Low-Cost Fabrication of Disposable Microfluidic ELISA Chips
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Microfluidic Enzyme-Linked Immunosorbent Assay (ELISA) technology represents a significant advancement in diagnostic testing, combining the sensitivity and specificity of traditional ELISA with the advantages of microfluidic systems. The evolution of this technology began in the late 1990s when researchers first explored miniaturizing conventional laboratory procedures onto chip-based platforms. Over the past two decades, microfluidic ELISA has progressed from academic research to commercial applications, driven by increasing demands for point-of-care diagnostics and personalized medicine.
The technological trajectory has been characterized by continuous improvements in materials science, fabrication techniques, and detection methodologies. Early microfluidic ELISA systems relied on glass or silicon substrates, which offered excellent chemical compatibility but presented challenges in terms of cost and mass production. The field subsequently shifted toward polymer-based materials such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and cyclic olefin copolymer (COC), which provided better scalability and reduced manufacturing costs.
Recent trends indicate a growing focus on disposable, low-cost microfluidic ELISA platforms that maintain analytical performance while addressing accessibility concerns. This shift aligns with global healthcare initiatives aimed at democratizing advanced diagnostic capabilities, particularly in resource-limited settings. The integration of smartphone-based detection systems and cloud connectivity has further expanded the potential applications of these devices, enabling remote monitoring and data analysis.
The primary technical objective for low-cost fabrication of disposable microfluidic ELISA chips is to develop manufacturing processes that significantly reduce per-unit costs while maintaining diagnostic accuracy comparable to laboratory-based ELISA. This includes exploring alternative materials, simplifying fabrication steps, and optimizing reagent consumption. Secondary objectives include enhancing user-friendliness for non-specialists, extending shelf life without refrigeration, and ensuring compatibility with various biological samples.
From a broader perspective, the development of these technologies aims to address several healthcare challenges: reducing diagnostic turnaround times from days to minutes, enabling testing in remote locations without sophisticated laboratory infrastructure, and facilitating early disease detection through more frequent and accessible testing. The ultimate goal is to create a platform that bridges the gap between high-performance laboratory diagnostics and the practical constraints of real-world healthcare delivery.
The convergence of advances in microfabrication, surface chemistry, and detection technologies positions microfluidic ELISA as a transformative approach to bioanalytical testing. As fabrication costs continue to decrease and performance improves, these systems are expected to play an increasingly important role in global health initiatives, particularly for infectious disease monitoring, cancer screening, and management of chronic conditions.
The technological trajectory has been characterized by continuous improvements in materials science, fabrication techniques, and detection methodologies. Early microfluidic ELISA systems relied on glass or silicon substrates, which offered excellent chemical compatibility but presented challenges in terms of cost and mass production. The field subsequently shifted toward polymer-based materials such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and cyclic olefin copolymer (COC), which provided better scalability and reduced manufacturing costs.
Recent trends indicate a growing focus on disposable, low-cost microfluidic ELISA platforms that maintain analytical performance while addressing accessibility concerns. This shift aligns with global healthcare initiatives aimed at democratizing advanced diagnostic capabilities, particularly in resource-limited settings. The integration of smartphone-based detection systems and cloud connectivity has further expanded the potential applications of these devices, enabling remote monitoring and data analysis.
The primary technical objective for low-cost fabrication of disposable microfluidic ELISA chips is to develop manufacturing processes that significantly reduce per-unit costs while maintaining diagnostic accuracy comparable to laboratory-based ELISA. This includes exploring alternative materials, simplifying fabrication steps, and optimizing reagent consumption. Secondary objectives include enhancing user-friendliness for non-specialists, extending shelf life without refrigeration, and ensuring compatibility with various biological samples.
From a broader perspective, the development of these technologies aims to address several healthcare challenges: reducing diagnostic turnaround times from days to minutes, enabling testing in remote locations without sophisticated laboratory infrastructure, and facilitating early disease detection through more frequent and accessible testing. The ultimate goal is to create a platform that bridges the gap between high-performance laboratory diagnostics and the practical constraints of real-world healthcare delivery.
