Research on Advanced Materials for Carbon Capture Technologies
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Materials Background and Objectives
Carbon capture technologies have evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The field began gaining serious attention in the 1970s with early research into carbon sequestration methods, but has accelerated dramatically since the early 2000s as climate change mitigation became a global priority. Today, carbon capture represents a critical frontier in environmental technology, with materials science playing a pivotal role in its advancement.
The evolution of carbon capture materials has progressed through several distinct phases. First-generation technologies primarily utilized liquid amine solutions, which while effective, suffered from high energy requirements and degradation issues. Second-generation approaches introduced solid sorbents and advanced membranes, improving efficiency but still facing challenges in durability and selectivity. Current third-generation materials are exploring nanoporous frameworks, biomimetic solutions, and hybrid systems that promise unprecedented performance metrics.
Market drivers for carbon capture technologies have intensified with strengthening global climate policies, carbon pricing mechanisms, and corporate sustainability commitments. The Paris Agreement and subsequent international frameworks have established clear targets for emissions reduction, creating substantial demand for effective carbon capture solutions across industrial sectors, particularly in power generation, cement production, and steel manufacturing.
The primary technical objective in this field is to develop materials that can selectively capture CO₂ with high capacity, rapid kinetics, and minimal energy requirements for regeneration. Specifically, researchers aim to create materials capable of capturing CO₂ at concentrations ranging from atmospheric levels (approximately 415 ppm) to concentrated industrial exhaust streams (up to 15%). These materials must maintain performance across thousands of capture-release cycles while resisting degradation from contaminants like SOx, NOx, and water vapor.
Secondary objectives include reducing the overall cost of carbon capture to below $50 per ton of CO₂, developing materials compatible with existing industrial infrastructure, and ensuring environmental sustainability across the full lifecycle of the materials themselves. The ultimate goal is to enable widespread deployment of carbon capture technologies that can meaningfully contribute to climate stabilization targets while remaining economically viable.
Recent technological breakthroughs in metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous polymers have dramatically expanded the design space for carbon capture materials. These advances, coupled with computational screening methods and high-throughput experimental techniques, are accelerating the discovery process and bringing the field closer to achieving its ambitious performance and cost targets.
The evolution of carbon capture materials has progressed through several distinct phases. First-generation technologies primarily utilized liquid amine solutions, which while effective, suffered from high energy requirements and degradation issues. Second-generation approaches introduced solid sorbents and advanced membranes, improving efficiency but still facing challenges in durability and selectivity. Current third-generation materials are exploring nanoporous frameworks, biomimetic solutions, and hybrid systems that promise unprecedented performance metrics.
Market drivers for carbon capture technologies have intensified with strengthening global climate policies, carbon pricing mechanisms, and corporate sustainability commitments. The Paris Agreement and subsequent international frameworks have established clear targets for emissions reduction, creating substantial demand for effective carbon capture solutions across industrial sectors, particularly in power generation, cement production, and steel manufacturing.
The primary technical objective in this field is to develop materials that can selectively capture CO₂ with high capacity, rapid kinetics, and minimal energy requirements for regeneration. Specifically, researchers aim to create materials capable of capturing CO₂ at concentrations ranging from atmospheric levels (approximately 415 ppm) to concentrated industrial exhaust streams (up to 15%). These materials must maintain performance across thousands of capture-release cycles while resisting degradation from contaminants like SOx, NOx, and water vapor.
Secondary objectives include reducing the overall cost of carbon capture to below $50 per ton of CO₂, developing materials compatible with existing industrial infrastructure, and ensuring environmental sustainability across the full lifecycle of the materials themselves. The ultimate goal is to enable widespread deployment of carbon capture technologies that can meaningfully contribute to climate stabilization targets while remaining economically viable.
Recent technological breakthroughs in metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous polymers have dramatically expanded the design space for carbon capture materials. These advances, coupled with computational screening methods and high-throughput experimental techniques, are accelerating the discovery process and bringing the field closer to achieving its ambitious performance and cost targets.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures. As of 2023, the market was valued at approximately $7.3 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This remarkable growth trajectory is primarily fueled by stringent carbon emission regulations across major economies and the rising adoption of carbon neutrality commitments by corporations worldwide.
