market opportunity, leveraging existing consumer technology to reduce overall system costs.
Healthcare systems worldwide are experiencing mounting pressure to reduce costs while improving accessibility, creating significant demand for decentralized testing solutions. Point-of-care testing (POCT) market research indicates that portable immunodiagnostic devices address critical needs in resource-limited settings, emergency response scenarios, and home healthcare environments where traditional laboratory infrastructure is unavailable or impractical.
Consumer behavior analysis reveals growing preference for self-monitoring health solutions, with patients increasingly seeking greater control over their healthcare management. This trend has accelerated following the COVID-19 pandemic, which demonstrated the critical importance of rapid, accessible diagnostic capabilities during public health emergencies and normalized the concept of at-home testing for many consumers.
The primary market segments for portable microfluidic ELISA technology include clinical diagnostics (hospitals, clinics, physician offices), home healthcare, research institutions, and pharmaceutical companies. Geographic market analysis shows particularly strong demand growth in developing regions where healthcare infrastructure remains limited but smartphone penetration is high, enabling integration with mobile health platforms.
Regulatory landscapes are evolving to accommodate innovative diagnostic technologies, with many countries establishing expedited approval pathways for point-of-care solutions that demonstrate significant public health benefits. This regulatory shift has created more favorable market conditions for novel portable immunodiagnostic technologies.
Key unmet needs identified through market research include improved sensitivity comparable to laboratory-based tests, reduced cost per test, simplified user interfaces for non-technical operators, expanded test menus on single platforms, and seamless data integration with electronic health records. Stakeholder interviews consistently highlight the importance of balancing analytical performance with practical considerations like shelf stability, minimal sample preparation, and intuitive result interpretation.
Market forecasts project continued strong growth for portable immunodiagnostic solutions, with microfluidic ELISA technologies positioned to capture significant market share due to their potential for multiplexed testing, reduced reagent consumption, and compatibility with various biomarkers. The convergence of microfluidics with smartphone-based detection systems represents a particularly promising market opportunity, leveraging existing consumer technology to reduce overall system costs.
Current Challenges in Microfluidic ELISA Development
Despite significant advancements in microfluidic ELISA technologies for portable immunodiagnostic testing, several critical challenges continue to impede widespread adoption and commercialization. The miniaturization of conventional ELISA protocols onto microfluidic platforms introduces complex fluid dynamics that affect assay performance. Surface tension effects, capillary forces, and laminar flow characteristics in microchannels create difficulties in achieving consistent reagent mixing and uniform analyte binding, leading to variability in test results.
Integration of multiple assay steps remains problematic, particularly the transition between washing, reagent addition, and detection phases. Current microfluidic designs struggle to incorporate all necessary ELISA components without compromising portability or increasing system complexity. The need for precise fluid control mechanisms often results in complicated chip designs requiring external pumps or multiple manual interventions, contradicting the goal of user-friendly point-of-care applications.
Sensitivity and detection limits present another significant hurdle. While conventional laboratory ELISA can achieve femtomolar detection limits, microfluidic versions frequently demonstrate reduced sensitivity due to smaller sample volumes and detection areas. This limitation becomes particularly problematic when diagnosing conditions where biomarkers appear at extremely low concentrations in early disease stages, potentially leading to false negatives in clinical settings.
Manufacturing scalability poses substantial challenges for commercialization. Many microfluidic ELISA designs employ sophisticated fabrication techniques like photolithography or laser ablation that are difficult to scale for mass production. Material compatibility issues arise when transitioning from laboratory prototypes to commercial products, affecting both performance and manufacturing costs. The lack of standardized fabrication protocols further complicates quality control across production batches.
Reagent stability represents a critical constraint for field deployment. Antibodies and enzymes used in ELISA reactions require specific storage conditions to maintain activity. Current preservation methods for on-chip reagent storage show limited shelf-life at ambient temperatures, restricting the practical utility of these devices in resource-limited settings without cold chain infrastructure.
Cross-platform compatibility and result standardization remain underdeveloped. The diversity of microfluidic ELISA designs has led to inconsistent reporting formats and calibration standards, making it difficult to compare results between different platforms or correlate them with conventional laboratory tests. This standardization gap hinders clinical validation and regulatory approval processes necessary for widespread adoption in healthcare settings.
Regulatory pathways for microfluidic diagnostic devices present complex hurdles, with requirements varying significantly across global markets. The novel nature of these integrated systems often places them in regulatory gray areas, requiring extensive validation studies and documentation that many research groups and startups lack resources to complete.
