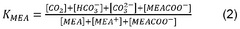Carbon Capture Technologies Enhancing Pharmaceutical Applications
OCT 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Background and Objectives
Carbon capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications across various industries. Initially developed to address environmental concerns related to greenhouse gas emissions, carbon capture has now expanded beyond traditional energy sectors into specialized fields such as pharmaceutical manufacturing. The evolution of this technology represents a convergence of environmental science, chemical engineering, and pharmaceutical innovation, creating new opportunities for sustainable drug development and manufacturing processes.
The pharmaceutical industry, traditionally focused on efficacy and safety, now faces increasing pressure to reduce its environmental footprint while maintaining stringent quality standards. Carbon capture technologies offer a promising solution by enabling the industry to reduce emissions while potentially enhancing production efficiency. Historical developments in carbon capture methods—from absorption and adsorption techniques to membrane separation and chemical looping—have created a foundation for pharmaceutical-specific applications that align with the industry's unique requirements.
Current technological objectives in this field center on developing carbon capture systems specifically optimized for pharmaceutical manufacturing environments. These objectives include creating capture methods compatible with Good Manufacturing Practice (GMP) standards, designing systems that can operate within the sterile conditions required for drug production, and developing capture technologies that can be integrated into existing pharmaceutical manufacturing infrastructure with minimal disruption to production processes.
A key goal is to transform captured carbon from a waste product into a valuable resource for pharmaceutical synthesis. This includes developing methods to purify captured CO2 to pharmaceutical-grade standards and creating novel synthetic pathways that utilize captured carbon as a feedstock for active pharmaceutical ingredients (APIs) and excipients. Such approaches could potentially reduce the industry's reliance on petroleum-derived raw materials while simultaneously decreasing its carbon footprint.
Research objectives also extend to exploring the economic viability of these technologies within the pharmaceutical context. This includes assessing the cost-effectiveness of various capture methods, evaluating potential regulatory incentives for adoption, and quantifying the long-term benefits of carbon-neutral or carbon-negative pharmaceutical manufacturing. The industry aims to establish carbon capture not merely as an environmental compliance measure but as a strategic advantage that enhances sustainability credentials while potentially reducing production costs through innovative carbon utilization pathways.
The pharmaceutical industry, traditionally focused on efficacy and safety, now faces increasing pressure to reduce its environmental footprint while maintaining stringent quality standards. Carbon capture technologies offer a promising solution by enabling the industry to reduce emissions while potentially enhancing production efficiency. Historical developments in carbon capture methods—from absorption and adsorption techniques to membrane separation and chemical looping—have created a foundation for pharmaceutical-specific applications that align with the industry's unique requirements.
Current technological objectives in this field center on developing carbon capture systems specifically optimized for pharmaceutical manufacturing environments. These objectives include creating capture methods compatible with Good Manufacturing Practice (GMP) standards, designing systems that can operate within the sterile conditions required for drug production, and developing capture technologies that can be integrated into existing pharmaceutical manufacturing infrastructure with minimal disruption to production processes.
A key goal is to transform captured carbon from a waste product into a valuable resource for pharmaceutical synthesis. This includes developing methods to purify captured CO2 to pharmaceutical-grade standards and creating novel synthetic pathways that utilize captured carbon as a feedstock for active pharmaceutical ingredients (APIs) and excipients. Such approaches could potentially reduce the industry's reliance on petroleum-derived raw materials while simultaneously decreasing its carbon footprint.
Research objectives also extend to exploring the economic viability of these technologies within the pharmaceutical context. This includes assessing the cost-effectiveness of various capture methods, evaluating potential regulatory incentives for adoption, and quantifying the long-term benefits of carbon-neutral or carbon-negative pharmaceutical manufacturing. The industry aims to establish carbon capture not merely as an environmental compliance measure but as a strategic advantage that enhances sustainability credentials while potentially reducing production costs through innovative carbon utilization pathways.
Pharmaceutical Market Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards sustainable practices, with carbon capture technologies emerging as a critical component in this transformation. Market analysis indicates that pharmaceutical companies are increasingly seeking carbon capture solutions to reduce their environmental footprint while maintaining operational efficiency. This demand is driven by multiple factors, including stringent regulatory requirements, corporate sustainability goals, and consumer preferences for environmentally responsible products.