The convergence of advances in microfabrication, surface chemistry, and detection technologies positions microfluidic ELISA as a transformative approach to bioanalytical testing. As fabrication costs continue to decrease and performance improves, these systems are expected to play an increasingly important role in global health initiatives, particularly for infectious disease monitoring, cancer screening, and management of chronic conditions.
Market Analysis for Disposable Diagnostic Platforms
The global market for disposable diagnostic platforms is experiencing robust growth, driven by increasing demand for point-of-care testing solutions and the rising prevalence of chronic diseases. The disposable microfluidic ELISA chip segment represents a particularly promising area within this broader market, with an estimated market value exceeding $3.5 billion in 2023 and projected to reach $7.2 billion by 2028, reflecting a compound annual growth rate of 15.6%.
Healthcare facilities worldwide are increasingly adopting disposable diagnostic platforms due to their advantages in preventing cross-contamination, reducing biohazardous waste management costs, and eliminating the need for complex sterilization procedures. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of rapid, accessible diagnostic solutions that can be deployed in diverse healthcare settings.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate over the next five years, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about the benefits of early diagnosis in countries like China and India.
The end-user landscape for disposable microfluidic ELISA chips spans across hospitals, diagnostic laboratories, academic and research institutions, and home healthcare settings. Hospitals remain the largest end-user segment, accounting for roughly 42% of the market share, while the home healthcare segment is projected to grow at the highest rate due to the increasing trend toward self-testing and remote patient monitoring.
Key market drivers include the growing geriatric population, rising incidence of infectious and chronic diseases, technological advancements in microfluidic fabrication techniques, and increasing healthcare expenditure globally. The push for decentralized healthcare delivery models is also contributing significantly to market expansion.
Pricing sensitivity remains a critical factor influencing market adoption, particularly in low and middle-income countries. The average cost per test using traditional ELISA methods ranges from $15-25, while current microfluidic ELISA platforms cost approximately $8-12 per test. Achieving a price point below $5 per test through low-cost fabrication methods could potentially unlock massive market opportunities in resource-limited settings, potentially expanding the addressable market by 40-50%.
Consumer preferences are increasingly shifting toward user-friendly, rapid diagnostic solutions that provide accurate results with minimal technical expertise. This trend favors disposable microfluidic platforms that can deliver laboratory-quality results in decentralized settings, creating significant opportunities for innovative product development in this space.
Healthcare facilities worldwide are increasingly adopting disposable diagnostic platforms due to their advantages in preventing cross-contamination, reducing biohazardous waste management costs, and eliminating the need for complex sterilization procedures. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of rapid, accessible diagnostic solutions that can be deployed in diverse healthcare settings.
Geographically, North America currently dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate over the next five years, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about the benefits of early diagnosis in countries like China and India.
The end-user landscape for disposable microfluidic ELISA chips spans across hospitals, diagnostic laboratories, academic and research institutions, and home healthcare settings. Hospitals remain the largest end-user segment, accounting for roughly 42% of the market share, while the home healthcare segment is projected to grow at the highest rate due to the increasing trend toward self-testing and remote patient monitoring.
Key market drivers include the growing geriatric population, rising incidence of infectious and chronic diseases, technological advancements in microfluidic fabrication techniques, and increasing healthcare expenditure globally. The push for decentralized healthcare delivery models is also contributing significantly to market expansion.
Pricing sensitivity remains a critical factor influencing market adoption, particularly in low and middle-income countries. The average cost per test using traditional ELISA methods ranges from $15-25, while current microfluidic ELISA platforms cost approximately $8-12 per test. Achieving a price point below $5 per test through low-cost fabrication methods could potentially unlock massive market opportunities in resource-limited settings, potentially expanding the addressable market by 40-50%.
Consumer preferences are increasingly shifting toward user-friendly, rapid diagnostic solutions that provide accurate results with minimal technical expertise. This trend favors disposable microfluidic platforms that can deliver laboratory-quality results in decentralized settings, creating significant opportunities for innovative product development in this space.
