Biocompatibility Trends in Vacuum Forming for Medical Devices
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocompatible Materials Evolution and Objectives
The field of biocompatible materials for medical devices has undergone significant evolution over the past few decades, driven by the increasing demand for safer and more effective medical treatments. The primary objective in this domain is to develop materials that can interact harmoniously with the human body, minimizing adverse reactions and promoting healing processes.
In the early stages of medical device development, the focus was primarily on materials that could withstand sterilization processes and maintain structural integrity within the body. However, as our understanding of the body's immune responses and cellular interactions advanced, the emphasis shifted towards creating materials that could actively promote positive biological responses.
The evolution of biocompatible materials has seen a transition from inert substances to bioactive and biomimetic materials. Inert materials, such as certain metals and ceramics, were initially favored for their stability and low reactivity. However, the field has progressed towards materials that can actively engage with the body's tissues, promoting cell adhesion, growth, and even tissue regeneration.
One of the key objectives in the development of biocompatible materials for vacuum-formed medical devices is to enhance the surface properties of the materials. This includes improving wettability, reducing protein adsorption, and minimizing bacterial adhesion. These surface modifications aim to reduce the risk of infection and improve the overall performance of the medical devices.
Another important goal is the development of materials with controlled degradation rates. This is particularly relevant for implantable devices or drug delivery systems, where the material needs to maintain its structural integrity for a specific period before being safely absorbed by the body.
The integration of antimicrobial properties into biocompatible materials has also become a significant objective. This aims to reduce the risk of device-associated infections, a major concern in healthcare settings. Researchers are exploring various approaches, including the incorporation of antimicrobial agents into the material matrix and the development of surface coatings with inherent antimicrobial properties.
As the field continues to advance, there is an increasing focus on personalized medicine. This has led to the development of biocompatible materials that can be tailored to individual patient needs, potentially through 3D printing or other advanced manufacturing techniques. The goal is to create custom-fit devices with optimized biocompatibility for each patient's unique physiology.
Looking ahead, the objectives for biocompatible materials in vacuum-formed medical devices include the development of smart materials that can respond to physiological changes, materials with improved mechanical properties that better mimic natural tissues, and materials that can facilitate non-invasive monitoring of device performance and patient health.
In the early stages of medical device development, the focus was primarily on materials that could withstand sterilization processes and maintain structural integrity within the body. However, as our understanding of the body's immune responses and cellular interactions advanced, the emphasis shifted towards creating materials that could actively promote positive biological responses.
The evolution of biocompatible materials has seen a transition from inert substances to bioactive and biomimetic materials. Inert materials, such as certain metals and ceramics, were initially favored for their stability and low reactivity. However, the field has progressed towards materials that can actively engage with the body's tissues, promoting cell adhesion, growth, and even tissue regeneration.
One of the key objectives in the development of biocompatible materials for vacuum-formed medical devices is to enhance the surface properties of the materials. This includes improving wettability, reducing protein adsorption, and minimizing bacterial adhesion. These surface modifications aim to reduce the risk of infection and improve the overall performance of the medical devices.
Another important goal is the development of materials with controlled degradation rates. This is particularly relevant for implantable devices or drug delivery systems, where the material needs to maintain its structural integrity for a specific period before being safely absorbed by the body.
The integration of antimicrobial properties into biocompatible materials has also become a significant objective. This aims to reduce the risk of device-associated infections, a major concern in healthcare settings. Researchers are exploring various approaches, including the incorporation of antimicrobial agents into the material matrix and the development of surface coatings with inherent antimicrobial properties.
As the field continues to advance, there is an increasing focus on personalized medicine. This has led to the development of biocompatible materials that can be tailored to individual patient needs, potentially through 3D printing or other advanced manufacturing techniques. The goal is to create custom-fit devices with optimized biocompatibility for each patient's unique physiology.
Looking ahead, the objectives for biocompatible materials in vacuum-formed medical devices include the development of smart materials that can respond to physiological changes, materials with improved mechanical properties that better mimic natural tissues, and materials that can facilitate non-invasive monitoring of device performance and patient health.
Market Demand Analysis for Biocompatible Medical Devices
The market demand for biocompatible medical devices formed through vacuum forming processes has been steadily increasing in recent years. This growth is primarily driven by the rising prevalence of chronic diseases, an aging global population, and advancements in medical technology. The healthcare industry's focus on patient safety and comfort has led to a surge in demand for devices that are not only effective but also biocompatible.
