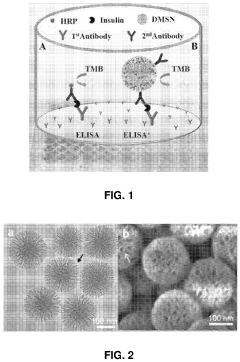
Microfluidic ELISA for High-Sensitivity Protein Profiling
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Microfluidic ELISA technology represents a significant advancement in the field of immunoassay techniques, evolving from traditional Enzyme-Linked Immunosorbent Assay (ELISA) methods that have been fundamental in clinical diagnostics since the 1970s. The integration of microfluidics with ELISA has emerged as a response to increasing demands for higher sensitivity, reduced sample volumes, faster analysis times, and point-of-care testing capabilities in protein detection and quantification.
The historical trajectory of this technology began with conventional ELISA platforms, which while effective, faced limitations in sensitivity, throughput, and resource requirements. The advent of microfluidic technologies in the late 1990s and early 2000s provided a pathway to miniaturize these assays, leading to the development of lab-on-a-chip devices capable of performing complex immunoassays with minimal reagent consumption.
Recent technological advancements have focused on enhancing detection limits, multiplexing capabilities, and automation of microfluidic ELISA systems. The incorporation of novel detection methods such as fluorescence, chemiluminescence, and electrochemical detection has pushed sensitivity boundaries to femtomolar and even attomolar levels, enabling the detection of low-abundance biomarkers critical for early disease diagnosis.
The primary objective of microfluidic ELISA development is to achieve ultra-high sensitivity protein profiling while maintaining assay specificity and reproducibility. This involves optimizing microfluidic channel designs, surface functionalization strategies, and detection methodologies to maximize signal-to-noise ratios and minimize non-specific binding events.
Another key goal is the development of integrated, automated systems that reduce operator dependency and enable standardized results across different laboratory settings. This includes the creation of sample-to-answer platforms that incorporate all necessary steps from sample preparation to result interpretation within a single device.
Portability and point-of-care applicability represent additional objectives, with efforts directed toward creating battery-operated, smartphone-compatible devices that can deliver laboratory-quality results in resource-limited settings. This democratization of advanced diagnostic capabilities has significant implications for global health initiatives and personalized medicine approaches.
The technology trend is moving toward multiplexed assays capable of simultaneously detecting multiple protein biomarkers from a single sample, providing comprehensive protein profiles that offer greater diagnostic and prognostic value than single-marker tests. This trend aligns with the broader movement toward precision medicine, where detailed molecular characterization guides therapeutic decision-making.
Looking forward, the field is expected to continue evolving toward greater integration with other analytical techniques, including mass spectrometry, genomic analysis, and artificial intelligence-driven data interpretation, creating powerful multi-modal diagnostic platforms that can address complex clinical questions with unprecedented accuracy and efficiency.
The historical trajectory of this technology began with conventional ELISA platforms, which while effective, faced limitations in sensitivity, throughput, and resource requirements. The advent of microfluidic technologies in the late 1990s and early 2000s provided a pathway to miniaturize these assays, leading to the development of lab-on-a-chip devices capable of performing complex immunoassays with minimal reagent consumption.
Recent technological advancements have focused on enhancing detection limits, multiplexing capabilities, and automation of microfluidic ELISA systems. The incorporation of novel detection methods such as fluorescence, chemiluminescence, and electrochemical detection has pushed sensitivity boundaries to femtomolar and even attomolar levels, enabling the detection of low-abundance biomarkers critical for early disease diagnosis.
The primary objective of microfluidic ELISA development is to achieve ultra-high sensitivity protein profiling while maintaining assay specificity and reproducibility. This involves optimizing microfluidic channel designs, surface functionalization strategies, and detection methodologies to maximize signal-to-noise ratios and minimize non-specific binding events.
Another key goal is the development of integrated, automated systems that reduce operator dependency and enable standardized results across different laboratory settings. This includes the creation of sample-to-answer platforms that incorporate all necessary steps from sample preparation to result interpretation within a single device.
Portability and point-of-care applicability represent additional objectives, with efforts directed toward creating battery-operated, smartphone-compatible devices that can deliver laboratory-quality results in resource-limited settings. This democratization of advanced diagnostic capabilities has significant implications for global health initiatives and personalized medicine approaches.