Integration of multiple assay steps remains problematic, particularly the transition between washing, reagent addition, and detection phases. Current microfluidic designs struggle to incorporate all necessary ELISA components without compromising portability or increasing system complexity. The need for precise fluid control mechanisms often results in complicated chip designs requiring external pumps or multiple manual interventions, contradicting the goal of user-friendly point-of-care applications.
Sensitivity and detection limits present another significant hurdle. While conventional laboratory ELISA can achieve femtomolar detection limits, microfluidic versions frequently demonstrate reduced sensitivity due to smaller sample volumes and detection areas. This limitation becomes particularly problematic when diagnosing conditions where biomarkers appear at extremely low concentrations in early disease stages, potentially leading to false negatives in clinical settings.
Manufacturing scalability poses substantial challenges for commercialization. Many microfluidic ELISA designs employ sophisticated fabrication techniques like photolithography or laser ablation that are difficult to scale for mass production. Material compatibility issues arise when transitioning from laboratory prototypes to commercial products, affecting both performance and manufacturing costs. The lack of standardized fabrication protocols further complicates quality control across production batches.
Reagent stability represents a critical constraint for field deployment. Antibodies and enzymes used in ELISA reactions require specific storage conditions to maintain activity. Current preservation methods for on-chip reagent storage show limited shelf-life at ambient temperatures, restricting the practical utility of these devices in resource-limited settings without cold chain infrastructure.
Cross-platform compatibility and result standardization remain underdeveloped. The diversity of microfluidic ELISA designs has led to inconsistent reporting formats and calibration standards, making it difficult to compare results between different platforms or correlate them with conventional laboratory tests. This standardization gap hinders clinical validation and regulatory approval processes necessary for widespread adoption in healthcare settings.
Regulatory pathways for microfluidic diagnostic devices present complex hurdles, with requirements varying significantly across global markets. The novel nature of these integrated systems often places them in regulatory gray areas, requiring extensive validation studies and documentation that many research groups and startups lack resources to complete.
Current Microfluidic ELISA Implementation Approaches
01 Portable microfluidic ELISA devices
Portable microfluidic ELISA devices have been developed to enable point-of-care testing outside of traditional laboratory settings. These devices integrate sample preparation, reagent handling, and detection systems into compact, portable platforms. The miniaturization of ELISA technology through microfluidics allows for reduced sample volumes, faster analysis times, and decreased power requirements, making them suitable for field use and resource-limited settings.- Portable microfluidic ELISA devices: Portable microfluidic ELISA devices have been developed to enable point-of-care testing outside of traditional laboratory settings. These devices integrate sample preparation, reagent handling, and detection systems into compact, portable platforms. The miniaturization allows for reduced sample volumes, faster analysis times, and lower power requirements, making them suitable for field use, remote locations, and resource-limited settings.
- Integrated sample preparation and analysis systems: Microfluidic ELISA platforms incorporate integrated sample preparation and analysis systems to enhance portability. These systems combine multiple analytical steps including sample collection, filtration, mixing with reagents, incubation, washing, and detection within a single device. The integration reduces the need for external equipment, minimizes user intervention, and enables automated workflows, contributing to improved portability and ease of use in field settings.
- Miniaturized detection systems: Miniaturized detection systems are crucial components of portable microfluidic ELISA devices. These systems utilize various detection methods including colorimetric, fluorescence, chemiluminescence, and electrochemical techniques that have been adapted for portable applications. The detection systems are designed to be compact, energy-efficient, and sensitive enough to detect low analyte concentrations, while maintaining compatibility with smartphone-based or other portable readout devices.
- Paper-based microfluidic ELISA platforms: Paper-based microfluidic ELISA platforms offer enhanced portability through the use of inexpensive, lightweight, and disposable materials. These platforms utilize paper substrates with patterned hydrophobic barriers to create microfluidic channels and reaction zones. The capillary action in paper eliminates the need for external pumps, and the platforms can be designed as foldable or stackable structures to facilitate multiple assay steps while maintaining a compact form factor for field deployment.
- Power-efficient microfluidic control systems: Power-efficient microfluidic control systems enhance the portability of ELISA devices by reducing energy requirements. These systems incorporate passive flow control mechanisms such as capillary forces, gravity-driven flow, or pre-stored vacuum to minimize or eliminate the need for external pumps. Additionally, some designs feature low-power microcontrollers, energy-harvesting technologies, or optimized battery systems to extend operational time in field settings where power sources may be limited.