Vacuum forming, as a cost-effective and versatile manufacturing process, has gained significant traction in the medical device industry. It allows for the production of complex shapes and designs while maintaining the biocompatibility of the materials used. This has opened up new possibilities for creating patient-specific devices and improving overall treatment outcomes.
The global market for biocompatible medical devices is projected to experience substantial growth over the next decade. Key factors contributing to this expansion include the increasing adoption of minimally invasive surgical procedures, the growing prevalence of cardiovascular and orthopedic disorders, and the rising demand for personalized medicine.
In terms of regional distribution, North America currently holds the largest market share for biocompatible medical devices, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates due to improving healthcare infrastructure and rising disposable incomes.
Specific segments within the biocompatible medical device market that are seeing particularly strong demand include cardiovascular devices, orthopedic implants, dental products, and drug delivery systems. The use of vacuum forming in these areas has enabled manufacturers to create devices that closely mimic natural tissue structures, reducing the risk of rejection and improving patient outcomes.
The trend towards miniaturization in medical devices has also fueled the demand for vacuum-formed biocompatible products. These smaller, more intricate devices require precise manufacturing techniques that vacuum forming can provide while maintaining the necessary biocompatibility standards.
As sustainability becomes an increasingly important consideration in healthcare, there is a growing demand for biocompatible medical devices that are also environmentally friendly. This has led to research into biodegradable materials that can be used in vacuum forming processes, potentially opening up new market opportunities.
The COVID-19 pandemic has further accelerated the demand for certain types of biocompatible medical devices, particularly those used in respiratory support and personal protective equipment. This has highlighted the importance of flexible manufacturing processes like vacuum forming in responding to sudden shifts in market demand.
Vacuum forming, as a cost-effective and versatile manufacturing process, has gained significant traction in the medical device industry. It allows for the production of complex shapes and designs while maintaining the biocompatibility of the materials used. This has opened up new possibilities for creating patient-specific devices and improving overall treatment outcomes.
The global market for biocompatible medical devices is projected to experience substantial growth over the next decade. Key factors contributing to this expansion include the increasing adoption of minimally invasive surgical procedures, the growing prevalence of cardiovascular and orthopedic disorders, and the rising demand for personalized medicine.
In terms of regional distribution, North America currently holds the largest market share for biocompatible medical devices, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates due to improving healthcare infrastructure and rising disposable incomes.
Specific segments within the biocompatible medical device market that are seeing particularly strong demand include cardiovascular devices, orthopedic implants, dental products, and drug delivery systems. The use of vacuum forming in these areas has enabled manufacturers to create devices that closely mimic natural tissue structures, reducing the risk of rejection and improving patient outcomes.
The trend towards miniaturization in medical devices has also fueled the demand for vacuum-formed biocompatible products. These smaller, more intricate devices require precise manufacturing techniques that vacuum forming can provide while maintaining the necessary biocompatibility standards.
As sustainability becomes an increasingly important consideration in healthcare, there is a growing demand for biocompatible medical devices that are also environmentally friendly. This has led to research into biodegradable materials that can be used in vacuum forming processes, potentially opening up new market opportunities.
The COVID-19 pandemic has further accelerated the demand for certain types of biocompatible medical devices, particularly those used in respiratory support and personal protective equipment. This has highlighted the importance of flexible manufacturing processes like vacuum forming in responding to sudden shifts in market demand.
Current Challenges in Vacuum Forming Biocompatibility
Vacuum forming has become a crucial process in the production of medical devices, offering advantages such as cost-effectiveness and versatility. However, ensuring biocompatibility in vacuum-formed medical devices presents several significant challenges that manufacturers and researchers must address.
One of the primary challenges is material selection. While many polymers are suitable for vacuum forming, not all meet the stringent biocompatibility requirements for medical applications. Materials must not only withstand the forming process but also remain inert when in contact with biological tissues and fluids. This necessitates extensive testing and validation of materials, which can be time-consuming and costly.
The vacuum forming process itself can introduce biocompatibility issues. High temperatures used during forming may cause degradation or chemical changes in the material, potentially leading to the release of harmful substances. Controlling these process parameters to maintain material integrity while achieving the desired shape is a delicate balance that requires precise engineering and quality control measures.
Surface properties of vacuum-formed devices pose another challenge. The process can create micro-textures or irregularities on the surface, which may affect cell adhesion or promote bacterial growth. Achieving smooth, uniform surfaces that meet biocompatibility standards often requires additional post-processing steps, adding complexity and cost to the manufacturing process.