Global pharmaceutical market projections show that companies implementing sustainable manufacturing processes, including carbon capture technologies, are likely to gain competitive advantages in terms of both regulatory compliance and market positioning. The pharmaceutical sector, valued at approximately 1.4 trillion USD globally, contributes significantly to industrial carbon emissions through energy-intensive manufacturing processes, creating substantial demand for integrated carbon capture solutions.
Market research reveals that pharmaceutical companies are particularly interested in carbon capture technologies that can be seamlessly integrated into existing manufacturing facilities without disrupting production workflows. There is specific demand for solutions that can capture carbon emissions from fermentation processes, chemical synthesis, and high-temperature operations common in pharmaceutical manufacturing.
The market demand extends beyond mere carbon capture to include technologies that enable carbon utilization within pharmaceutical processes. Companies are exploring opportunities to convert captured carbon into valuable pharmaceutical precursors or excipients, creating closed-loop systems that enhance sustainability while potentially reducing production costs. This represents a significant market opportunity for technologies that can bridge carbon capture with pharmaceutical manufacturing processes.
Geographically, market demand is strongest in regions with established pharmaceutical manufacturing hubs combined with progressive environmental regulations, particularly in Western Europe, North America, and increasingly in parts of Asia. Countries with carbon pricing mechanisms or emissions trading schemes show accelerated adoption rates for pharmaceutical carbon capture technologies.
Investment trends indicate growing venture capital interest in startups developing specialized carbon capture solutions for pharmaceutical applications. Market forecasts suggest that the pharmaceutical carbon capture technology segment could grow at a compound annual rate exceeding the broader carbon capture market, reflecting the industry's unique requirements and willingness to invest in sustainable technologies that align with both environmental goals and operational needs.
Consumer and stakeholder pressure is another significant market driver, with pharmaceutical companies facing increasing scrutiny regarding their environmental impact. Market research indicates that companies demonstrating tangible commitments to carbon reduction through technologies like carbon capture enjoy enhanced brand reputation and stakeholder trust, translating to measurable market advantages in an increasingly environmentally conscious marketplace.
Global pharmaceutical market projections show that companies implementing sustainable manufacturing processes, including carbon capture technologies, are likely to gain competitive advantages in terms of both regulatory compliance and market positioning. The pharmaceutical sector, valued at approximately 1.4 trillion USD globally, contributes significantly to industrial carbon emissions through energy-intensive manufacturing processes, creating substantial demand for integrated carbon capture solutions.
Market research reveals that pharmaceutical companies are particularly interested in carbon capture technologies that can be seamlessly integrated into existing manufacturing facilities without disrupting production workflows. There is specific demand for solutions that can capture carbon emissions from fermentation processes, chemical synthesis, and high-temperature operations common in pharmaceutical manufacturing.
The market demand extends beyond mere carbon capture to include technologies that enable carbon utilization within pharmaceutical processes. Companies are exploring opportunities to convert captured carbon into valuable pharmaceutical precursors or excipients, creating closed-loop systems that enhance sustainability while potentially reducing production costs. This represents a significant market opportunity for technologies that can bridge carbon capture with pharmaceutical manufacturing processes.
Geographically, market demand is strongest in regions with established pharmaceutical manufacturing hubs combined with progressive environmental regulations, particularly in Western Europe, North America, and increasingly in parts of Asia. Countries with carbon pricing mechanisms or emissions trading schemes show accelerated adoption rates for pharmaceutical carbon capture technologies.
Investment trends indicate growing venture capital interest in startups developing specialized carbon capture solutions for pharmaceutical applications. Market forecasts suggest that the pharmaceutical carbon capture technology segment could grow at a compound annual rate exceeding the broader carbon capture market, reflecting the industry's unique requirements and willingness to invest in sustainable technologies that align with both environmental goals and operational needs.
Consumer and stakeholder pressure is another significant market driver, with pharmaceutical companies facing increasing scrutiny regarding their environmental impact. Market research indicates that companies demonstrating tangible commitments to carbon reduction through technologies like carbon capture enjoy enhanced brand reputation and stakeholder trust, translating to measurable market advantages in an increasingly environmentally conscious marketplace.