Current Fabrication Challenges and Limitations
Despite significant advancements in microfluidic ELISA chip technology, the fabrication of these devices continues to face substantial challenges that impede widespread adoption, particularly in resource-limited settings. The conventional manufacturing processes for microfluidic ELISA chips predominantly rely on cleanroom facilities, photolithography techniques, and specialized equipment, resulting in high production costs that contradict the fundamental goal of creating truly disposable diagnostic tools.
Material selection presents a critical challenge, as the biocompatibility requirements must be balanced with cost considerations. Traditional materials like polydimethylsiloxane (PDMS) offer excellent optical properties and biocompatibility but involve complex preparation processes including mixing, degassing, and curing that are difficult to scale economically for mass production. Alternative materials such as polymethyl methacrylate (PMMA) and cyclic olefin copolymer (COC) may offer cost advantages but present their own fabrication complexities.
Surface modification and functionalization represent another significant hurdle. The creation of consistent hydrophilic or hydrophobic surfaces for controlled fluid flow and the immobilization of capture antibodies require precise chemical treatments that are difficult to standardize in low-cost production environments. These processes often involve plasma treatment or chemical modification steps that add complexity and cost to the manufacturing workflow.
Channel dimension precision and reproducibility remain problematic in low-cost fabrication scenarios. Current techniques struggle to consistently produce microchannels with the exact dimensions required for reliable ELISA reactions, particularly when transitioning from prototype to mass production. Variations in channel geometry can significantly impact fluid flow dynamics, reaction kinetics, and ultimately test sensitivity and specificity.
Integration of detection components presents additional complications. While sophisticated ELISA chips may incorporate electrochemical or optical detection elements, incorporating these components in a cost-effective manner while maintaining detection sensitivity poses considerable engineering challenges. The miniaturization of detection systems often requires specialized microfabrication techniques that are inherently expensive.
Scaling production from laboratory prototypes to commercial manufacturing introduces further limitations. Many promising fabrication approaches demonstrated in research settings fail to translate effectively to high-throughput production environments. Techniques like 3D printing offer potential solutions but currently lack the resolution and material compatibility required for reliable microfluidic ELISA applications.
Quality control and standardization represent persistent challenges in low-cost fabrication scenarios. The absence of established quality metrics and testing protocols specifically designed for disposable microfluidic ELISA chips complicates the validation process and regulatory approval pathway, creating additional barriers to commercialization and widespread implementation.
Material selection presents a critical challenge, as the biocompatibility requirements must be balanced with cost considerations. Traditional materials like polydimethylsiloxane (PDMS) offer excellent optical properties and biocompatibility but involve complex preparation processes including mixing, degassing, and curing that are difficult to scale economically for mass production. Alternative materials such as polymethyl methacrylate (PMMA) and cyclic olefin copolymer (COC) may offer cost advantages but present their own fabrication complexities.
Surface modification and functionalization represent another significant hurdle. The creation of consistent hydrophilic or hydrophobic surfaces for controlled fluid flow and the immobilization of capture antibodies require precise chemical treatments that are difficult to standardize in low-cost production environments. These processes often involve plasma treatment or chemical modification steps that add complexity and cost to the manufacturing workflow.
Channel dimension precision and reproducibility remain problematic in low-cost fabrication scenarios. Current techniques struggle to consistently produce microchannels with the exact dimensions required for reliable ELISA reactions, particularly when transitioning from prototype to mass production. Variations in channel geometry can significantly impact fluid flow dynamics, reaction kinetics, and ultimately test sensitivity and specificity.
Integration of detection components presents additional complications. While sophisticated ELISA chips may incorporate electrochemical or optical detection elements, incorporating these components in a cost-effective manner while maintaining detection sensitivity poses considerable engineering challenges. The miniaturization of detection systems often requires specialized microfabrication techniques that are inherently expensive.
Scaling production from laboratory prototypes to commercial manufacturing introduces further limitations. Many promising fabrication approaches demonstrated in research settings fail to translate effectively to high-throughput production environments. Techniques like 3D printing offer potential solutions but currently lack the resolution and material compatibility required for reliable microfluidic ELISA applications.