Sterilization compatibility is a critical concern for medical devices. Vacuum-formed products must withstand sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving without compromising their biocompatibility or structural integrity. This requirement limits the range of usable materials and necessitates thorough testing of sterilization effects on the final product.
Leaching of additives or residual monomers from the formed material is another significant challenge. Plasticizers, stabilizers, and other additives used to enhance material properties may migrate to the surface over time, potentially causing adverse biological reactions. Manufacturers must carefully select and test additives to ensure long-term biocompatibility.
Regulatory compliance adds another layer of complexity to vacuum forming for medical devices. Meeting standards set by organizations such as the FDA and ISO requires extensive documentation, testing, and validation processes. This regulatory burden can significantly impact development timelines and costs, particularly for novel materials or applications.
Lastly, the challenge of scalability and reproducibility in vacuum forming biocompatible devices cannot be overlooked. Ensuring consistent biocompatibility across large production runs requires robust quality control systems and may necessitate investments in advanced manufacturing technologies and monitoring systems.
One of the primary challenges is material selection. While many polymers are suitable for vacuum forming, not all meet the stringent biocompatibility requirements for medical applications. Materials must not only withstand the forming process but also remain inert when in contact with biological tissues and fluids. This necessitates extensive testing and validation of materials, which can be time-consuming and costly.
The vacuum forming process itself can introduce biocompatibility issues. High temperatures used during forming may cause degradation or chemical changes in the material, potentially leading to the release of harmful substances. Controlling these process parameters to maintain material integrity while achieving the desired shape is a delicate balance that requires precise engineering and quality control measures.
Surface properties of vacuum-formed devices pose another challenge. The process can create micro-textures or irregularities on the surface, which may affect cell adhesion or promote bacterial growth. Achieving smooth, uniform surfaces that meet biocompatibility standards often requires additional post-processing steps, adding complexity and cost to the manufacturing process.
Sterilization compatibility is a critical concern for medical devices. Vacuum-formed products must withstand sterilization methods such as ethylene oxide treatment, gamma irradiation, or autoclaving without compromising their biocompatibility or structural integrity. This requirement limits the range of usable materials and necessitates thorough testing of sterilization effects on the final product.
Leaching of additives or residual monomers from the formed material is another significant challenge. Plasticizers, stabilizers, and other additives used to enhance material properties may migrate to the surface over time, potentially causing adverse biological reactions. Manufacturers must carefully select and test additives to ensure long-term biocompatibility.
Regulatory compliance adds another layer of complexity to vacuum forming for medical devices. Meeting standards set by organizations such as the FDA and ISO requires extensive documentation, testing, and validation processes. This regulatory burden can significantly impact development timelines and costs, particularly for novel materials or applications.
Lastly, the challenge of scalability and reproducibility in vacuum forming biocompatible devices cannot be overlooked. Ensuring consistent biocompatibility across large production runs requires robust quality control systems and may necessitate investments in advanced manufacturing technologies and monitoring systems.
Existing Biocompatible Vacuum Forming Solutions
01 Biocompatible materials for vacuum forming
Various biocompatible materials can be used in vacuum forming processes to create medical devices or implants. These materials are selected for their ability to interact safely with biological systems and maintain their properties under vacuum conditions. Common biocompatible materials include certain polymers, metals, and ceramics that have been extensively tested for their compatibility with living tissues.- Biocompatible materials for vacuum forming: Various biocompatible materials can be used in vacuum forming processes to create medical devices or implants. These materials are selected for their ability to interact safely with biological systems and maintain their properties under vacuum conditions. Common materials include certain polymers, metals, and ceramics that have been proven to be non-toxic and non-immunogenic.
- Surface modification techniques for improved biocompatibility: Surface modification techniques can be applied to vacuum-formed materials to enhance their biocompatibility. These methods may include plasma treatment, chemical etching, or coating with bioactive substances. Such modifications can improve cell adhesion, reduce foreign body response, and promote integration with surrounding tissues.
- Vacuum forming processes for biomedical applications: Specialized vacuum forming processes have been developed for biomedical applications. These processes focus on maintaining material integrity and biocompatibility while achieving desired shapes and structures. Techniques may include low-temperature vacuum forming, sterile processing environments, and precise control of forming parameters to ensure consistent product quality.
- Testing and evaluation of biocompatibility in vacuum-formed products: Methods for testing and evaluating the biocompatibility of vacuum-formed products have been established. These include in vitro cytotoxicity assays, cell adhesion studies, and in vivo implantation tests. Such evaluations are crucial to ensure the safety and efficacy of vacuum-formed biomedical devices before clinical use.