Current Status and Challenges in Carbon Capture
Carbon capture technologies have evolved significantly over the past decade, with current global CO2 capture capacity reaching approximately 40 million tonnes annually. However, this represents less than 0.1% of global emissions, highlighting the substantial gap between existing capabilities and climate mitigation needs. Within the pharmaceutical sector, carbon capture implementation remains particularly limited despite the industry's significant carbon footprint, estimated at 52 million tonnes CO2 equivalent annually.
The dominant carbon capture approaches currently deployed include post-combustion capture using amine-based solvents, pre-combustion capture, and oxy-fuel combustion. While these technologies have demonstrated technical feasibility at industrial scale, their application in pharmaceutical manufacturing faces unique challenges due to stringent product purity requirements and complex production environments. Current capture efficiency rates typically range between 85-95%, but pharmaceutical applications often require higher performance metrics.
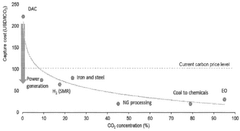
Cost remains a primary barrier to widespread adoption, with current carbon capture expenses ranging from $40-120 per tonne of CO2 captured, significantly impacting pharmaceutical production economics. Energy penalties associated with capture processes—often requiring 15-30% additional energy—further complicate implementation in energy-intensive pharmaceutical operations. The pharmaceutical industry's distributed emission sources, rather than concentrated point sources, create additional technical complexity for efficient capture systems.
Regulatory frameworks governing carbon capture in pharmaceutical applications remain underdeveloped globally, creating uncertainty for potential investors and technology developers. The absence of standardized protocols for measuring carbon capture performance specifically in pharmaceutical contexts hampers technology assessment and comparison. Additionally, integration challenges with existing Good Manufacturing Practice (GMP) requirements create significant compliance hurdles.
Material science limitations present another significant challenge, as current sorbent materials demonstrate degradation issues when exposed to pharmaceutical manufacturing environments containing various solvents and chemical compounds. Scalability concerns persist, with most pharmaceutical-specific carbon capture technologies remaining at laboratory or pilot scale, lacking demonstration at commercial production volumes.
Recent technological developments show promise, including advanced membrane systems with pharmaceutical-compatible materials, enzymatic capture systems with potential for integration with biopharmaceutical processes, and metal-organic frameworks (MOFs) offering exceptional selectivity. However, these emerging solutions require substantial further development to address pharmaceutical-specific requirements for purity, reliability, and regulatory compliance before achieving widespread industry adoption.
The dominant carbon capture approaches currently deployed include post-combustion capture using amine-based solvents, pre-combustion capture, and oxy-fuel combustion. While these technologies have demonstrated technical feasibility at industrial scale, their application in pharmaceutical manufacturing faces unique challenges due to stringent product purity requirements and complex production environments. Current capture efficiency rates typically range between 85-95%, but pharmaceutical applications often require higher performance metrics.
Cost remains a primary barrier to widespread adoption, with current carbon capture expenses ranging from $40-120 per tonne of CO2 captured, significantly impacting pharmaceutical production economics. Energy penalties associated with capture processes—often requiring 15-30% additional energy—further complicate implementation in energy-intensive pharmaceutical operations. The pharmaceutical industry's distributed emission sources, rather than concentrated point sources, create additional technical complexity for efficient capture systems.
Regulatory frameworks governing carbon capture in pharmaceutical applications remain underdeveloped globally, creating uncertainty for potential investors and technology developers. The absence of standardized protocols for measuring carbon capture performance specifically in pharmaceutical contexts hampers technology assessment and comparison. Additionally, integration challenges with existing Good Manufacturing Practice (GMP) requirements create significant compliance hurdles.
Material science limitations present another significant challenge, as current sorbent materials demonstrate degradation issues when exposed to pharmaceutical manufacturing environments containing various solvents and chemical compounds. Scalability concerns persist, with most pharmaceutical-specific carbon capture technologies remaining at laboratory or pilot scale, lacking demonstration at commercial production volumes.
Recent technological developments show promise, including advanced membrane systems with pharmaceutical-compatible materials, enzymatic capture systems with potential for integration with biopharmaceutical processes, and metal-organic frameworks (MOFs) offering exceptional selectivity. However, these emerging solutions require substantial further development to address pharmaceutical-specific requirements for purity, reliability, and regulatory compliance before achieving widespread industry adoption.
