Microfluidic ELISA for Viral Antigen Detection
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Microfluidic Enzyme-Linked Immunosorbent Assay (ELISA) represents a significant advancement in diagnostic technology, evolving from traditional ELISA methods first developed in the 1970s. This miniaturized platform integrates the principles of microfluidics—the manipulation of fluids at the microscale—with the high specificity and sensitivity of immunoassays, creating a powerful tool for viral antigen detection.
The evolution of microfluidic ELISA has been driven by increasing demands for rapid, sensitive, and point-of-care diagnostic solutions, particularly during global health emergencies such as the SARS, MERS, and COVID-19 pandemics. Traditional ELISA techniques, while reliable, are limited by lengthy processing times, laboratory dependence, and substantial reagent consumption.
Technological progression in this field has followed several key trends: miniaturization of reaction chambers, integration of multiple analytical steps, automation of fluid handling, and enhancement of detection sensitivity. The convergence of microfluidics with advanced materials science, nanotechnology, and biosensor development has accelerated innovation in this domain.
The fundamental principle of microfluidic ELISA involves capturing target viral antigens using specific antibodies immobilized within microchannels or microchambers, followed by detection using enzyme-labeled secondary antibodies. The confined reaction environment significantly reduces diffusion distances, enhancing reaction kinetics and detection efficiency.
Recent advancements have incorporated novel detection methods including electrochemical, fluorescence, chemiluminescence, and colorimetric approaches, each offering unique advantages in terms of sensitivity, equipment requirements, and quantification capabilities. Additionally, smartphone-based detection systems have emerged, democratizing access to sophisticated diagnostic capabilities in resource-limited settings.
The primary objectives of current microfluidic ELISA research for viral antigen detection include: reducing detection time from hours to minutes; achieving sensitivity comparable to or exceeding PCR-based methods; developing fully integrated sample-to-answer systems; ensuring compatibility with diverse biological samples; and creating cost-effective, mass-producible platforms suitable for global deployment.
Furthermore, research aims to address challenges related to non-specific binding, cross-reactivity with similar viral antigens, and matrix effects from complex biological samples. The development of multiplexed systems capable of simultaneously detecting multiple viral antigens represents another critical research direction, particularly valuable for differential diagnosis during co-circulating outbreaks.
The ultimate goal is to establish microfluidic ELISA as a standard diagnostic tool that combines the specificity of traditional immunoassays with the speed, portability, and minimal resource requirements necessary for effective point-of-care testing in diverse healthcare settings worldwide.
The evolution of microfluidic ELISA has been driven by increasing demands for rapid, sensitive, and point-of-care diagnostic solutions, particularly during global health emergencies such as the SARS, MERS, and COVID-19 pandemics. Traditional ELISA techniques, while reliable, are limited by lengthy processing times, laboratory dependence, and substantial reagent consumption.
Technological progression in this field has followed several key trends: miniaturization of reaction chambers, integration of multiple analytical steps, automation of fluid handling, and enhancement of detection sensitivity. The convergence of microfluidics with advanced materials science, nanotechnology, and biosensor development has accelerated innovation in this domain.
The fundamental principle of microfluidic ELISA involves capturing target viral antigens using specific antibodies immobilized within microchannels or microchambers, followed by detection using enzyme-labeled secondary antibodies. The confined reaction environment significantly reduces diffusion distances, enhancing reaction kinetics and detection efficiency.
Recent advancements have incorporated novel detection methods including electrochemical, fluorescence, chemiluminescence, and colorimetric approaches, each offering unique advantages in terms of sensitivity, equipment requirements, and quantification capabilities. Additionally, smartphone-based detection systems have emerged, democratizing access to sophisticated diagnostic capabilities in resource-limited settings.
The primary objectives of current microfluidic ELISA research for viral antigen detection include: reducing detection time from hours to minutes; achieving sensitivity comparable to or exceeding PCR-based methods; developing fully integrated sample-to-answer systems; ensuring compatibility with diverse biological samples; and creating cost-effective, mass-producible platforms suitable for global deployment.