Quality control and standardization represent persistent challenges in low-cost fabrication scenarios. The absence of established quality metrics and testing protocols specifically designed for disposable microfluidic ELISA chips complicates the validation process and regulatory approval pathway, creating additional barriers to commercialization and widespread implementation.
Current Low-Cost Fabrication Methodologies
01 Cost-effective manufacturing methods for microfluidic ELISA chips
Various manufacturing techniques have been developed to reduce the cost of microfluidic ELISA chips. These include using inexpensive materials like polymers instead of glass or silicon, implementing mass production methods such as injection molding, and designing simplified fabrication processes that require fewer steps. These approaches significantly lower the production costs while maintaining the functionality and reliability of the chips for diagnostic applications.- Cost-effective manufacturing methods for microfluidic ELISA chips: Various manufacturing techniques have been developed to reduce the cost of microfluidic ELISA chips. These include using inexpensive materials like polymers instead of glass or silicon, implementing mass production methods such as injection molding or hot embossing, and designing simpler chip architectures that require fewer fabrication steps. These approaches significantly lower the production costs while maintaining the analytical performance of the chips.
- Integration of low-cost detection systems: Microfluidic ELISA chips can be made more affordable by integrating cost-effective detection systems. These include smartphone-based optical detection, simplified electrochemical sensors, and paper-based colorimetric detection methods. By eliminating the need for expensive laboratory equipment, these integrated detection systems make microfluidic ELISA technology more accessible for point-of-care applications and resource-limited settings.
- Reusable microfluidic ELISA platforms: Developing reusable microfluidic ELISA platforms can significantly reduce the per-test cost. These platforms feature washable channels, replaceable reaction chambers, or modular designs where only certain components need replacement between tests. Some designs incorporate regenerable sensing surfaces that can be used for multiple assays, thereby distributing the initial manufacturing cost across numerous tests.
- Miniaturization and reagent reduction strategies: Miniaturization of microfluidic ELISA chips leads to cost savings through reduced reagent consumption. Advanced chip designs incorporate microchannel geometries that optimize fluid flow and mixing, allowing for efficient reactions with minimal sample and reagent volumes. Some chips feature integrated reagent storage or automated dispensing systems that precisely control reagent usage, further reducing operational costs.
- Mass production and commercial scaling approaches: Strategies for scaling up production of microfluidic ELISA chips focus on reducing unit costs through economies of scale. These include standardized manufacturing protocols, automated assembly lines, and partnerships with established manufacturing facilities. Some approaches involve adapting existing industrial processes from other sectors to microfluidic chip production, enabling higher throughput and lower costs per chip.
02 Integration of low-cost detection systems
Integrating cost-effective detection systems with microfluidic ELISA chips helps reduce the overall expense of the testing platform. These systems include smartphone-based optical detection, simplified electrochemical sensors, and miniaturized spectrophotometric components. By eliminating the need for expensive laboratory equipment, these integrated detection approaches make microfluidic ELISA more accessible for point-of-care applications and resource-limited settings.Expand Specific Solutions03 Reusable microfluidic ELISA chip designs
Developing reusable microfluidic ELISA chips significantly reduces the per-test cost. These designs incorporate durable materials, cleanable surfaces, and replaceable components that allow multiple uses without compromising test accuracy. Some approaches include chips with removable reaction chambers, regenerable sensing surfaces, or modular designs where only certain components need replacement between tests, extending the lifespan of the more expensive components.Expand Specific Solutions04 Reagent reduction strategies