The industrial sector represents the largest demand segment for carbon capture technologies, accounting for roughly 45% of the current market. Power generation follows closely at 30%, with the remaining market share distributed across oil and gas, cement manufacturing, and other carbon-intensive industries. Geographically, North America leads the market with approximately 38% share, followed by Europe at 32%, Asia-Pacific at 22%, and the rest of the world comprising the remaining 8%.
Post-combustion capture technologies currently dominate the market, representing about 65% of installations due to their compatibility with existing infrastructure. However, pre-combustion and oxy-fuel combustion technologies are gaining traction, particularly in new facility constructions where integration costs can be minimized during the design phase.
A notable market trend is the increasing interest in direct air capture (DAC) technologies, which have seen investment growth of over 200% in the past three years. Though currently accounting for less than 5% of the total carbon capture market, DAC is projected to be the fastest-growing segment with a CAGR exceeding 30% through 2030.
The economic viability of carbon capture solutions remains a significant market challenge. Current capture costs range from $40-120 per ton of CO2, depending on the source and technology employed. For widespread commercial adoption, industry analysts suggest these costs need to decrease to $25-30 per ton. Advanced materials research is critical to achieving this cost reduction, with novel sorbents and membranes potentially reducing energy penalties by 30-40%.
Government incentives are playing a crucial role in market development. The U.S. 45Q tax credit offering up to $85 per ton for captured and sequestered CO2 has catalyzed numerous projects. Similarly, the EU's Innovation Fund and carbon pricing mechanisms have stimulated market growth in Europe, while China's inclusion of CCUS in its latest Five-Year Plan signals expanding opportunities in Asia.
The industrial sector represents the largest demand segment for carbon capture technologies, accounting for roughly 45% of the current market. Power generation follows closely at 30%, with the remaining market share distributed across oil and gas, cement manufacturing, and other carbon-intensive industries. Geographically, North America leads the market with approximately 38% share, followed by Europe at 32%, Asia-Pacific at 22%, and the rest of the world comprising the remaining 8%.
Post-combustion capture technologies currently dominate the market, representing about 65% of installations due to their compatibility with existing infrastructure. However, pre-combustion and oxy-fuel combustion technologies are gaining traction, particularly in new facility constructions where integration costs can be minimized during the design phase.
A notable market trend is the increasing interest in direct air capture (DAC) technologies, which have seen investment growth of over 200% in the past three years. Though currently accounting for less than 5% of the total carbon capture market, DAC is projected to be the fastest-growing segment with a CAGR exceeding 30% through 2030.
The economic viability of carbon capture solutions remains a significant market challenge. Current capture costs range from $40-120 per ton of CO2, depending on the source and technology employed. For widespread commercial adoption, industry analysts suggest these costs need to decrease to $25-30 per ton. Advanced materials research is critical to achieving this cost reduction, with novel sorbents and membranes potentially reducing energy penalties by 30-40%.
Government incentives are playing a crucial role in market development. The U.S. 45Q tax credit offering up to $85 per ton for captured and sequestered CO2 has catalyzed numerous projects. Similarly, the EU's Innovation Fund and carbon pricing mechanisms have stimulated market growth in Europe, while China's inclusion of CCUS in its latest Five-Year Plan signals expanding opportunities in Asia.
Current State and Challenges in Carbon Capture Materials
Carbon capture technologies have evolved significantly over the past decades, with materials research playing a pivotal role in advancing efficiency and reducing costs. Currently, the field employs several categories of materials including amine-based sorbents, metal-organic frameworks (MOFs), zeolites, and various membrane technologies. Each material class presents unique advantages and limitations in practical carbon capture applications.
Amine-based materials remain the most commercially deployed solution, particularly in post-combustion capture systems. These materials offer high CO2 selectivity and established operational protocols. However, they face significant challenges including high regeneration energy requirements (typically 3-4 GJ/tonne CO2), thermal degradation issues, and corrosion concerns that limit long-term durability in industrial settings.