- Innovations in biodegradable vacuum-formed materials: Recent innovations have focused on developing biodegradable materials suitable for vacuum forming in biomedical applications. These materials are designed to maintain biocompatibility while gradually breaking down in the body, eliminating the need for removal surgeries. Research in this area includes the development of novel polymer blends and composite materials with controlled degradation rates.
02 Surface modification techniques for improved biocompatibility
Surface modification techniques can be applied to vacuum-formed materials to enhance their biocompatibility. These methods may include plasma treatment, chemical etching, or coating with bioactive substances. Such modifications can improve cell adhesion, reduce immune responses, and promote integration with surrounding tissues, making the vacuum-formed products more suitable for medical applications.Expand Specific Solutions03 Vacuum forming processes for biomedical applications
Specialized vacuum forming processes have been developed for creating biocompatible medical devices and implants. These processes often involve precise temperature control, sterile environments, and specific mold designs to ensure the final product meets biocompatibility standards. The vacuum forming technique allows for the creation of complex shapes while maintaining the material's biocompatible properties.Expand Specific Solutions04 Testing methods for biocompatibility of vacuum-formed products
Various testing methods are employed to assess the biocompatibility of vacuum-formed products. These may include in vitro cell culture tests, animal studies, and long-term implantation trials. Standardized protocols are often used to evaluate cytotoxicity, allergic reactions, and potential for inflammation or rejection. These tests ensure that the vacuum-formed products meet regulatory requirements for medical use.Expand Specific Solutions05 Innovations in biodegradable vacuum-formed materials
Recent innovations have focused on developing biodegradable materials suitable for vacuum forming processes. These materials are designed to maintain biocompatibility while gradually breaking down in the body over time. This approach is particularly useful for temporary implants or drug delivery systems, where the material's degradation can be synchronized with the healing process or drug release profile.Expand Specific Solutions
Key Players in Biocompatible Medical Device Manufacturing
The biocompatibility trends in vacuum forming for medical devices are evolving rapidly, reflecting the industry's growth phase and increasing market demand. The global market for biocompatible medical devices is expanding, driven by technological advancements and an aging population. Companies like Medtronic Vascular, Inc., Boston Scientific Scimed, Inc., and Covidien Pte Ltd. are at the forefront of innovation, leveraging their expertise to develop advanced biocompatible materials and manufacturing processes. The technology's maturity is progressing, with research institutions such as Zhejiang University and Wake Forest University contributing to advancements in material science and bioengineering. Collaboration between industry leaders and academic institutions is accelerating the development of novel biocompatible solutions, pushing the boundaries of what's possible in medical device manufacturing.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific has pioneered the use of hydrophilic coatings in vacuum-formed medical devices to improve biocompatibility and reduce thrombogenicity[2]. Their technology involves a multi-layer approach, with an inner layer optimized for mechanical properties and an outer layer designed for optimal biological interaction[4]. The company has developed a proprietary vacuum forming process that allows for the incorporation of bioactive agents directly into the device structure, promoting tissue healing and reducing the risk of infection[6]. Boston Scientific has also made significant progress in developing bioresorbable vacuum-formed devices for temporary implantation, addressing the need for short-term interventions without long-term foreign body presence[8].
Strengths: Strong focus on minimally invasive devices, extensive patent portfolio in biocompatible materials, and established global distribution network. Weaknesses: Potential for increased device complexity leading to higher manufacturing costs, and challenges in scaling up production of advanced biocompatible materials.
Johnson & Johnson Regenerative Therapeutics LLC
Technical Solution: Johnson & Johnson has focused on developing biocompatible materials for vacuum-formed regenerative medicine applications. Their approach combines advanced biomaterials with vacuum forming techniques to create scaffolds that promote tissue regeneration[10]. The company has pioneered the use of decellularized extracellular matrix (ECM) components in vacuum-formed devices, enhancing their biocompatibility and ability to support cell growth[12]. J&J has also developed a novel vacuum forming process that allows for the incorporation of growth factors and other bioactive molecules into the device structure, creating a microenvironment conducive to tissue regeneration[14]. Recent advancements include the development of smart, stimuli-responsive materials that can change their properties in response to physiological cues, improving the overall biocompatibility and functionality of vacuum-formed implants[16].