The technology trend is moving toward multiplexed assays capable of simultaneously detecting multiple protein biomarkers from a single sample, providing comprehensive protein profiles that offer greater diagnostic and prognostic value than single-marker tests. This trend aligns with the broader movement toward precision medicine, where detailed molecular characterization guides therapeutic decision-making.
Looking forward, the field is expected to continue evolving toward greater integration with other analytical techniques, including mass spectrometry, genomic analysis, and artificial intelligence-driven data interpretation, creating powerful multi-modal diagnostic platforms that can address complex clinical questions with unprecedented accuracy and efficiency.
Market Demand Analysis for High-Sensitivity Protein Detection
The global market for high-sensitivity protein detection technologies has experienced substantial growth in recent years, driven primarily by increasing demand in clinical diagnostics, pharmaceutical research, and academic studies. The protein detection market was valued at approximately $28.4 billion in 2022 and is projected to reach $44.9 billion by 2028, growing at a CAGR of 7.9% during the forecast period.
Microfluidic ELISA technology addresses critical market needs across multiple sectors. In clinical diagnostics, there is growing demand for early disease detection capabilities, particularly for conditions where protein biomarkers appear at ultra-low concentrations during initial disease stages. Cancer diagnostics represents a particularly significant segment, with the cancer biomarker market alone expected to reach $33.7 billion by 2025, growing at 13.8% annually.
The pharmaceutical industry constitutes another major market driver, with increasing requirements for high-sensitivity protein analysis during drug development and validation processes. The drug discovery market, valued at $71.5 billion in 2021, heavily relies on advanced protein detection methods for target identification and validation, creating substantial demand for microfluidic ELISA platforms.
Point-of-care testing represents a rapidly expanding application area, with the global POC diagnostics market projected to reach $50.6 billion by 2025. Microfluidic ELISA's potential to deliver laboratory-quality results in decentralized settings aligns perfectly with this market trend, particularly in resource-limited environments where conventional laboratory infrastructure is unavailable.
Precision medicine initiatives worldwide are further accelerating demand for high-sensitivity protein profiling technologies. The global precision medicine market, valued at $66.1 billion in 2021, is expected to grow at 11.5% annually through 2030, creating sustained demand for advanced protein detection platforms capable of identifying individual patient biomarker profiles.
Emerging economies present significant growth opportunities, with healthcare infrastructure development and increasing research activities driving adoption of advanced diagnostic technologies. China's IVD market alone is growing at approximately 15% annually, significantly outpacing global averages.
Key market challenges include cost considerations, with many high-sensitivity detection platforms remaining prohibitively expensive for widespread adoption, particularly in resource-limited settings. Additionally, regulatory hurdles for novel diagnostic technologies can delay market entry, with approval processes often taking 3-5 years in major markets.
Consumer trends indicate growing preference for non-invasive testing methods and home-based monitoring solutions, creating opportunities for microfluidic ELISA technologies that can operate with minimal sample volumes from less invasive collection methods.
Microfluidic ELISA technology addresses critical market needs across multiple sectors. In clinical diagnostics, there is growing demand for early disease detection capabilities, particularly for conditions where protein biomarkers appear at ultra-low concentrations during initial disease stages. Cancer diagnostics represents a particularly significant segment, with the cancer biomarker market alone expected to reach $33.7 billion by 2025, growing at 13.8% annually.
The pharmaceutical industry constitutes another major market driver, with increasing requirements for high-sensitivity protein analysis during drug development and validation processes. The drug discovery market, valued at $71.5 billion in 2021, heavily relies on advanced protein detection methods for target identification and validation, creating substantial demand for microfluidic ELISA platforms.
Point-of-care testing represents a rapidly expanding application area, with the global POC diagnostics market projected to reach $50.6 billion by 2025. Microfluidic ELISA's potential to deliver laboratory-quality results in decentralized settings aligns perfectly with this market trend, particularly in resource-limited environments where conventional laboratory infrastructure is unavailable.
Precision medicine initiatives worldwide are further accelerating demand for high-sensitivity protein profiling technologies. The global precision medicine market, valued at $66.1 billion in 2021, is expected to grow at 11.5% annually through 2030, creating sustained demand for advanced protein detection platforms capable of identifying individual patient biomarker profiles.
Emerging economies present significant growth opportunities, with healthcare infrastructure development and increasing research activities driving adoption of advanced diagnostic technologies. China's IVD market alone is growing at approximately 15% annually, significantly outpacing global averages.