MOFs represent a promising frontier with exceptional surface areas exceeding 7,000 m²/g and highly tunable pore structures. Recent advancements have produced MOFs with CO2 adsorption capacities reaching 1.5-2 mmol/g under ambient conditions. Despite these impressive properties, MOFs continue to struggle with hydrothermal stability issues and manufacturing scalability, limiting their industrial implementation despite laboratory success.
Zeolites and porous carbon materials offer robust alternatives with good thermal stability, but generally demonstrate lower CO2 selectivity in the presence of water vapor—a critical limitation for flue gas applications where moisture is invariably present. Recent modifications incorporating amine functionalization have shown promise in addressing these selectivity issues.
Membrane technologies have advanced considerably, with polymeric and mixed matrix membranes achieving CO2 permeabilities above 1,000 Barrer while maintaining selectivity. However, the performance trade-off between permeability and selectivity (Robeson upper bound) continues to constrain practical applications, particularly at industrial scales requiring high throughput.
Geographically, research leadership in carbon capture materials shows distinct patterns. North America and Europe dominate in fundamental research and patent filings, particularly for novel MOFs and advanced membrane materials. China has rapidly expanded its research footprint, focusing heavily on scaled applications and cost reduction for existing technologies. Japan leads in specialized areas including ceramic-based membranes and hybrid capture systems.
Key technical challenges across all material classes include: energy penalty reduction (targeting below 2 GJ/tonne CO2), material stability under realistic industrial conditions (high temperatures, contaminants, cycling), manufacturing scalability, and cost-effectiveness (current materials contribute to capture costs of $40-80/tonne CO2, significantly above economically viable targets of $20-30/tonne).
Additionally, most current materials are optimized for post-combustion capture at atmospheric pressure, leaving significant gaps in materials designed specifically for pre-combustion or direct air capture applications, which represent growing market segments requiring specialized material properties.
Amine-based materials remain the most commercially deployed solution, particularly in post-combustion capture systems. These materials offer high CO2 selectivity and established operational protocols. However, they face significant challenges including high regeneration energy requirements (typically 3-4 GJ/tonne CO2), thermal degradation issues, and corrosion concerns that limit long-term durability in industrial settings.
MOFs represent a promising frontier with exceptional surface areas exceeding 7,000 m²/g and highly tunable pore structures. Recent advancements have produced MOFs with CO2 adsorption capacities reaching 1.5-2 mmol/g under ambient conditions. Despite these impressive properties, MOFs continue to struggle with hydrothermal stability issues and manufacturing scalability, limiting their industrial implementation despite laboratory success.
Zeolites and porous carbon materials offer robust alternatives with good thermal stability, but generally demonstrate lower CO2 selectivity in the presence of water vapor—a critical limitation for flue gas applications where moisture is invariably present. Recent modifications incorporating amine functionalization have shown promise in addressing these selectivity issues.
Membrane technologies have advanced considerably, with polymeric and mixed matrix membranes achieving CO2 permeabilities above 1,000 Barrer while maintaining selectivity. However, the performance trade-off between permeability and selectivity (Robeson upper bound) continues to constrain practical applications, particularly at industrial scales requiring high throughput.
Geographically, research leadership in carbon capture materials shows distinct patterns. North America and Europe dominate in fundamental research and patent filings, particularly for novel MOFs and advanced membrane materials. China has rapidly expanded its research footprint, focusing heavily on scaled applications and cost reduction for existing technologies. Japan leads in specialized areas including ceramic-based membranes and hybrid capture systems.
Key technical challenges across all material classes include: energy penalty reduction (targeting below 2 GJ/tonne CO2), material stability under realistic industrial conditions (high temperatures, contaminants, cycling), manufacturing scalability, and cost-effectiveness (current materials contribute to capture costs of $40-80/tonne CO2, significantly above economically viable targets of $20-30/tonne).
Additionally, most current materials are optimized for post-combustion capture at atmospheric pressure, leaving significant gaps in materials designed specifically for pre-combustion or direct air capture applications, which represent growing market segments requiring specialized material properties.