Strengths: Extensive resources for research and development, strong presence in both pharmaceutical and medical device markets, and a comprehensive approach to regenerative medicine. Weaknesses: Complex regulatory landscape for combination products, potential for longer development timelines due to the integration of multiple technologies.
Innovative Biocompatible Material Developments
Microlayer coextrusion to create a time-release drug substance delivery product
PatentActiveUS20200315920A1
Innovation
- The development of medical devices through microlayer coextrusion, which creates laminated flow streams with amplified laminations, allowing for the formation of thin or nanometer-range geometries with controlled drug release, using biocompatible polymers and layers with varying drug concentrations and diffusivity to achieve time-dependent drug delivery.
Implantable materials having engineered surfaces and method of making same
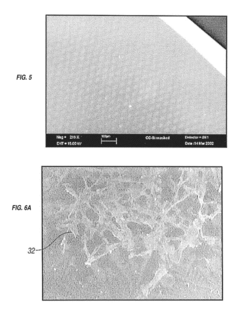
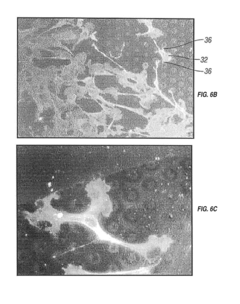
PatentInactiveUS20190038815A1
Innovation
- The implementation of vacuum-deposited biocompatible materials with patterned arrays of geometric physiologically functional features on the surface of implantable devices to enhance endothelial cell binding, proliferation, and migration, promoting complete endothelialization by creating a favorable environment for cell attachment and growth.
Regulatory Framework for Biocompatible Medical Devices
The regulatory framework for biocompatible medical devices is a critical aspect of the medical device industry, ensuring patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the approval and regulation of medical devices. The FDA's regulatory process is based on a risk-based classification system, with Class I devices being low-risk and Class III devices being high-risk.
For biocompatible medical devices, manufacturers must comply with ISO 10993, a set of standards for evaluating the biocompatibility of medical devices. This standard outlines various tests and evaluation procedures to assess the potential biological risks associated with device materials and their interaction with human tissues.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2021 and May 2022, respectively. These regulations have significantly increased the requirements for clinical evidence and post-market surveillance of medical devices, including those manufactured using vacuum forming techniques.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices under the Pharmaceutical and Medical Device Act. The PMDA has specific requirements for biocompatibility testing and documentation, which must be submitted as part of the device approval process.
Globally, there is a trend towards harmonization of regulatory requirements through initiatives like the International Medical Device Regulators Forum (IMDRF). This organization aims to accelerate international medical device regulatory harmonization and convergence, potentially streamlining the approval process for manufacturers operating in multiple markets.
Regulatory bodies are increasingly focusing on the entire lifecycle of medical devices, from design and development to post-market surveillance. This approach requires manufacturers to implement robust quality management systems and maintain ongoing vigilance for potential safety issues.
For vacuum-formed medical devices, specific regulatory considerations include material selection, sterilization processes, and the potential for leachables and extractables. Manufacturers must demonstrate that their vacuum forming processes do not introduce harmful substances or alter the biocompatibility of the materials used.
As the field of biocompatible materials advances, regulatory frameworks are evolving to keep pace with new technologies. This includes the development of guidance documents for novel materials and manufacturing processes, as well as updated testing protocols to assess long-term biocompatibility and potential systemic effects of implantable devices.
For biocompatible medical devices, manufacturers must comply with ISO 10993, a set of standards for evaluating the biocompatibility of medical devices. This standard outlines various tests and evaluation procedures to assess the potential biological risks associated with device materials and their interaction with human tissues.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2021 and May 2022, respectively. These regulations have significantly increased the requirements for clinical evidence and post-market surveillance of medical devices, including those manufactured using vacuum forming techniques.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices under the Pharmaceutical and Medical Device Act. The PMDA has specific requirements for biocompatibility testing and documentation, which must be submitted as part of the device approval process.
Globally, there is a trend towards harmonization of regulatory requirements through initiatives like the International Medical Device Regulators Forum (IMDRF). This organization aims to accelerate international medical device regulatory harmonization and convergence, potentially streamlining the approval process for manufacturers operating in multiple markets.
Regulatory bodies are increasingly focusing on the entire lifecycle of medical devices, from design and development to post-market surveillance. This approach requires manufacturers to implement robust quality management systems and maintain ongoing vigilance for potential safety issues.
For vacuum-formed medical devices, specific regulatory considerations include material selection, sterilization processes, and the potential for leachables and extractables. Manufacturers must demonstrate that their vacuum forming processes do not introduce harmful substances or alter the biocompatibility of the materials used.