Furthermore, research aims to address challenges related to non-specific binding, cross-reactivity with similar viral antigens, and matrix effects from complex biological samples. The development of multiplexed systems capable of simultaneously detecting multiple viral antigens represents another critical research direction, particularly valuable for differential diagnosis during co-circulating outbreaks.
The ultimate goal is to establish microfluidic ELISA as a standard diagnostic tool that combines the specificity of traditional immunoassays with the speed, portability, and minimal resource requirements necessary for effective point-of-care testing in diverse healthcare settings worldwide.
Market Demand Analysis for Rapid Viral Antigen Detection
The global market for rapid viral antigen detection has experienced unprecedented growth, particularly accelerated by the COVID-19 pandemic. Current market valuations indicate that the viral diagnostics sector reached approximately 19.2 billion USD in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2030. This substantial growth reflects the critical need for efficient, accurate, and accessible viral detection technologies across healthcare systems worldwide.
Microfluidic ELISA technology addresses several key market demands that traditional laboratory-based testing cannot fulfill. Primary among these is the need for point-of-care testing capabilities, which has become increasingly important in resource-limited settings, emergency response scenarios, and decentralized healthcare models. The ability to deliver laboratory-quality results without sophisticated infrastructure represents a significant market advantage.
Healthcare providers are increasingly demanding rapid turnaround times for viral antigen detection, with ideal solutions delivering results in under 30 minutes. This demand is driven by clinical requirements for quick decision-making in treatment protocols and infection control measures. Traditional ELISA methods typically require 3-5 hours for completion, creating a substantial market opportunity for microfluidic solutions that can dramatically reduce this timeframe.
Cost considerations also drive market demand, particularly in low and middle-income countries where diagnostic budgets are constrained. The global market shows strong preference for solutions that can reduce per-test costs below 5 USD while maintaining high sensitivity and specificity. Microfluidic ELISA platforms offer potential cost advantages through reduced reagent consumption and simplified operational requirements.
The market increasingly demands multiplex testing capabilities, allowing for simultaneous detection of multiple viral antigens from a single sample. This trend is particularly evident in respiratory virus diagnostics, where differentiation between influenza, respiratory syncytial virus, and SARS-CoV-2 from a single test provides significant clinical and operational value.
Regulatory and procurement agencies worldwide have established stringent performance requirements, typically demanding sensitivity above 95% and specificity above 98% for viral antigen detection. These benchmarks represent the minimum market entry threshold for new technologies seeking widespread adoption.
Consumer and healthcare provider preferences are shifting toward user-friendly diagnostic platforms that require minimal training and technical expertise. This trend favors microfluidic solutions that automate complex processes and reduce user-dependent variables, potentially expanding the addressable market beyond traditional laboratory settings to include pharmacies, workplace clinics, and home testing environments.
Microfluidic ELISA technology addresses several key market demands that traditional laboratory-based testing cannot fulfill. Primary among these is the need for point-of-care testing capabilities, which has become increasingly important in resource-limited settings, emergency response scenarios, and decentralized healthcare models. The ability to deliver laboratory-quality results without sophisticated infrastructure represents a significant market advantage.
Healthcare providers are increasingly demanding rapid turnaround times for viral antigen detection, with ideal solutions delivering results in under 30 minutes. This demand is driven by clinical requirements for quick decision-making in treatment protocols and infection control measures. Traditional ELISA methods typically require 3-5 hours for completion, creating a substantial market opportunity for microfluidic solutions that can dramatically reduce this timeframe.
Cost considerations also drive market demand, particularly in low and middle-income countries where diagnostic budgets are constrained. The global market shows strong preference for solutions that can reduce per-test costs below 5 USD while maintaining high sensitivity and specificity. Microfluidic ELISA platforms offer potential cost advantages through reduced reagent consumption and simplified operational requirements.