Current Advanced Material Solutions for Carbon Capture
01 Metal-Organic Frameworks (MOFs) for Carbon Capture
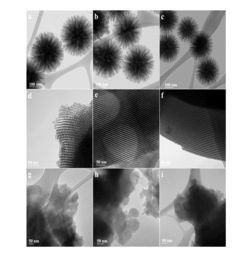
Metal-Organic Frameworks represent a class of advanced porous materials with exceptional CO2 adsorption capabilities due to their high surface area and tunable pore structures. These crystalline materials consist of metal ions coordinated to organic ligands, creating three-dimensional structures with precisely engineered pore sizes that can selectively capture carbon dioxide molecules. MOFs can be designed with specific functional groups to enhance CO2 binding affinity and selectivity over other gases, making them highly efficient for carbon capture applications in both pre-combustion and post-combustion processes.- Metal-Organic Frameworks (MOFs) for Carbon Capture: Metal-Organic Frameworks represent a class of advanced porous materials with exceptional surface area and tunable pore structures that make them highly effective for carbon dioxide adsorption. These crystalline materials consist of metal ions or clusters coordinated with organic ligands, creating three-dimensional structures with customizable pore sizes and chemical functionalities. MOFs can be designed with specific binding sites for CO2, allowing for selective capture even in mixed gas streams. Their high adsorption capacity and regeneration capabilities make them promising candidates for industrial-scale carbon capture applications.
- Carbon-Based Adsorbents and Composites: Advanced carbon-based materials, including activated carbons, carbon nanotubes, graphene, and carbon composites, offer significant potential for carbon capture applications. These materials can be functionalized with various chemical groups to enhance CO2 selectivity and adsorption capacity. Carbon-based adsorbents typically feature high surface areas, good thermal stability, and can be produced from sustainable or waste sources. Composite materials combining carbon with other components such as polymers or metal oxides can achieve synergistic effects, improving capture performance while maintaining mechanical stability and reducing regeneration energy requirements.
- Membrane Technologies for CO2 Separation: Advanced membrane materials offer a promising approach for carbon capture through selective gas separation. These membranes can be fabricated from polymers, ceramics, or hybrid materials with tailored pore structures and surface chemistries that allow CO2 to permeate while blocking other gases. Mixed matrix membranes incorporating nanomaterials can achieve enhanced selectivity and permeability. Membrane-based carbon capture systems offer advantages including continuous operation, compact design, and potentially lower energy requirements compared to traditional absorption processes. Recent innovations focus on improving membrane stability under industrial conditions and scaling up for commercial applications.
- Novel Sorbents and Chemical Looping Materials: Innovative sorbent materials and chemical looping systems represent cutting-edge approaches for carbon capture. These include advanced solid sorbents like amine-functionalized silicas, hydrotalcites, and calcium-based materials that can chemically bind CO2. Chemical looping combustion and reforming processes utilize metal oxides as oxygen carriers that can simultaneously capture carbon dioxide. These materials are designed to operate across various temperature ranges with high CO2 selectivity and minimal degradation over multiple capture-regeneration cycles. Research focuses on enhancing sorption capacity, improving kinetics, and developing materials that require less energy for regeneration.
- Biomimetic and Bioinspired Carbon Capture Materials: Biomimetic approaches to carbon capture draw inspiration from natural systems that efficiently process CO2, such as enzymes like carbonic anhydrase. These advanced materials incorporate biological principles or components to achieve selective and energy-efficient carbon capture. Enzyme-immobilized systems, artificial photosynthesis platforms, and materials that mimic plant or marine organism CO2 processing mechanisms represent this emerging category. Bioinspired materials often operate under mild conditions and can achieve high selectivity. Research in this area focuses on improving stability, scaling up production, and integrating these materials into practical carbon capture systems that can function in industrial environments.