Key market challenges include cost considerations, with many high-sensitivity detection platforms remaining prohibitively expensive for widespread adoption, particularly in resource-limited settings. Additionally, regulatory hurdles for novel diagnostic technologies can delay market entry, with approval processes often taking 3-5 years in major markets.
Consumer trends indicate growing preference for non-invasive testing methods and home-based monitoring solutions, creating opportunities for microfluidic ELISA technologies that can operate with minimal sample volumes from less invasive collection methods.
Current Challenges in Microfluidic ELISA Implementation
Despite the significant advancements in microfluidic ELISA technology for high-sensitivity protein profiling, several critical challenges continue to impede its widespread implementation and commercialization. These challenges span multiple domains including technical limitations, standardization issues, and practical deployment barriers.
Sample preparation remains a fundamental challenge in microfluidic ELISA systems. The miniaturized nature of these platforms requires highly purified samples with minimal matrix effects, as contaminants can significantly impact assay performance at microscale volumes. Current sample preparation protocols often require complex pre-processing steps that are difficult to integrate into automated microfluidic workflows, creating a bottleneck in the analytical process.
Surface chemistry optimization presents another significant hurdle. The high surface-to-volume ratio in microfluidic channels magnifies the importance of surface properties. Non-specific protein adsorption frequently leads to background noise and reduced sensitivity. While various surface modification strategies exist, achieving consistent, stable, and biocompatible surfaces across different microfluidic materials remains problematic, particularly for long-term storage and operation.
Flow control precision represents a persistent technical challenge. Maintaining consistent and precise fluid handling at nanoliter to picoliter volumes requires sophisticated pumping and valving systems. Pressure fluctuations, bubble formation, and channel clogging can dramatically affect assay reproducibility. Current solutions often involve complex external equipment that contradicts the goal of creating portable, point-of-care devices.
Detection sensitivity limitations continue to constrain the full potential of microfluidic ELISA. While miniaturization theoretically improves detection limits through reduced diffusion distances and reaction volumes, practical implementation often suffers from reduced optical path lengths and increased background interference. The integration of sensitive detection methods without compromising the simplicity and portability of microfluidic platforms remains challenging.
Manufacturing scalability presents significant commercialization barriers. Many microfluidic ELISA platforms rely on fabrication techniques that are suitable for laboratory prototyping but challenging to scale for mass production. Techniques like soft lithography using PDMS offer excellent prototyping capabilities but present challenges in terms of batch-to-batch reproducibility and industrial-scale manufacturing.
Standardization across the field is notably lacking. The diversity of microfluidic designs, materials, and detection methods has resulted in fragmented approaches with limited cross-platform compatibility. This absence of standardized protocols and reference materials complicates result comparison between different microfluidic ELISA platforms and traditional plate-based methods, hindering clinical validation and regulatory approval processes.
Sample preparation remains a fundamental challenge in microfluidic ELISA systems. The miniaturized nature of these platforms requires highly purified samples with minimal matrix effects, as contaminants can significantly impact assay performance at microscale volumes. Current sample preparation protocols often require complex pre-processing steps that are difficult to integrate into automated microfluidic workflows, creating a bottleneck in the analytical process.
Surface chemistry optimization presents another significant hurdle. The high surface-to-volume ratio in microfluidic channels magnifies the importance of surface properties. Non-specific protein adsorption frequently leads to background noise and reduced sensitivity. While various surface modification strategies exist, achieving consistent, stable, and biocompatible surfaces across different microfluidic materials remains problematic, particularly for long-term storage and operation.
Flow control precision represents a persistent technical challenge. Maintaining consistent and precise fluid handling at nanoliter to picoliter volumes requires sophisticated pumping and valving systems. Pressure fluctuations, bubble formation, and channel clogging can dramatically affect assay reproducibility. Current solutions often involve complex external equipment that contradicts the goal of creating portable, point-of-care devices.
Detection sensitivity limitations continue to constrain the full potential of microfluidic ELISA. While miniaturization theoretically improves detection limits through reduced diffusion distances and reaction volumes, practical implementation often suffers from reduced optical path lengths and increased background interference. The integration of sensitive detection methods without compromising the simplicity and portability of microfluidic platforms remains challenging.