Current Carbon Capture Solutions for Pharmaceuticals
01 Chemical absorption methods for carbon capture
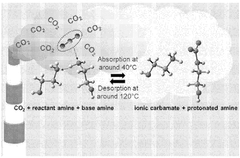
Chemical absorption is a widely used method for capturing carbon dioxide from various sources. This approach involves the use of solvents, typically amine-based compounds, that chemically react with CO2 to form stable compounds. The CO2 can later be released through heating or pressure changes, allowing for the regeneration of the solvent and the collection of concentrated CO2. This technology is particularly effective for post-combustion capture from power plants and industrial facilities, offering high capture efficiency despite energy requirements for solvent regeneration.- Direct Air Capture Technologies: Direct air capture (DAC) technologies involve systems that extract carbon dioxide directly from the atmosphere. These technologies typically use sorbent materials or chemical solutions to selectively capture CO2 from ambient air. After capture, the CO2 can be concentrated, compressed, and either stored underground or utilized in various applications. DAC systems can be deployed in various locations regardless of emission sources and offer a solution for addressing historical emissions.
- Post-Combustion Carbon Capture: Post-combustion carbon capture technologies focus on removing CO2 from flue gases after the combustion process in power plants and industrial facilities. These systems typically employ chemical absorption using amine-based solvents or other capture media to selectively remove CO2 from exhaust streams. The captured CO2 is then separated from the capture medium, compressed, and prepared for transport and storage. This approach allows retrofitting existing facilities without major modifications to the core combustion process.
- Carbon Utilization and Conversion: Carbon utilization technologies focus on converting captured CO2 into valuable products rather than simply storing it. These processes transform CO2 into fuels, chemicals, building materials, or other commercial products. Methods include catalytic conversion, electrochemical reduction, mineralization, and biological conversion using microorganisms. By creating economic value from captured carbon, these technologies aim to offset the costs of carbon capture while reducing net emissions and creating a circular carbon economy.
- Biological Carbon Sequestration: Biological carbon sequestration leverages natural processes to remove CO2 from the atmosphere. These approaches include enhanced forestry management, soil carbon enhancement, algae cultivation, and ocean-based solutions. Biological systems naturally absorb CO2 through photosynthesis, converting it into biomass or storing it in soils and sediments. These methods can be enhanced through targeted interventions such as biochar application, regenerative agricultural practices, or engineered biological systems that accelerate natural carbon fixation processes.
- Carbon Storage and Sequestration Infrastructure: Carbon storage technologies focus on the long-term containment of captured CO2 to prevent its release into the atmosphere. These include geological storage in depleted oil and gas reservoirs, deep saline formations, or unminable coal seams. The infrastructure encompasses injection wells, monitoring systems, and transportation networks such as pipelines for moving captured CO2 from emission sources to storage sites. Advanced monitoring technologies ensure the integrity of storage sites and prevent leakage, while regulatory frameworks govern site selection, operation, and long-term stewardship.
02 Direct air capture (DAC) technologies
Direct air capture technologies are designed to extract carbon dioxide directly from ambient air rather than from point sources like industrial emissions. These systems typically use specialized sorbents or solutions that selectively bind with CO2 from the atmosphere. After capture, the CO2 can be released through heating or other processes, concentrated, and then stored or utilized. While DAC offers flexibility in location and addresses historical emissions, current challenges include high energy requirements and costs compared to point-source capture methods.Expand Specific Solutions03 Membrane-based carbon capture systems
Membrane-based carbon capture systems utilize selective permeable barriers that allow CO2 to pass through while blocking other gases. These membranes can be made from polymers, ceramics, or composite materials with specific pore sizes and chemical properties designed to maximize CO2 separation efficiency. The technology offers advantages including modular design, lower energy consumption compared to some alternative methods, and continuous operation capability. Current research focuses on improving membrane durability, selectivity, and permeability to enhance overall system performance.Expand Specific Solutions04 Biological carbon capture methods