Microfluidic ELISA chips employ various strategies to minimize reagent consumption, which is a major contributor to test costs. These include precise microfluidic channel designs that require smaller sample volumes, reagent recycling systems, multiplexed assay formats that test multiple analytes simultaneously, and concentrated reagent formulations. By reducing the amount of expensive antibodies and other biochemicals needed per test, these approaches significantly lower the operational costs of microfluidic ELISA.Expand Specific Solutions05 Automated production and operation systems
Automation in both the production and operation of microfluidic ELISA chips helps reduce labor costs and improve efficiency. Automated manufacturing systems enable high-throughput production with consistent quality, while automated sample processing and analysis minimize the need for skilled technicians. These systems include robotic assembly lines, digital microfluidic control mechanisms, and software-driven analysis tools that streamline the entire testing process from chip fabrication to result interpretation.Expand Specific Solutions
Key Industry Players and Research Institutions
The microfluidic ELISA chip fabrication market is currently in a growth phase, with increasing demand for low-cost disposable diagnostic solutions. The market size is expanding rapidly due to rising applications in point-of-care testing and personalized medicine, estimated to reach several billion dollars by 2025. Technologically, the field shows varying maturity levels across different players. Research institutions like Duke University, California Institute of Technology, and Boston University are pioneering fundamental innovations, while companies like IBM, Caliper Life Sciences, and Tecan Trading AG are developing commercial applications. Industrial players such as ITRI and Fraunhofer USA are bridging the gap between academic research and industrial implementation, focusing on scalable manufacturing processes. The competitive landscape features a mix of academic institutions, established technology corporations, and specialized biotech companies collaborating to overcome fabrication challenges and reduce production costs.
Caliper Life Sciences, Inc.
Technical Solution: Caliper Life Sciences has developed a comprehensive microfluidic platform for disposable ELISA chips utilizing their LabChip technology. Their approach incorporates polymer-based microfluidic channels with integrated detection systems for point-of-care diagnostics. The fabrication process employs hot embossing and injection molding techniques to create low-cost, mass-producible PDMS (polydimethylsiloxane) and COC (cyclic olefin copolymer) chips. Their proprietary surface modification methods enable protein immobilization without compromising bioactivity. The system integrates capillary-driven flow mechanisms, eliminating the need for external pumps, and incorporates colorimetric detection compatible with smartphone cameras for result analysis. This technology reduces sample volume requirements to nanoliter scales while maintaining sensitivity comparable to traditional ELISA methods.
Strengths: Established manufacturing infrastructure for mass production; proprietary surface chemistry expertise; integrated detection systems. Weaknesses: Relatively higher cost compared to paper-based alternatives; requires specialized equipment for fabrication; limited multiplexing capabilities compared to newer technologies.
William Marsh Rice University
Technical Solution: Rice University has pioneered a revolutionary approach to low-cost microfluidic ELISA chip fabrication using 3D printing combined with paper microfluidics. Their technology employs direct-ink writing of hydrophobic barriers on cellulose substrates, followed by selective deposition of functional biomaterials using inkjet printing. This process creates precisely defined reaction zones with controlled flow paths without requiring clean room facilities. The fabrication incorporates a proprietary nanocellulose composite that enhances protein binding while maintaining capillary flow properties. Rice's chips feature vertical flow architecture with sequential reagent delivery zones that automatically time the ELISA reaction steps without user intervention. The system includes smartphone-compatible colorimetric detection enhanced by plasmonic nanoparticles that amplify signal intensity. Their manufacturing approach requires minimal capital equipment investment (under $10,000 for complete setup) and achieves per-chip costs below $0.25 at production scales. Field testing has demonstrated performance comparable to conventional ELISA for detecting infectious disease biomarkers with detection limits in the ng/mL range.
Strengths: Minimal infrastructure requirements for manufacturing; highly adaptable design process; environmentally sustainable materials. Weaknesses: Lower throughput production compared to industrial methods; more limited channel geometry options; potential for material variability affecting performance.