Manufacturing scalability presents significant commercialization barriers. Many microfluidic ELISA platforms rely on fabrication techniques that are suitable for laboratory prototyping but challenging to scale for mass production. Techniques like soft lithography using PDMS offer excellent prototyping capabilities but present challenges in terms of batch-to-batch reproducibility and industrial-scale manufacturing.
Standardization across the field is notably lacking. The diversity of microfluidic designs, materials, and detection methods has resulted in fragmented approaches with limited cross-platform compatibility. This absence of standardized protocols and reference materials complicates result comparison between different microfluidic ELISA platforms and traditional plate-based methods, hindering clinical validation and regulatory approval processes.
Current Technical Solutions for Microfluidic Protein Profiling
01 Microfluidic chip designs for enhanced ELISA sensitivity
Various microfluidic chip designs can significantly improve ELISA sensitivity. These include specialized channel geometries, integrated detection chambers, and optimized flow patterns that enhance antigen-antibody interactions. The miniaturized reaction environment reduces diffusion distances and increases the surface-to-volume ratio, leading to faster reaction kinetics and improved detection limits. Some designs incorporate multiple reaction chambers for parallel processing or sequential reaction steps, further enhancing sensitivity.- Microfluidic chip designs for enhanced ELISA sensitivity: Various microfluidic chip designs can significantly improve ELISA sensitivity. These designs include specialized channel geometries, integrated reaction chambers, and optimized flow patterns that enhance antigen-antibody interactions. By controlling fluid dynamics at the microscale, these chips enable more efficient binding, reduced sample volumes, and improved signal-to-noise ratios, ultimately leading to lower detection limits and higher sensitivity compared to conventional ELISA methods.
- Signal amplification strategies in microfluidic ELISA: Signal amplification techniques are crucial for improving microfluidic ELISA sensitivity. These include enzymatic amplification cascades, nanoparticle-based enhancement, and novel reporter systems that generate stronger detectable signals. By amplifying the detection signal while maintaining low background noise, these strategies can achieve detection limits in the femtomolar or even attomolar range, representing orders of magnitude improvement over traditional ELISA methods.
- Integration of nanomaterials for sensitivity enhancement: Incorporating nanomaterials into microfluidic ELISA platforms significantly enhances detection sensitivity. Materials such as quantum dots, gold nanoparticles, carbon nanotubes, and magnetic nanoparticles can serve as signal enhancers, capture substrates, or detection elements. These nanomaterials provide larger surface areas for biomolecule immobilization, enhanced optical properties for detection, and improved capture efficiency, collectively leading to substantial improvements in assay sensitivity.
- Automated sample processing and detection systems: Automated microfluidic ELISA systems integrate sample preparation, reagent handling, incubation, washing, and detection steps into a single platform. These systems minimize human error, reduce contamination risks, and ensure consistent assay conditions. Advanced automation features include precise flow control, temperature regulation, and integrated optical or electrochemical detection systems, all contributing to improved reproducibility and enhanced sensitivity for detecting low-abundance biomarkers.
- Novel detection methods for ultrasensitive microfluidic ELISA: Innovative detection methods have revolutionized microfluidic ELISA sensitivity. These include digital ELISA approaches that count individual enzyme-labeled molecules, electrochemical detection systems that measure electrical signals from enzymatic reactions, and advanced optical techniques like surface plasmon resonance or fluorescence enhancement. These novel detection strategies, when integrated with microfluidic platforms, can achieve unprecedented sensitivity levels for protein biomarker detection, enabling early disease diagnosis and monitoring.