The market increasingly demands multiplex testing capabilities, allowing for simultaneous detection of multiple viral antigens from a single sample. This trend is particularly evident in respiratory virus diagnostics, where differentiation between influenza, respiratory syncytial virus, and SARS-CoV-2 from a single test provides significant clinical and operational value.
Regulatory and procurement agencies worldwide have established stringent performance requirements, typically demanding sensitivity above 95% and specificity above 98% for viral antigen detection. These benchmarks represent the minimum market entry threshold for new technologies seeking widespread adoption.
Consumer and healthcare provider preferences are shifting toward user-friendly diagnostic platforms that require minimal training and technical expertise. This trend favors microfluidic solutions that automate complex processes and reduce user-dependent variables, potentially expanding the addressable market beyond traditional laboratory settings to include pharmacies, workplace clinics, and home testing environments.
Current Status and Challenges in Microfluidic ELISA Development
Microfluidic ELISA technology for viral antigen detection has witnessed significant advancements globally, yet faces several critical challenges that impede its widespread clinical adoption. Currently, research institutions across North America, Europe, and Asia have developed various microfluidic platforms capable of performing ELISA with reduced sample volumes (1-10 μL) and accelerated reaction times (15-30 minutes) compared to conventional ELISA methods that require hours to complete.
The miniaturization of ELISA onto microfluidic chips has successfully demonstrated enhanced sensitivity, with detection limits reaching picogram to femtogram levels for viral antigens including SARS-CoV-2, influenza, and dengue. This represents a 10-100 fold improvement over traditional plate-based methods. However, this sensitivity enhancement is not consistent across different viral targets and sample matrices, presenting a significant technical hurdle.
A major challenge in microfluidic ELISA development is achieving reproducible surface functionalization for antibody immobilization. Current approaches including physical adsorption, covalent binding, and bioaffinity immobilization each present trade-offs between binding stability, antibody orientation, and retention of biological activity. The microscale environment introduces complex fluid dynamics that affect binding kinetics and washing efficiency, further complicating assay standardization.
Integration of sample preparation remains problematic, with most current systems requiring off-chip sample processing before introduction to the microfluidic device. This preprocessing step diminishes the point-of-care advantages of microfluidic platforms and introduces potential for contamination and human error. Only approximately 30% of published microfluidic ELISA systems incorporate on-chip sample preparation capabilities.
Manufacturing scalability presents another significant barrier. Many academic prototypes utilize materials and fabrication techniques that are not amenable to mass production, such as PDMS molding or complex surface treatments. The transition from laboratory prototypes to commercially viable products faces challenges in materials selection, manufacturing processes, and quality control that maintain the performance characteristics demonstrated in research settings.
Detection methods vary widely across platforms, from optical (colorimetric, fluorescence, chemiluminescence) to electrochemical approaches. While electrochemical detection offers advantages in miniaturization and electronic integration, optical methods currently dominate due to their established protocols and readout systems. The lack of standardization in detection methodologies complicates cross-platform comparison and validation.
Regulatory pathways for microfluidic diagnostic devices remain complex, with few microfluidic ELISA systems having achieved FDA approval or equivalent authorizations globally. The novel nature of these integrated systems often requires extensive validation studies and sometimes new regulatory frameworks, significantly extending development timelines and increasing costs for commercialization.
The miniaturization of ELISA onto microfluidic chips has successfully demonstrated enhanced sensitivity, with detection limits reaching picogram to femtogram levels for viral antigens including SARS-CoV-2, influenza, and dengue. This represents a 10-100 fold improvement over traditional plate-based methods. However, this sensitivity enhancement is not consistent across different viral targets and sample matrices, presenting a significant technical hurdle.
A major challenge in microfluidic ELISA development is achieving reproducible surface functionalization for antibody immobilization. Current approaches including physical adsorption, covalent binding, and bioaffinity immobilization each present trade-offs between binding stability, antibody orientation, and retention of biological activity. The microscale environment introduces complex fluid dynamics that affect binding kinetics and washing efficiency, further complicating assay standardization.