Critical Patents and Innovations in Material Science
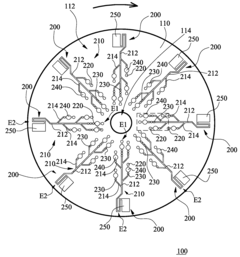
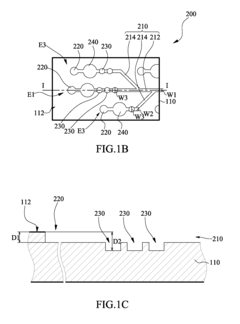
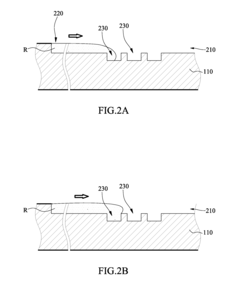
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Pathway for Disposable Diagnostic Devices
The regulatory landscape for disposable microfluidic ELISA diagnostic devices presents a complex pathway that manufacturers must navigate to bring products to market. In the United States, the FDA classifies these devices primarily under Class II medical devices, requiring a 510(k) premarket notification unless they qualify for specific exemptions. For novel technologies with no predicate devices, a De Novo classification request may be necessary, which involves more extensive clinical validation.
European market access requires CE marking under the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDR directive in May 2022. This transition significantly increased requirements for clinical evidence and post-market surveillance, particularly affecting low-cost disposable diagnostic platforms. Manufacturers must now implement more robust quality management systems and risk classification procedures.
The regulatory pathway typically begins with analytical validation, demonstrating the device's precision, accuracy, and limit of detection. For microfluidic ELISA chips, this includes validation of flow characteristics, reagent stability, and cross-reactivity assessments. Clinical validation follows, requiring studies that compare performance against reference methods, often necessitating multi-center trials for broader market approval.
Quality system requirements present another critical regulatory component. In the US, manufacturers must comply with 21 CFR Part 820 (Quality System Regulation), while ISO 13485 certification is essential for global markets. For low-cost disposable devices, balancing quality assurance with cost constraints requires strategic approaches to validation and verification protocols.
Environmental considerations have emerged as increasingly important regulatory factors. Disposable diagnostic devices face growing scrutiny regarding material selection, waste management, and end-of-life considerations. Several jurisdictions now require environmental impact assessments and waste management plans as part of the approval process.
Regulatory pathways for emerging markets present additional considerations. Countries like China, India, and Brazil have established their own regulatory frameworks, often requiring country-specific testing and documentation. The WHO Prequalification program offers an alternative pathway for diagnostic devices intended for use in resource-limited settings, potentially accelerating access to these markets.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline global approvals, though significant regional differences persist. Manufacturers of low-cost microfluidic ELISA chips must develop comprehensive regulatory strategies that account for these variations while maintaining cost-effectiveness throughout the product lifecycle.
European market access requires CE marking under the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDR directive in May 2022. This transition significantly increased requirements for clinical evidence and post-market surveillance, particularly affecting low-cost disposable diagnostic platforms. Manufacturers must now implement more robust quality management systems and risk classification procedures.
The regulatory pathway typically begins with analytical validation, demonstrating the device's precision, accuracy, and limit of detection. For microfluidic ELISA chips, this includes validation of flow characteristics, reagent stability, and cross-reactivity assessments. Clinical validation follows, requiring studies that compare performance against reference methods, often necessitating multi-center trials for broader market approval.
Quality system requirements present another critical regulatory component. In the US, manufacturers must comply with 21 CFR Part 820 (Quality System Regulation), while ISO 13485 certification is essential for global markets. For low-cost disposable devices, balancing quality assurance with cost constraints requires strategic approaches to validation and verification protocols.
Environmental considerations have emerged as increasingly important regulatory factors. Disposable diagnostic devices face growing scrutiny regarding material selection, waste management, and end-of-life considerations. Several jurisdictions now require environmental impact assessments and waste management plans as part of the approval process.
Regulatory pathways for emerging markets present additional considerations. Countries like China, India, and Brazil have established their own regulatory frameworks, often requiring country-specific testing and documentation. The WHO Prequalification program offers an alternative pathway for diagnostic devices intended for use in resource-limited settings, potentially accelerating access to these markets.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to streamline global approvals, though significant regional differences persist. Manufacturers of low-cost microfluidic ELISA chips must develop comprehensive regulatory strategies that account for these variations while maintaining cost-effectiveness throughout the product lifecycle.