02 Surface modification techniques for improved biomolecule capture
Surface modification of microfluidic channels can significantly enhance ELISA sensitivity by improving biomolecule capture efficiency. Techniques include chemical functionalization with reactive groups, coating with capture proteins or antibodies, and creating nanostructured surfaces that increase the effective binding area. These modifications reduce non-specific binding while maximizing specific interactions, leading to lower background signals and improved detection limits. Some approaches use polymer brushes or hydrogels to create three-dimensional capture environments within the microchannels.Expand Specific Solutions03 Signal amplification methods for ultrasensitive detection
Various signal amplification strategies can be integrated into microfluidic ELISA platforms to achieve ultrasensitive detection. These include enzymatic amplification cascades, nanoparticle-based signal enhancement, and digital ELISA approaches that enable single-molecule detection. Other methods incorporate quantum dots, fluorescent polymers, or chemiluminescent reagents that produce stronger signals than traditional colorimetric detection. These amplification techniques can improve detection limits by several orders of magnitude compared to conventional ELISA methods.Expand Specific Solutions04 Integration of nanomaterials for sensitivity enhancement
Nanomaterials can be incorporated into microfluidic ELISA systems to significantly enhance detection sensitivity. Materials such as gold nanoparticles, magnetic nanoparticles, carbon nanotubes, and quantum dots provide increased surface area for biomolecule immobilization and can generate stronger optical or electrochemical signals. Some nanomaterials exhibit unique properties like surface plasmon resonance or superparamagnetism that can be exploited for improved detection. These materials can be used as labels, signal amplifiers, or surface modifiers within the microfluidic channels.Expand Specific Solutions05 Sample preparation and concentration techniques
Advanced sample preparation and concentration techniques integrated into microfluidic platforms can significantly improve ELISA sensitivity. These include on-chip filtration, dielectrophoretic concentration, isotachophoresis, and immunomagnetic separation to isolate and concentrate target analytes from complex biological samples. By increasing the effective concentration of target molecules before detection, these techniques can lower detection limits by several orders of magnitude. Some systems incorporate multiple preparation steps in a single integrated device, minimizing sample loss and contamination risks.Expand Specific Solutions
Key Industry Players in Microfluidic ELISA Development
Microfluidic ELISA for high-sensitivity protein profiling is emerging as a transformative technology in the early-stage growth phase of the precision diagnostics market. The global market is expanding rapidly, projected to reach significant value as healthcare systems increasingly adopt personalized medicine approaches. Technologically, the field shows varying maturity levels across players: academic institutions (Johns Hopkins University, MIT, Harvard) lead fundamental research while commercial entities demonstrate different development stages. Companies like Exosome Diagnostics and Samsung Electronics are advancing toward commercialization, with Enzo Biochem and Beijing Wantai offering market-ready solutions. Research institutions in Asia (Fudan University, Sun Yat-Sen University) are accelerating innovation, while European players (Institut Curie, EPFL) contribute specialized expertise. This competitive landscape reflects a technology transitioning from research to clinical application.
The Johns Hopkins University
Technical Solution: Johns Hopkins has developed a sophisticated microfluidic ELISA platform that combines digital detection principles with advanced microfluidic engineering for ultrasensitive protein analysis. Their system utilizes a network of thousands of nanoliter-sized reaction chambers that enable digital quantification of enzyme-labeled immunocomplexes, dramatically improving detection sensitivity. The platform incorporates proprietary surface chemistry modifications that minimize non-specific binding while maximizing capture antibody density and orientation. Johns Hopkins researchers have implemented magnetic bead-based capture and separation techniques within their microfluidic channels, enabling efficient target enrichment from complex biological matrices. The system features integrated sample preparation modules including automated dilution, mixing, and incubation steps, significantly reducing hands-on time and improving reproducibility[9][10]. Their platform also incorporates novel enzymatic amplification strategies that generate fluorescent, chemiluminescent, or electrochemical signals, providing flexibility for different detection modalities. The technology has demonstrated detection limits in the femtomolar to attomolar range for various cancer biomarkers, inflammatory mediators, and infectious disease markers.
Strengths: Exceptional sensitivity through digital detection principles; versatile detection modalities (fluorescent, chemiluminescent, electrochemical); efficient target enrichment from complex samples; high reproducibility through automation. Weaknesses: Complex microfluidic architecture increasing manufacturing challenges; higher cost compared to conventional ELISA; specialized training required for operation; potential for false positives in digital detection if contamination occurs.
The Regents of the University of California
Technical Solution: UC system researchers have developed a comprehensive microfluidic ELISA platform that integrates nanomaterial-enhanced detection with advanced microfluidic architectures. Their technology utilizes a multilayer polydimethylsiloxane (PDMS) design with pneumatically actuated valves and pumps to enable precise fluid handling and reagent delivery. The platform incorporates quantum dot-based signal amplification strategies that provide superior sensitivity and photostability compared to traditional enzymatic reporters. UC researchers have implemented innovative surface patterning techniques to create high-density antibody arrays within microfluidic channels, enabling multiplexed detection of up to 20 different protein targets simultaneously. The system features integrated sample preparation modules including on-chip blood filtration, cell lysis, and protein extraction capabilities, allowing direct processing of complex biological samples[6][8]. Their platform also incorporates machine learning algorithms for automated image analysis and quantification, reducing operator-dependent variability and improving reproducibility across different testing sites. Detection limits in the sub-picomolar range have been consistently achieved for various clinically relevant biomarkers.