Integration of sample preparation remains problematic, with most current systems requiring off-chip sample processing before introduction to the microfluidic device. This preprocessing step diminishes the point-of-care advantages of microfluidic platforms and introduces potential for contamination and human error. Only approximately 30% of published microfluidic ELISA systems incorporate on-chip sample preparation capabilities.
Manufacturing scalability presents another significant barrier. Many academic prototypes utilize materials and fabrication techniques that are not amenable to mass production, such as PDMS molding or complex surface treatments. The transition from laboratory prototypes to commercially viable products faces challenges in materials selection, manufacturing processes, and quality control that maintain the performance characteristics demonstrated in research settings.
Detection methods vary widely across platforms, from optical (colorimetric, fluorescence, chemiluminescence) to electrochemical approaches. While electrochemical detection offers advantages in miniaturization and electronic integration, optical methods currently dominate due to their established protocols and readout systems. The lack of standardization in detection methodologies complicates cross-platform comparison and validation.
Regulatory pathways for microfluidic diagnostic devices remain complex, with few microfluidic ELISA systems having achieved FDA approval or equivalent authorizations globally. The novel nature of these integrated systems often requires extensive validation studies and sometimes new regulatory frameworks, significantly extending development timelines and increasing costs for commercialization.
Current Technical Solutions for Microfluidic ELISA Implementation
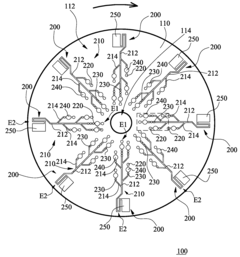
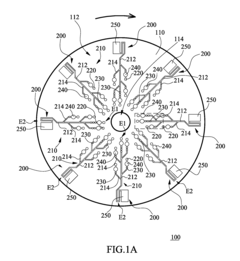
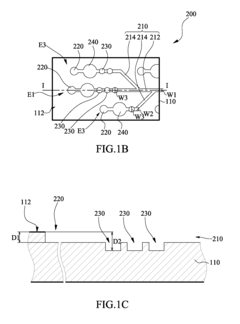
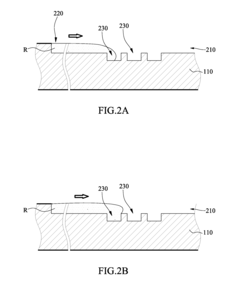
01 Microfluidic chip designs for viral antigen detection
Various microfluidic chip designs have been developed specifically for viral antigen detection using ELISA techniques. These designs incorporate channels, chambers, and reaction zones optimized for sample processing, reagent mixing, and signal detection. The chips often feature integrated valves, pumps, and flow control mechanisms to automate the ELISA process, reducing sample volume requirements and enhancing detection sensitivity. Some designs include multiple detection zones for simultaneous testing of different viral antigens.- Microfluidic chip designs for viral antigen detection: Various microfluidic chip designs have been developed specifically for viral antigen detection using ELISA techniques. These designs incorporate channels, chambers, and reaction zones optimized for sample preparation, reagent mixing, and signal detection. The microfluidic architecture enables precise control of fluid flow, reduced sample volumes, and enhanced sensitivity compared to traditional ELISA methods. These chips often integrate multiple functional components to achieve automated processing from sample input to result readout.
- Integration of detection systems with microfluidic ELISA: Detection systems integrated with microfluidic ELISA platforms enable rapid and sensitive viral antigen detection. These systems may incorporate optical, electrochemical, or fluorescence-based detection methods that are miniaturized to work with microfluidic chips. Signal amplification techniques are often employed to enhance detection sensitivity, allowing for identification of viral antigens at low concentrations. The integration of these detection systems with microfluidic platforms creates portable and user-friendly diagnostic tools for point-of-care applications.