02 Integrated sample preparation and analysis systems
Microfluidic ELISA platforms incorporate integrated sample preparation and analysis systems to enhance portability. These systems combine multiple laboratory steps into a single device, including sample collection, filtration, mixing with reagents, incubation, and detection. The integration reduces the need for external equipment and minimizes user intervention, making the devices more suitable for non-laboratory environments and operation by minimally trained personnel.Expand Specific Solutions03 Miniaturized detection systems for portable ELISA
Miniaturized detection systems have been developed specifically for portable microfluidic ELISA applications. These systems include compact optical sensors, smartphone-based detection platforms, and integrated electrochemical sensors that can detect and quantify ELISA results without bulky laboratory equipment. The miniaturized detection systems enable real-time analysis and data processing, contributing significantly to the overall portability of microfluidic ELISA technology.Expand Specific Solutions04 Power-efficient microfluidic ELISA designs
Power-efficient designs are crucial for portable microfluidic ELISA devices. These designs incorporate low-power components, energy-harvesting technologies, and optimized fluid handling mechanisms that require minimal external power. Some systems utilize passive capillary flow or gravity-driven mechanisms to move fluids through the microchannels, while others employ efficient micropumps and valves that consume minimal energy, enabling battery operation or even solar-powered functionality in field settings.Expand Specific Solutions05 Ruggedized and field-deployable ELISA platforms
Ruggedized and field-deployable microfluidic ELISA platforms are designed to withstand challenging environmental conditions while maintaining analytical performance. These platforms incorporate robust materials, sealed components, and temperature stabilization features to ensure reliable operation in diverse settings. The designs focus on resistance to physical shock, temperature variations, humidity, and contamination, making them suitable for use in remote locations, disaster areas, or resource-limited healthcare facilities.Expand Specific Solutions
Key Industry Players in Microfluidic Diagnostics
Microfluidic ELISA for portable immunodiagnostic testing is currently in a growth phase, with the market expanding due to increasing demand for point-of-care diagnostics. The global market size is projected to reach significant value as healthcare systems seek cost-effective, rapid testing solutions. Technologically, the field is advancing from early-stage development toward maturity, with key players demonstrating various levels of innovation. Companies like Revvity Health Sciences and F. Hoffmann-La Roche are leading commercial development with established diagnostic portfolios, while Samsung Electronics brings expertise in miniaturization and consumer electronics integration. Academic institutions including Beijing University of Chemical Technology, Sun Yat-Sen University, and Fudan University are contributing fundamental research advances, particularly in novel microfluidic designs and detection methods. Research organizations like AIT Austrian Institute of Technology and Industrial Technology Research Institute are bridging the gap between academic innovation and commercial application.
Revvity Health Sciences, Inc.
Technical Solution: Revvity has pioneered microfluidic ELISA technology with their integrated lab-on-a-chip platforms designed specifically for point-of-care diagnostics. Their approach utilizes proprietary microfluidic cartridges with pre-loaded reagents that enable complete sample-to-answer workflows with minimal user intervention. The technology incorporates advanced flow control mechanisms including passive capillary valves and active pneumatic controls to precisely manage sample and reagent movement through the analytical pathway. Revvity's systems feature highly sensitive chemiluminescence detection methods that achieve sub-picogram/mL detection limits while maintaining portability. Their platforms incorporate automated quality control measures including internal calibration standards and process verification steps to ensure reliable results in field settings. The company has developed specialized surface treatments that minimize non-specific binding while maximizing capture antibody density, significantly improving signal-to-noise ratios compared to conventional ELISA methods[2][5].
Strengths: Exceptional sensitivity approaching laboratory-grade instruments; highly automated workflow reducing user error potential; robust validation across diverse environmental conditions ensuring field reliability. Weaknesses: Higher per-test costs compared to traditional laboratory methods; limited customization options for specialized applications; dependence on proprietary consumables increasing operational costs.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed advanced microfluidic ELISA platforms that integrate multiple analytical steps onto a single chip. Their technology utilizes precision-engineered microchannels with optimized surface chemistry for antibody immobilization, enhancing sensitivity while reducing sample volume requirements to nanoliter scales. The company's portable immunodiagnostic systems incorporate automated fluid handling with integrated optical detection systems capable of quantitative analysis. Roche's platforms feature multiplexed detection capabilities, allowing simultaneous testing for multiple biomarkers from a single sample. Their systems employ sophisticated signal amplification techniques to achieve detection limits comparable to laboratory-based systems while maintaining field portability. Recent innovations include integration with smartphone-based readout systems for telemedicine applications and cloud connectivity for remote data analysis and patient monitoring[1][3].