As the field of biocompatible materials advances, regulatory frameworks are evolving to keep pace with new technologies. This includes the development of guidance documents for novel materials and manufacturing processes, as well as updated testing protocols to assess long-term biocompatibility and potential systemic effects of implantable devices.
Environmental Impact of Biocompatible Materials Production
The production of biocompatible materials for medical devices, particularly those used in vacuum forming processes, has significant environmental implications. As the demand for these materials increases, it is crucial to assess and mitigate their environmental impact throughout the entire lifecycle.
Raw material extraction and processing for biocompatible polymers often involve energy-intensive processes and the use of non-renewable resources. For instance, the production of polyethylene (PE) and polypropylene (PP), commonly used in medical devices, relies heavily on fossil fuels. This dependency contributes to greenhouse gas emissions and resource depletion.
Manufacturing processes for biocompatible materials, including extrusion and molding, consume substantial amounts of energy and water. Additionally, these processes may generate hazardous waste and emissions, requiring careful management and disposal. The use of additives and plasticizers to enhance material properties can also introduce potential environmental contaminants.
The increasing focus on biodegradable and bio-based materials as alternatives to traditional petroleum-based polymers presents both opportunities and challenges. While these materials may reduce reliance on non-renewable resources, their production can still have significant environmental impacts, such as land use changes for crop cultivation and water consumption in processing.
Waste management of biocompatible materials poses another environmental concern. Many medical devices are designed for single-use applications, contributing to the growing problem of plastic waste in healthcare settings. Proper disposal and recycling of these materials are often complicated by contamination risks and regulatory requirements.
The environmental impact extends to the transportation and distribution of raw materials and finished products. The global nature of the medical device supply chain results in substantial carbon emissions from long-distance shipping and air freight.
To address these environmental challenges, the industry is exploring various strategies. These include improving energy efficiency in production processes, developing closed-loop recycling systems for medical-grade plastics, and investing in renewable energy sources for manufacturing facilities.
Life cycle assessment (LCA) studies are increasingly being conducted to quantify the environmental footprint of biocompatible materials and identify hotspots for improvement. These assessments consider factors such as carbon emissions, water usage, and ecosystem impacts across the entire product lifecycle.
As regulations and consumer awareness regarding environmental sustainability grow, manufacturers are under pressure to adopt more eco-friendly practices. This has led to innovations in green chemistry approaches for material synthesis and the exploration of novel, environmentally benign materials that maintain the necessary biocompatibility for medical applications.
Raw material extraction and processing for biocompatible polymers often involve energy-intensive processes and the use of non-renewable resources. For instance, the production of polyethylene (PE) and polypropylene (PP), commonly used in medical devices, relies heavily on fossil fuels. This dependency contributes to greenhouse gas emissions and resource depletion.
Manufacturing processes for biocompatible materials, including extrusion and molding, consume substantial amounts of energy and water. Additionally, these processes may generate hazardous waste and emissions, requiring careful management and disposal. The use of additives and plasticizers to enhance material properties can also introduce potential environmental contaminants.
The increasing focus on biodegradable and bio-based materials as alternatives to traditional petroleum-based polymers presents both opportunities and challenges. While these materials may reduce reliance on non-renewable resources, their production can still have significant environmental impacts, such as land use changes for crop cultivation and water consumption in processing.
Waste management of biocompatible materials poses another environmental concern. Many medical devices are designed for single-use applications, contributing to the growing problem of plastic waste in healthcare settings. Proper disposal and recycling of these materials are often complicated by contamination risks and regulatory requirements.
The environmental impact extends to the transportation and distribution of raw materials and finished products. The global nature of the medical device supply chain results in substantial carbon emissions from long-distance shipping and air freight.
To address these environmental challenges, the industry is exploring various strategies. These include improving energy efficiency in production processes, developing closed-loop recycling systems for medical-grade plastics, and investing in renewable energy sources for manufacturing facilities.
Life cycle assessment (LCA) studies are increasingly being conducted to quantify the environmental footprint of biocompatible materials and identify hotspots for improvement. These assessments consider factors such as carbon emissions, water usage, and ecosystem impacts across the entire product lifecycle.
As regulations and consumer awareness regarding environmental sustainability grow, manufacturers are under pressure to adopt more eco-friendly practices. This has led to innovations in green chemistry approaches for material synthesis and the exploration of novel, environmentally benign materials that maintain the necessary biocompatibility for medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!