- Sample preparation and processing for viral detection: Effective sample preparation is crucial for successful viral antigen detection in microfluidic ELISA systems. Innovations in this area include methods for cell lysis, viral particle concentration, and removal of interfering substances from clinical samples. Microfluidic platforms may incorporate filtration membranes, magnetic bead-based separation, or chemical treatment zones to process raw samples before analysis. These sample preparation techniques improve detection sensitivity and reduce false results by providing purified viral antigens for the ELISA reaction.
- Multiplexed detection of viral antigens: Multiplexed detection systems allow for simultaneous identification of multiple viral antigens in a single microfluidic ELISA test. These systems utilize spatial separation of detection zones, different labeled antibodies, or array-based approaches to distinguish between various viral targets. Multiplexing capabilities are particularly valuable during outbreaks or co-circulation of multiple pathogens, enabling comprehensive diagnostic information from a single sample. This approach improves diagnostic efficiency while conserving precious sample material and reducing testing time.
- Portable and rapid diagnostic systems: Portable microfluidic ELISA systems have been developed for rapid viral antigen detection in resource-limited settings or point-of-care applications. These systems integrate sample processing, ELISA reactions, and result readout into compact, user-friendly devices that require minimal laboratory infrastructure. Innovations in this area focus on reducing assay time, simplifying operation procedures, and developing battery-powered or smartphone-integrated readout systems. These portable diagnostic tools enable timely detection of viral infections in field settings, supporting disease surveillance and control efforts.
02 Enhanced signal amplification methods for microfluidic ELISA
Signal amplification techniques have been developed to improve the sensitivity of microfluidic ELISA for viral antigen detection. These methods include enzymatic amplification cascades, nanoparticle-based signal enhancement, and novel substrate systems that produce intensified colorimetric, fluorescent, or electrochemical signals. The amplification strategies enable detection of viral antigens at lower concentrations, which is particularly important for early-stage infection diagnosis when viral loads may be minimal.Expand Specific Solutions03 Integration of sample preparation with microfluidic ELISA
Integrated systems that combine sample preparation with microfluidic ELISA have been developed for viral antigen detection. These systems incorporate modules for sample collection, filtration, cell lysis, and target enrichment prior to the ELISA reaction. The integration minimizes sample handling, reduces contamination risks, and streamlines the workflow from raw sample to detection result. Some systems include blood separation, nucleic acid extraction, and viral particle concentration capabilities within the same microfluidic platform.Expand Specific Solutions04 Multiplexed detection of viral antigens
Multiplexed microfluidic ELISA platforms enable simultaneous detection of multiple viral antigens in a single sample. These systems utilize spatial separation of detection zones, different detection antibodies, or distinct signal reporters to differentiate between various viral targets. Multiplexed detection is particularly valuable for differential diagnosis of infections with similar clinical presentations or for identifying co-infections. The technology reduces testing time, sample volume requirements, and reagent consumption compared to running multiple individual tests.Expand Specific Solutions05 Portable and point-of-care microfluidic ELISA systems
Portable microfluidic ELISA systems have been developed for point-of-care viral antigen detection. These compact devices integrate sample processing, ELISA reactions, and result readout in a user-friendly format suitable for field use or resource-limited settings. The systems often incorporate smartphone-based detection, battery operation, and simplified user interfaces. Some designs include disposable test cartridges with pre-loaded reagents to minimize user intervention and training requirements, enabling rapid viral detection outside of laboratory environments.Expand Specific Solutions
Critical Patents and Innovations in Microfluidic Viral Detection
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Framework for Point-of-Care Diagnostic Devices
The regulatory landscape for point-of-care diagnostic devices, particularly those utilizing microfluidic ELISA for viral antigen detection, presents a complex framework that manufacturers must navigate. In the United States, the Food and Drug Administration (FDA) classifies these devices under the In Vitro Diagnostic (IVD) regulatory pathway, with most viral detection systems falling under Class II or Class III, requiring either 510(k) clearance or Premarket Approval (PMA) respectively. During the COVID-19 pandemic, the FDA introduced Emergency Use Authorization (EUA) pathways, providing valuable precedents for expedited approval of novel microfluidic viral detection platforms.