Strengths: Exceptional manufacturing quality control ensuring reproducible results; extensive clinical validation across diverse biomarkers; comprehensive ecosystem of reagents and consumables optimized for their platforms. Weaknesses: Higher cost compared to simpler systems; proprietary reagent requirements limiting flexibility; relatively complex user interface requiring more training than some competing solutions.
Core Patents and Innovations in Portable Immunoassays
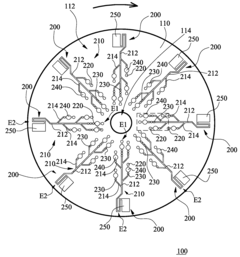
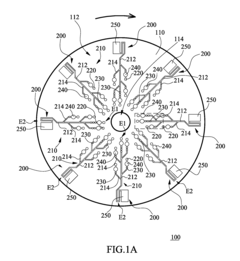
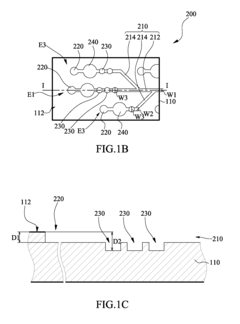
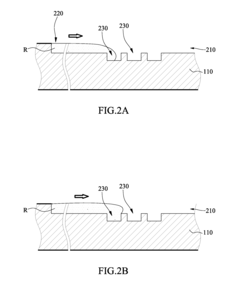
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Framework for Point-of-Care Diagnostic Devices
The regulatory landscape for point-of-care diagnostic devices, particularly microfluidic ELISA systems for portable immunodiagnostic testing, presents a complex framework that manufacturers must navigate to bring products to market. In the United States, the Food and Drug Administration (FDA) classifies these devices under the In Vitro Diagnostic (IVD) regulatory pathway, with most portable ELISA systems falling into Class II, requiring a 510(k) premarket notification unless granted a CLIA waiver for point-of-care use.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, most microfluidic immunoassay devices are classified as Class C, requiring conformity assessment by a Notified Body.
In emerging markets, regulatory frameworks vary significantly. China's National Medical Products Administration (NMPA) has established specific pathways for IVD devices, while India's Central Drugs Standard Control Organization (CDSCO) regulates these products under medical device rules implemented in 2017, with specific provisions for near-patient testing devices.
Quality management systems compliance represents a critical regulatory requirement across jurisdictions. ISO 13485:2016 serves as the international standard for medical device quality management systems, while specific standards like ISO 14971 for risk management and IEC 62304 for software lifecycle processes apply to devices with digital components, which is increasingly common in modern microfluidic platforms.
Performance validation requirements present particular challenges for portable immunodiagnostic systems. Regulatory bodies typically require demonstration of analytical performance (sensitivity, specificity, precision), clinical performance (clinical sensitivity and specificity), and stability under various environmental conditions—especially critical for portable devices designed for field use in diverse settings.
The regulatory pathway for novel microfluidic ELISA technologies often benefits from early engagement with regulatory authorities. The FDA's Pre-Submission Program and the EU's Scientific Advice procedure provide opportunities for manufacturers to discuss development plans before formal submission, potentially streamlining the approval process for innovative diagnostic platforms.
Recent regulatory trends indicate increasing focus on real-world performance data and cybersecurity considerations for connected diagnostic devices. Additionally, regulatory frameworks are evolving to accommodate artificial intelligence and machine learning components that may be integrated into advanced immunodiagnostic systems for result interpretation and quality control.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, most microfluidic immunoassay devices are classified as Class C, requiring conformity assessment by a Notified Body.
In emerging markets, regulatory frameworks vary significantly. China's National Medical Products Administration (NMPA) has established specific pathways for IVD devices, while India's Central Drugs Standard Control Organization (CDSCO) regulates these products under medical device rules implemented in 2017, with specific provisions for near-patient testing devices.
Quality management systems compliance represents a critical regulatory requirement across jurisdictions. ISO 13485:2016 serves as the international standard for medical device quality management systems, while specific standards like ISO 14971 for risk management and IEC 62304 for software lifecycle processes apply to devices with digital components, which is increasingly common in modern microfluidic platforms.
Performance validation requirements present particular challenges for portable immunodiagnostic systems. Regulatory bodies typically require demonstration of analytical performance (sensitivity, specificity, precision), clinical performance (clinical sensitivity and specificity), and stability under various environmental conditions—especially critical for portable devices designed for field use in diverse settings.