Biological carbon capture methods leverage natural biological processes to remove CO2 from the atmosphere or flue gases. These approaches include engineered microalgae systems, enhanced forest management, biochar production, and microbial conversion technologies. Microalgae, for example, can capture CO2 through photosynthesis at rates higher than terrestrial plants, while producing valuable biomass. These methods offer advantages of being renewable and potentially more environmentally friendly than some industrial approaches, though challenges remain in scaling and process optimization.Expand Specific Solutions05 Carbon capture utilization and storage (CCUS) integration
Carbon capture utilization and storage (CCUS) integration focuses on comprehensive systems that not only capture CO2 but also convert it into valuable products or securely store it. These integrated approaches include converting captured carbon into construction materials, chemicals, fuels, or enhancing oil recovery. Storage methods involve injecting CO2 into geological formations such as depleted oil and gas reservoirs or saline aquifers. The integration of capture with utilization and storage creates economic incentives for carbon capture while ensuring long-term climate benefits through permanent sequestration of greenhouse gases.Expand Specific Solutions
Key Industry Players and Competitors
Carbon capture technologies in pharmaceutical applications are evolving rapidly, with the market currently in an early growth phase. The global market size is expanding, driven by increasing environmental regulations and sustainability initiatives, with projections suggesting significant growth over the next decade. Technologically, the field shows varying maturity levels across different approaches. Leading petroleum companies like China Petroleum & Chemical Corp. (Sinopec) and Saudi Aramco are advancing industrial-scale carbon capture solutions, while research institutions such as Korea Institute of Energy Research and Dalian University of Technology are developing novel pharmaceutical-specific applications. Specialized firms like Compact Membrane Systems and Ardent Process Technologies are creating innovative membrane technologies for pharmaceutical carbon capture, while major pharmaceutical stakeholders are increasingly adopting these technologies to reduce their carbon footprint and enhance manufacturing sustainability.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced carbon capture technologies specifically tailored for pharmaceutical applications. Their proprietary amine-based solvent systems achieve over 90% CO2 capture efficiency while minimizing energy penalties. Sinopec's integrated approach combines post-combustion capture with utilization pathways that convert captured CO2 into pharmaceutical precursors and excipients. Their modular carbon capture units can be retrofitted to existing pharmaceutical manufacturing facilities, capturing emissions from fermentation processes, chemical synthesis, and utility systems. Sinopec has demonstrated successful implementation at several pharmaceutical production sites, where captured CO2 is purified to pharmaceutical-grade quality (99.9%+ purity) for use in drug formulation, controlled atmosphere packaging, and as a sustainable feedstock for API synthesis.
Strengths: High capture efficiency with pharmaceutical-grade CO2 output; integration capabilities with existing pharma infrastructure; established commercial implementations. Weaknesses: Higher initial capital costs compared to conventional systems; requires significant technical expertise for optimal operation; energy requirements still present challenges for smaller facilities.
China National Petroleum Corp.
Technical Solution: China National Petroleum Corp. (CNPC) has developed a comprehensive carbon capture technology platform targeting pharmaceutical applications. Their system employs advanced physical adsorption using proprietary metal-organic frameworks (MOFs) specifically designed to capture CO2 from pharmaceutical manufacturing processes. CNPC's technology achieves selective carbon capture even in complex gas mixtures typical in pharmaceutical production environments. The captured CO2 undergoes multi-stage purification to meet USP-NF and EP standards for pharmaceutical applications. CNPC has implemented this technology in several pharmaceutical manufacturing facilities, where the captured and purified CO2 is utilized for supercritical fluid extraction, controlled atmosphere packaging, and as a reagent in API synthesis. Their integrated approach includes heat recovery systems that reduce the energy penalty by approximately 30% compared to conventional amine-based systems.
Strengths: Highly selective capture even in complex gas mixtures; energy-efficient operation with heat integration; produces pharmaceutical-grade CO2 meeting regulatory standards. Weaknesses: Higher upfront capital costs; requires specialized expertise for operation and maintenance; technology still scaling to accommodate varying facility sizes.
Core Patents and Technical Literature Analysis
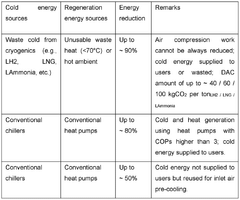
Systems and methods for combined carbon capture and thermal energy storage
PatentWO2025095856A1
Innovation
- A method and system for combined carbon capture and thermal energy storage, where CO2 is captured using a carbon capture medium that generates heat through an exothermic reaction, and this heat is utilized for thermal energy storage, with cooling applied to maintain the capture medium at a temperature below the CO2 regeneration temperature.