The European Union regulates these devices under the In Vitro Diagnostic Medical Devices Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022. This transition introduced more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic ELISA systems for viral detection typically fall under Class C or D, requiring notified body involvement and comprehensive technical documentation.
International standards play a crucial role in regulatory compliance, with ISO 13485 for quality management systems and ISO 14971 for risk management being particularly relevant. For microfluidic ELISA systems, additional standards such as ISO 18113 (labeling requirements) and ISO 23640 (stability testing) must be considered. The Clinical and Laboratory Standards Institute (CLSI) also provides guidelines specific to point-of-care testing that influence regulatory expectations.
Emerging markets present varying regulatory approaches. China's National Medical Products Administration (NMPA) has established a registration pathway similar to the FDA but with country-specific requirements for clinical trials. India's Central Drugs Standard Control Organization (CDSCO) classifies most diagnostic devices under Class C or D, requiring substantial clinical evidence for approval.
Regulatory challenges specific to microfluidic ELISA platforms include demonstrating analytical and clinical performance across diverse viral strains, establishing appropriate quality control measures for miniaturized systems, and validating shelf-life stability in various environmental conditions. The integration of digital components for result interpretation introduces additional regulatory considerations related to software validation and cybersecurity.
Future regulatory trends indicate movement toward harmonization through the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives. Additionally, regulators are developing frameworks for AI-enhanced diagnostic systems, which may impact next-generation microfluidic ELISA platforms incorporating machine learning for result interpretation or predictive analytics.
The European Union regulates these devices under the In Vitro Diagnostic Medical Devices Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022. This transition introduced more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic ELISA systems for viral detection typically fall under Class C or D, requiring notified body involvement and comprehensive technical documentation.
International standards play a crucial role in regulatory compliance, with ISO 13485 for quality management systems and ISO 14971 for risk management being particularly relevant. For microfluidic ELISA systems, additional standards such as ISO 18113 (labeling requirements) and ISO 23640 (stability testing) must be considered. The Clinical and Laboratory Standards Institute (CLSI) also provides guidelines specific to point-of-care testing that influence regulatory expectations.
Emerging markets present varying regulatory approaches. China's National Medical Products Administration (NMPA) has established a registration pathway similar to the FDA but with country-specific requirements for clinical trials. India's Central Drugs Standard Control Organization (CDSCO) classifies most diagnostic devices under Class C or D, requiring substantial clinical evidence for approval.
Regulatory challenges specific to microfluidic ELISA platforms include demonstrating analytical and clinical performance across diverse viral strains, establishing appropriate quality control measures for miniaturized systems, and validating shelf-life stability in various environmental conditions. The integration of digital components for result interpretation introduces additional regulatory considerations related to software validation and cybersecurity.
Future regulatory trends indicate movement toward harmonization through the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives. Additionally, regulators are developing frameworks for AI-enhanced diagnostic systems, which may impact next-generation microfluidic ELISA platforms incorporating machine learning for result interpretation or predictive analytics.
Cost-Benefit Analysis of Microfluidic ELISA vs. Conventional Methods
The economic implications of implementing microfluidic ELISA technology for viral antigen detection require thorough examination when compared to conventional ELISA methods. Initial investment costs for microfluidic systems are significantly higher, with specialized equipment ranging from $50,000 to $150,000 depending on automation level and throughput capacity. Additionally, the development of custom microfluidic chips may require specialized manufacturing facilities, further increasing upfront expenses.
However, operational cost analysis reveals substantial long-term advantages. Microfluidic ELISA typically reduces reagent consumption by 80-95% compared to conventional methods, translating to approximately $3-5 savings per test in high-volume settings. Sample volumes are similarly reduced from microliters to nanoliters, enabling more tests from limited patient samples and decreasing collection costs.