Strengths: Comprehensive sample-to-answer capability; high-level multiplexing for comprehensive protein profiling; robust performance with complex biological samples; automated analysis reducing operator variability. Weaknesses: Complex multilayer fabrication increasing manufacturing costs; reliance on external pneumatic systems for valve actuation; quantum dot reagents more expensive than traditional enzymatic reporters; potential for cross-reactivity in highly multiplexed assays.
Critical Patents and Innovations in Microfluidic ELISA
Detecting an analyte
PatentActiveUS11867699B2
Innovation
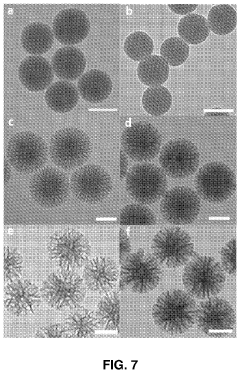
- The use of mesoporous silica nanoparticles with radial pore channels for enhanced enzyme loading and accessibility, and quantum dots immobilized within these nanoparticles to improve signal amplification and light efficiency in detection methods and displays.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Manufacturing Scalability and Cost Considerations
The scalability of microfluidic ELISA manufacturing represents a critical challenge for widespread adoption in clinical and research settings. Current production methods for microfluidic devices often rely on cleanroom-based photolithography and soft lithography techniques, which involve significant capital investment and specialized expertise. These approaches, while precise, create bottlenecks in mass production and contribute to high per-unit costs that limit accessibility for routine diagnostic applications.
Material selection significantly impacts both manufacturing scalability and operational costs. Traditional polydimethylsiloxane (PDMS) offers excellent optical properties and biocompatibility but presents challenges for high-volume production due to its curing time requirements. Alternative thermoplastics like cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) enable injection molding and hot embossing techniques that are more amenable to mass production, though they may require different surface modification strategies to maintain protein binding efficiency.
Automation represents another crucial factor in scaling microfluidic ELISA manufacturing. Current assembly processes often involve manual bonding, reagent loading, and quality control steps that introduce variability and limit throughput. Investments in automated assembly lines, robotic handling systems, and inline quality verification can significantly enhance production capacity but require substantial initial capital expenditure that smaller companies may struggle to secure.
Cost considerations extend beyond manufacturing to include reagent consumption and operational efficiency. Microfluidic platforms typically reduce reagent volumes by orders of magnitude compared to conventional ELISA, potentially offering significant cost savings in high-sensitivity applications. However, this advantage must be balanced against the higher unit cost of microfluidic chips and the need for specialized detection equipment designed for smaller sample volumes.
Economies of scale present both opportunities and challenges for microfluidic ELISA technologies. While increased production volumes would theoretically reduce per-unit costs, the specialized nature of microfluidic manufacturing means that achieving true economies of scale requires substantial market demand. This creates a chicken-and-egg problem where high initial costs limit adoption, which in turn prevents the volume production needed to drive costs down.
Standardization efforts across the industry could significantly impact manufacturing scalability. The development of common design specifications, materials standards, and quality control metrics would enable more contract manufacturers to enter the space, potentially reducing production costs through increased competition. Additionally, modular approaches to microfluidic design could allow for more flexible manufacturing processes that adapt to varying production volumes and application requirements.
Material selection significantly impacts both manufacturing scalability and operational costs. Traditional polydimethylsiloxane (PDMS) offers excellent optical properties and biocompatibility but presents challenges for high-volume production due to its curing time requirements. Alternative thermoplastics like cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) enable injection molding and hot embossing techniques that are more amenable to mass production, though they may require different surface modification strategies to maintain protein binding efficiency.
Automation represents another crucial factor in scaling microfluidic ELISA manufacturing. Current assembly processes often involve manual bonding, reagent loading, and quality control steps that introduce variability and limit throughput. Investments in automated assembly lines, robotic handling systems, and inline quality verification can significantly enhance production capacity but require substantial initial capital expenditure that smaller companies may struggle to secure.
Cost considerations extend beyond manufacturing to include reagent consumption and operational efficiency. Microfluidic platforms typically reduce reagent volumes by orders of magnitude compared to conventional ELISA, potentially offering significant cost savings in high-sensitivity applications. However, this advantage must be balanced against the higher unit cost of microfluidic chips and the need for specialized detection equipment designed for smaller sample volumes.