Method and apparatus for carbon capture coupled hydrogen production
PatentPendingUS20250018339A1
Innovation
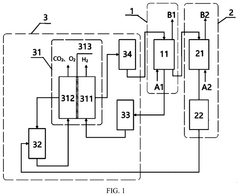
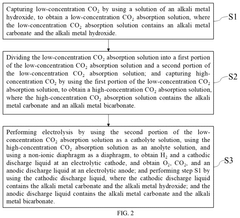
- A method and apparatus utilizing an alkali metal hydroxide solution to capture CO2 across a wide concentration range, followed by electrolysis using a non-ionic diaphragm to regenerate the absorption solution and produce hydrogen and oxygen, reducing the need for expensive ion exchange membranes and simplifying the purification process.
Environmental Impact Assessment
The implementation of carbon capture technologies in pharmaceutical applications necessitates a comprehensive environmental impact assessment to understand both positive and negative consequences. Carbon capture systems significantly reduce greenhouse gas emissions from pharmaceutical manufacturing facilities, with potential reductions of 30-90% depending on the specific technology deployed. This represents a substantial contribution to the industry's sustainability goals, particularly important as pharmaceutical manufacturing accounts for approximately 4.5% of global industrial carbon emissions.
When evaluating lifecycle impacts, carbon capture technologies demonstrate net environmental benefits despite their energy requirements. Modern systems have improved efficiency ratios, consuming 0.2-0.4 GJ of energy per ton of CO2 captured, representing a 40% improvement over earlier generation technologies. The environmental footprint is further optimized when renewable energy sources power these systems, creating a truly sustainable carbon management solution.
Water usage presents a notable environmental consideration, as certain carbon capture methods, particularly amine-based systems, require significant water resources for cooling and processing. Advanced water recycling systems integrated with carbon capture technologies can reduce freshwater consumption by up to 60%, mitigating this environmental pressure. Additionally, closed-loop systems prevent contamination of local water bodies with chemical solvents used in the capture process.
Land use impacts vary considerably based on technology selection. While direct air capture systems may require substantial space for installation, integration with existing pharmaceutical manufacturing infrastructure minimizes additional land requirements. Post-capture carbon utilization pathways, such as conversion to pharmaceutical precursors, can reduce the need for virgin material extraction, further decreasing environmental footprint across the supply chain.
Chemical safety represents another critical environmental consideration. Amine degradation products and other capture solvents must be carefully managed to prevent environmental contamination. New biodegradable capture agents show promising environmental profiles, with toxicity levels 80% lower than conventional options and significantly reduced persistence in environmental systems.
Biodiversity impacts near pharmaceutical manufacturing sites implementing carbon capture can be positive when properly managed. Buffer zones around capture facilities can be designed as ecological corridors, supporting local biodiversity while the reduction in acidifying emissions benefits sensitive ecosystems. Studies indicate pH stabilization in adjacent aquatic systems within 2-3 years of implementing comprehensive carbon capture systems at manufacturing sites.
When evaluating lifecycle impacts, carbon capture technologies demonstrate net environmental benefits despite their energy requirements. Modern systems have improved efficiency ratios, consuming 0.2-0.4 GJ of energy per ton of CO2 captured, representing a 40% improvement over earlier generation technologies. The environmental footprint is further optimized when renewable energy sources power these systems, creating a truly sustainable carbon management solution.
Water usage presents a notable environmental consideration, as certain carbon capture methods, particularly amine-based systems, require significant water resources for cooling and processing. Advanced water recycling systems integrated with carbon capture technologies can reduce freshwater consumption by up to 60%, mitigating this environmental pressure. Additionally, closed-loop systems prevent contamination of local water bodies with chemical solvents used in the capture process.
Land use impacts vary considerably based on technology selection. While direct air capture systems may require substantial space for installation, integration with existing pharmaceutical manufacturing infrastructure minimizes additional land requirements. Post-capture carbon utilization pathways, such as conversion to pharmaceutical precursors, can reduce the need for virgin material extraction, further decreasing environmental footprint across the supply chain.
Chemical safety represents another critical environmental consideration. Amine degradation products and other capture solvents must be carefully managed to prevent environmental contamination. New biodegradable capture agents show promising environmental profiles, with toxicity levels 80% lower than conventional options and significantly reduced persistence in environmental systems.
Biodiversity impacts near pharmaceutical manufacturing sites implementing carbon capture can be positive when properly managed. Buffer zones around capture facilities can be designed as ecological corridors, supporting local biodiversity while the reduction in acidifying emissions benefits sensitive ecosystems. Studies indicate pH stabilization in adjacent aquatic systems within 2-3 years of implementing comprehensive carbon capture systems at manufacturing sites.