Labor efficiency represents another significant economic benefit. Conventional ELISA protocols require 3-5 hours of technician time with multiple manual intervention steps. In contrast, automated microfluidic platforms can reduce hands-on time to 15-30 minutes, allowing laboratory personnel to manage multiple tests simultaneously and increasing throughput by 300-400%.
Time-to-result metrics strongly favor microfluidic approaches, with results typically available in 30-60 minutes versus 3-5 hours for conventional methods. This rapid turnaround enables faster clinical decision-making, particularly valuable during disease outbreaks when timely intervention affects patient outcomes and containment efforts.
Infrastructure requirements present contrasting profiles. Conventional ELISA demands dedicated laboratory space with specialized washing stations and incubators. Microfluidic systems offer a smaller footprint, potentially reducing facility costs by 40-60%, though they require reliable power sources and potentially specialized maintenance contracts.
Scalability analysis indicates microfluidic ELISA becomes increasingly cost-effective at higher testing volumes. The break-even point typically occurs at 5,000-10,000 tests annually, after which the per-test cost advantage becomes pronounced. For high-throughput settings processing >20,000 samples annually, cost savings may reach 60-70% compared to conventional methods.
Environmental impact assessment reveals microfluidic approaches generate approximately 75% less plastic waste and chemical effluent. While difficult to quantify economically, these sustainability benefits align with increasingly stringent environmental regulations and institutional sustainability goals, potentially avoiding future compliance costs.
When considering total cost of ownership over a five-year period, microfluidic ELISA systems demonstrate 30-45% lower costs despite higher initial investment, primarily through reagent savings, increased throughput, and reduced labor requirements. This economic advantage becomes particularly compelling for resource-limited settings where operational efficiency is paramount.
However, operational cost analysis reveals substantial long-term advantages. Microfluidic ELISA typically reduces reagent consumption by 80-95% compared to conventional methods, translating to approximately $3-5 savings per test in high-volume settings. Sample volumes are similarly reduced from microliters to nanoliters, enabling more tests from limited patient samples and decreasing collection costs.
Labor efficiency represents another significant economic benefit. Conventional ELISA protocols require 3-5 hours of technician time with multiple manual intervention steps. In contrast, automated microfluidic platforms can reduce hands-on time to 15-30 minutes, allowing laboratory personnel to manage multiple tests simultaneously and increasing throughput by 300-400%.
Time-to-result metrics strongly favor microfluidic approaches, with results typically available in 30-60 minutes versus 3-5 hours for conventional methods. This rapid turnaround enables faster clinical decision-making, particularly valuable during disease outbreaks when timely intervention affects patient outcomes and containment efforts.
Infrastructure requirements present contrasting profiles. Conventional ELISA demands dedicated laboratory space with specialized washing stations and incubators. Microfluidic systems offer a smaller footprint, potentially reducing facility costs by 40-60%, though they require reliable power sources and potentially specialized maintenance contracts.
Scalability analysis indicates microfluidic ELISA becomes increasingly cost-effective at higher testing volumes. The break-even point typically occurs at 5,000-10,000 tests annually, after which the per-test cost advantage becomes pronounced. For high-throughput settings processing >20,000 samples annually, cost savings may reach 60-70% compared to conventional methods.
Environmental impact assessment reveals microfluidic approaches generate approximately 75% less plastic waste and chemical effluent. While difficult to quantify economically, these sustainability benefits align with increasingly stringent environmental regulations and institutional sustainability goals, potentially avoiding future compliance costs.
When considering total cost of ownership over a five-year period, microfluidic ELISA systems demonstrate 30-45% lower costs despite higher initial investment, primarily through reagent savings, increased throughput, and reduced labor requirements. This economic advantage becomes particularly compelling for resource-limited settings where operational efficiency is paramount.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!