Sustainability and Environmental Impact Assessment
The environmental impact of disposable microfluidic ELISA chips represents a critical consideration in their development and deployment. These devices, while offering significant advantages in point-of-care diagnostics, generate substantial waste streams that require careful assessment. Current fabrication methods predominantly utilize polymers such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and polycarbonate, which present end-of-life disposal challenges due to their non-biodegradable nature and potential for microplastic generation.
Life cycle assessment (LCA) studies indicate that the environmental footprint of these chips extends beyond disposal concerns to include resource extraction, manufacturing energy requirements, and transportation impacts. Comparative analyses reveal that traditional laboratory-based ELISA testing consumes approximately 5-10 times more water and generates significantly higher carbon emissions per test than microfluidic alternatives. However, the single-use nature of disposable chips creates a volume challenge that offsets some of these efficiency gains.
Recent innovations in sustainable materials show promising directions for environmental impact reduction. Paper-based microfluidic platforms utilizing cellulose derivatives demonstrate enhanced biodegradability while maintaining diagnostic performance. Similarly, biopolymer alternatives such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs) offer improved end-of-life characteristics with decomposition rates 50-200 times faster than conventional petroleum-based polymers under optimal conditions.
Waste management strategies for these devices must address both biological hazard containment and material recovery. Current protocols typically recommend incineration, which effectively neutralizes biohazards but contributes to atmospheric emissions. Advanced waste treatment technologies including chemical decontamination followed by material separation show potential for recovering up to 60% of chip components for recycling or repurposing.
Regulatory frameworks increasingly incorporate environmental considerations into medical device approval processes. The European Union's Medical Device Regulation now includes sustainability criteria, while the FDA has initiated programs encouraging "green chemistry" approaches in medical diagnostics. These regulatory shifts are driving manufacturers toward eco-design principles that consider environmental impacts throughout the product lifecycle.
Economic analyses demonstrate that environmentally optimized designs need not increase production costs significantly. Studies indicate that material substitution with biodegradable alternatives typically adds 5-15% to manufacturing costs, which can be offset through optimized production processes and economies of scale. Furthermore, emerging circular economy models for healthcare products suggest potential for closed-loop systems where device components are recovered and reintegrated into production streams.
Life cycle assessment (LCA) studies indicate that the environmental footprint of these chips extends beyond disposal concerns to include resource extraction, manufacturing energy requirements, and transportation impacts. Comparative analyses reveal that traditional laboratory-based ELISA testing consumes approximately 5-10 times more water and generates significantly higher carbon emissions per test than microfluidic alternatives. However, the single-use nature of disposable chips creates a volume challenge that offsets some of these efficiency gains.
Recent innovations in sustainable materials show promising directions for environmental impact reduction. Paper-based microfluidic platforms utilizing cellulose derivatives demonstrate enhanced biodegradability while maintaining diagnostic performance. Similarly, biopolymer alternatives such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs) offer improved end-of-life characteristics with decomposition rates 50-200 times faster than conventional petroleum-based polymers under optimal conditions.
Waste management strategies for these devices must address both biological hazard containment and material recovery. Current protocols typically recommend incineration, which effectively neutralizes biohazards but contributes to atmospheric emissions. Advanced waste treatment technologies including chemical decontamination followed by material separation show potential for recovering up to 60% of chip components for recycling or repurposing.
Regulatory frameworks increasingly incorporate environmental considerations into medical device approval processes. The European Union's Medical Device Regulation now includes sustainability criteria, while the FDA has initiated programs encouraging "green chemistry" approaches in medical diagnostics. These regulatory shifts are driving manufacturers toward eco-design principles that consider environmental impacts throughout the product lifecycle.
Economic analyses demonstrate that environmentally optimized designs need not increase production costs significantly. Studies indicate that material substitution with biodegradable alternatives typically adds 5-15% to manufacturing costs, which can be offset through optimized production processes and economies of scale. Furthermore, emerging circular economy models for healthcare products suggest potential for closed-loop systems where device components are recovered and reintegrated into production streams.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!