Regulatory Framework and Compliance
The regulatory landscape for carbon capture technologies in pharmaceutical applications is complex and evolving rapidly as governments worldwide recognize the dual benefits of reducing carbon emissions while enhancing pharmaceutical manufacturing processes. Current regulatory frameworks primarily focus on environmental compliance, with the EPA in the United States and the European Environment Agency establishing stringent guidelines for carbon emissions and capture methodologies in industrial settings.
Pharmaceutical companies implementing carbon capture technologies must navigate multiple regulatory domains, including Good Manufacturing Practices (GMP), which now increasingly incorporate sustainability metrics. The FDA and EMA have begun developing specific guidance documents addressing the integration of carbon capture systems within pharmaceutical manufacturing facilities, particularly focusing on ensuring that captured carbon does not compromise product quality or safety when repurposed within production processes.
Compliance requirements typically include extensive documentation of carbon capture efficiency, purity of captured carbon, and validation of any processes utilizing captured CO2 in pharmaceutical applications. Companies must demonstrate through rigorous testing that pharmaceutical products manufactured using captured carbon meet the same quality standards as traditional methods. This often necessitates additional quality control measures and stability studies.
International standards organizations, including ISO and ICH, are currently developing harmonized guidelines specifically addressing carbon capture in pharmaceutical contexts. These emerging standards aim to create consistent global requirements for validation, verification, and reporting of carbon capture initiatives within pharmaceutical manufacturing.
Tax incentives and carbon credit systems represent another significant regulatory consideration. Many jurisdictions offer substantial financial benefits for pharmaceutical companies implementing effective carbon capture technologies, though qualifying for these incentives requires adherence to specific measurement and reporting protocols that vary by region.
Risk assessment frameworks specifically for pharmaceutical carbon capture applications are being developed by regulatory bodies, focusing on potential contamination risks, process integration challenges, and long-term stability concerns. Companies must conduct comprehensive risk analyses before implementing carbon capture technologies in GMP environments.
Regulatory trends indicate movement toward mandatory carbon capture requirements for pharmaceutical manufacturing facilities exceeding certain emission thresholds, with implementation timelines varying by country. Forward-thinking pharmaceutical companies are proactively developing compliance strategies that anticipate these evolving requirements while leveraging current incentive programs to offset implementation costs.
Pharmaceutical companies implementing carbon capture technologies must navigate multiple regulatory domains, including Good Manufacturing Practices (GMP), which now increasingly incorporate sustainability metrics. The FDA and EMA have begun developing specific guidance documents addressing the integration of carbon capture systems within pharmaceutical manufacturing facilities, particularly focusing on ensuring that captured carbon does not compromise product quality or safety when repurposed within production processes.
Compliance requirements typically include extensive documentation of carbon capture efficiency, purity of captured carbon, and validation of any processes utilizing captured CO2 in pharmaceutical applications. Companies must demonstrate through rigorous testing that pharmaceutical products manufactured using captured carbon meet the same quality standards as traditional methods. This often necessitates additional quality control measures and stability studies.
International standards organizations, including ISO and ICH, are currently developing harmonized guidelines specifically addressing carbon capture in pharmaceutical contexts. These emerging standards aim to create consistent global requirements for validation, verification, and reporting of carbon capture initiatives within pharmaceutical manufacturing.
Tax incentives and carbon credit systems represent another significant regulatory consideration. Many jurisdictions offer substantial financial benefits for pharmaceutical companies implementing effective carbon capture technologies, though qualifying for these incentives requires adherence to specific measurement and reporting protocols that vary by region.
Risk assessment frameworks specifically for pharmaceutical carbon capture applications are being developed by regulatory bodies, focusing on potential contamination risks, process integration challenges, and long-term stability concerns. Companies must conduct comprehensive risk analyses before implementing carbon capture technologies in GMP environments.
Regulatory trends indicate movement toward mandatory carbon capture requirements for pharmaceutical manufacturing facilities exceeding certain emission thresholds, with implementation timelines varying by country. Forward-thinking pharmaceutical companies are proactively developing compliance strategies that anticipate these evolving requirements while leveraging current incentive programs to offset implementation costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!