The regulatory pathway for novel microfluidic ELISA technologies often benefits from early engagement with regulatory authorities. The FDA's Pre-Submission Program and the EU's Scientific Advice procedure provide opportunities for manufacturers to discuss development plans before formal submission, potentially streamlining the approval process for innovative diagnostic platforms.
Recent regulatory trends indicate increasing focus on real-world performance data and cybersecurity considerations for connected diagnostic devices. Additionally, regulatory frameworks are evolving to accommodate artificial intelligence and machine learning components that may be integrated into advanced immunodiagnostic systems for result interpretation and quality control.
Cost-Benefit Analysis of Microfluidic ELISA Adoption
The adoption of microfluidic ELISA technology for portable immunodiagnostic testing requires careful economic evaluation to determine its viability across different healthcare settings. Initial implementation costs include specialized equipment acquisition, staff training, and facility modifications, which can range from $50,000 to $200,000 depending on scale and sophistication of the system.
Operational expenses must be considered alongside capital investments. While traditional ELISA testing typically costs $15-25 per test in laboratory settings, microfluidic platforms can potentially reduce this to $5-10 per test through decreased reagent consumption (up to 90% reduction) and minimized labor requirements. Additionally, the compact nature of microfluidic systems reduces physical space requirements by approximately 75% compared to conventional laboratory setups.
Time efficiency represents a significant benefit, with microfluidic ELISA reducing testing turnaround from hours to 15-30 minutes. This acceleration enables point-of-care applications that can dramatically improve patient management decisions and reduce unnecessary hospitalizations, estimated to save $300-500 per patient in acute care settings.
Maintenance considerations reveal that microfluidic systems generally require less frequent calibration and quality control procedures than traditional immunoassay equipment. However, specialized components may necessitate technical support that could be challenging in resource-limited environments, potentially adding 10-15% to annual operational costs.
Scalability analysis demonstrates that microfluidic ELISA platforms offer favorable economics at both low and high testing volumes. Small clinics processing 10-50 tests daily achieve cost-effectiveness within 12-18 months, while high-volume settings (>200 tests daily) may see return on investment within 6-9 months.
Long-term economic impact assessment indicates that widespread adoption could reduce overall healthcare expenditures by enabling earlier disease detection and more targeted therapeutic interventions. Studies suggest potential savings of $1,200-3,000 per patient annually for conditions requiring regular immunological monitoring, such as autoimmune disorders or therapeutic drug monitoring.
Risk factors include potential technology obsolescence as the field rapidly evolves, regulatory compliance costs, and variable reimbursement policies across healthcare systems. These uncertainties must be factored into comprehensive adoption planning with contingency budgeting of 15-20% recommended.
Operational expenses must be considered alongside capital investments. While traditional ELISA testing typically costs $15-25 per test in laboratory settings, microfluidic platforms can potentially reduce this to $5-10 per test through decreased reagent consumption (up to 90% reduction) and minimized labor requirements. Additionally, the compact nature of microfluidic systems reduces physical space requirements by approximately 75% compared to conventional laboratory setups.
Time efficiency represents a significant benefit, with microfluidic ELISA reducing testing turnaround from hours to 15-30 minutes. This acceleration enables point-of-care applications that can dramatically improve patient management decisions and reduce unnecessary hospitalizations, estimated to save $300-500 per patient in acute care settings.
Maintenance considerations reveal that microfluidic systems generally require less frequent calibration and quality control procedures than traditional immunoassay equipment. However, specialized components may necessitate technical support that could be challenging in resource-limited environments, potentially adding 10-15% to annual operational costs.
Scalability analysis demonstrates that microfluidic ELISA platforms offer favorable economics at both low and high testing volumes. Small clinics processing 10-50 tests daily achieve cost-effectiveness within 12-18 months, while high-volume settings (>200 tests daily) may see return on investment within 6-9 months.
Long-term economic impact assessment indicates that widespread adoption could reduce overall healthcare expenditures by enabling earlier disease detection and more targeted therapeutic interventions. Studies suggest potential savings of $1,200-3,000 per patient annually for conditions requiring regular immunological monitoring, such as autoimmune disorders or therapeutic drug monitoring.
Risk factors include potential technology obsolescence as the field rapidly evolves, regulatory compliance costs, and variable reimbursement policies across healthcare systems. These uncertainties must be factored into comprehensive adoption planning with contingency budgeting of 15-20% recommended.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!