Economies of scale present both opportunities and challenges for microfluidic ELISA technologies. While increased production volumes would theoretically reduce per-unit costs, the specialized nature of microfluidic manufacturing means that achieving true economies of scale requires substantial market demand. This creates a chicken-and-egg problem where high initial costs limit adoption, which in turn prevents the volume production needed to drive costs down.
Standardization efforts across the industry could significantly impact manufacturing scalability. The development of common design specifications, materials standards, and quality control metrics would enable more contract manufacturers to enter the space, potentially reducing production costs through increased competition. Additionally, modular approaches to microfluidic design could allow for more flexible manufacturing processes that adapt to varying production volumes and application requirements.
Clinical Validation and Regulatory Approval Pathways
The regulatory landscape for microfluidic ELISA technologies presents significant challenges due to their novel integration of microfluidics with traditional immunoassay methods. For clinical implementation, these high-sensitivity protein profiling platforms must navigate a complex approval process that varies by region. In the United States, the FDA typically classifies such diagnostic devices under Class II or Class III, requiring either 510(k) clearance or Premarket Approval (PMA), depending on the intended clinical application and risk profile.
Clinical validation represents the cornerstone of regulatory approval, requiring robust demonstration of analytical performance metrics including sensitivity, specificity, precision, and reproducibility. For microfluidic ELISA systems, establishing limits of detection (LOD) that consistently outperform conventional ELISA methods is particularly critical. Validation studies must include comparison against gold standard methods using clinically relevant samples across diverse patient populations.
The regulatory pathway often necessitates phased clinical trials, beginning with exploratory studies to establish preliminary performance characteristics, followed by larger validation cohorts. Prospective studies demonstrating clinical utility and impact on patient outcomes may be required, particularly for novel biomarkers or diagnostic applications. The complexity increases when the technology is intended for multiplex protein profiling, as each biomarker may require individual validation.
Quality control and manufacturing consistency present unique challenges for microfluidic platforms. Regulatory bodies require comprehensive documentation of manufacturing processes, quality management systems, and risk management protocols. The miniaturized nature of these devices demands exceptional precision in fabrication, with stringent tolerances that must be consistently maintained and documented.
International regulatory harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining approval processes across major markets. However, significant regional variations persist, with the European Union's Medical Device Regulation (MDR) imposing particularly rigorous clinical evidence requirements for novel diagnostic technologies.
Laboratory-developed test (LDT) pathways may offer an initial route to clinical implementation in certain markets, though regulatory agencies worldwide are increasingly scrutinizing this approach. For widespread clinical adoption, manufacturers of microfluidic ELISA platforms must develop comprehensive regulatory strategies that address both immediate approval requirements and anticipate evolving standards for novel diagnostic technologies.
Clinical validation represents the cornerstone of regulatory approval, requiring robust demonstration of analytical performance metrics including sensitivity, specificity, precision, and reproducibility. For microfluidic ELISA systems, establishing limits of detection (LOD) that consistently outperform conventional ELISA methods is particularly critical. Validation studies must include comparison against gold standard methods using clinically relevant samples across diverse patient populations.
The regulatory pathway often necessitates phased clinical trials, beginning with exploratory studies to establish preliminary performance characteristics, followed by larger validation cohorts. Prospective studies demonstrating clinical utility and impact on patient outcomes may be required, particularly for novel biomarkers or diagnostic applications. The complexity increases when the technology is intended for multiplex protein profiling, as each biomarker may require individual validation.
Quality control and manufacturing consistency present unique challenges for microfluidic platforms. Regulatory bodies require comprehensive documentation of manufacturing processes, quality management systems, and risk management protocols. The miniaturized nature of these devices demands exceptional precision in fabrication, with stringent tolerances that must be consistently maintained and documented.
International regulatory harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining approval processes across major markets. However, significant regional variations persist, with the European Union's Medical Device Regulation (MDR) imposing particularly rigorous clinical evidence requirements for novel diagnostic technologies.
Laboratory-developed test (LDT) pathways may offer an initial route to clinical implementation in certain markets, though regulatory agencies worldwide are increasingly scrutinizing this approach. For widespread clinical adoption, manufacturers of microfluidic ELISA platforms must develop comprehensive regulatory strategies that address both immediate approval requirements and anticipate evolving standards for novel diagnostic technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!