02 Amine-Functionalized Adsorbents
Amine-functionalized materials represent a significant advancement in carbon capture technology, utilizing chemical adsorption mechanisms to bind CO2. These materials incorporate various amine groups onto support structures such as silica, polymers, or porous carbons, creating strong chemical bonds with carbon dioxide molecules. The amine functionality can be introduced through impregnation, grafting, or polymerization methods, resulting in materials with high CO2 selectivity even at low concentrations. These adsorbents demonstrate excellent performance in humid conditions and can be regenerated at relatively low temperatures, making them suitable for industrial carbon capture applications.Expand Specific Solutions03 Carbon-Based Nanomaterials for CO2 Adsorption
Advanced carbon-based nanomaterials, including graphene, carbon nanotubes, and porous carbon structures, offer promising solutions for carbon capture. These materials feature high surface areas, controllable pore structures, and can be functionalized to enhance CO2 adsorption capacity. The carbon framework provides excellent thermal and chemical stability while allowing for rapid adsorption-desorption cycles. Various modification techniques, such as nitrogen doping, metal incorporation, and surface oxidation, can significantly improve the CO2 selectivity and capacity of these carbon-based materials, making them effective for both direct air capture and point-source carbon capture applications.Expand Specific Solutions04 Membrane Technologies for CO2 Separation
Advanced membrane technologies represent an energy-efficient approach to carbon capture, utilizing selective permeation to separate CO2 from gas mixtures. These membranes can be fabricated from polymers, ceramics, or composite materials with specifically engineered pore structures and surface chemistry to enhance CO2 transport while restricting other gases. Mixed matrix membranes, which incorporate selective fillers like MOFs or zeolites into polymer matrices, offer improved permeability and selectivity. Facilitated transport membranes containing carriers that reversibly react with CO2 provide enhanced separation performance. These membrane technologies can operate continuously with lower energy requirements compared to traditional solvent-based capture systems.Expand Specific Solutions05 Hybrid and Composite Materials for Enhanced Carbon Capture
Hybrid and composite materials combine the advantages of different material classes to create synergistic carbon capture systems with superior performance. These include polymer-inorganic composites, hierarchical porous structures, and multi-functional adsorbents that integrate physical and chemical capture mechanisms. By combining materials with complementary properties, these hybrids can achieve higher CO2 selectivity, improved stability under various operating conditions, and enhanced regeneration capabilities. Advanced manufacturing techniques like 3D printing and controlled assembly methods enable precise structural control of these composite materials, optimizing their performance for specific carbon capture applications while minimizing energy requirements for regeneration.Expand Specific Solutions
Leading Organizations in Carbon Capture Materials Research
The carbon capture technology market is currently in a growth phase, with increasing global focus on climate change mitigation driving expansion. The market is projected to reach significant scale as carbon reduction commitments accelerate worldwide. Technologically, the field shows varying maturity levels across different capture approaches. Leading players include established energy corporations like Sinopec, ExxonMobil, and PETRONAS, who leverage their industrial infrastructure for large-scale implementation. Specialized innovators such as Climeworks AG are pioneering direct air capture technologies, while academic institutions including MIT, Rice University, and KAUST contribute cutting-edge research on novel materials and processes. The competitive landscape reflects a blend of traditional energy companies pivoting toward sustainability and purpose-built carbon capture ventures developing next-generation solutions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced carbon capture materials including modified zeolites and metal-organic frameworks (MOFs) with exceptional CO2 selectivity. Their proprietary amine-functionalized mesoporous silica materials demonstrate CO2 adsorption capacities exceeding 5.2 mmol/g under flue gas conditions[5]. Sinopec has pioneered composite membrane materials that combine the mechanical strength of polymers with the selectivity of inorganic components, achieving CO2/N2 selectivity ratios above 50 with good permeability[6]. Their research includes carbon molecular sieve membranes that can operate at temperatures up to 200°C while maintaining structural integrity. For post-combustion capture, Sinopec has developed novel solvent formulations that reduce regeneration energy by approximately 30% compared to conventional MEA solvents, with demonstrated stability over 5,000+ hours of operation at their Sinopec Qilu Petrochemical demonstration project, which captures 40,000 tons of CO2 annually[7].
Strengths: Extensive industrial infrastructure allows for rapid scaling and implementation across multiple facilities. Their materials are designed for integration with existing industrial processes, reducing retrofit costs. Weaknesses: Their materials optimization focuses primarily on point-source capture rather than direct air capture. Some of their advanced materials face challenges with moisture sensitivity and long-term stability in industrial environments.
Climeworks AG
Technical Solution: Climeworks has pioneered direct air capture (DAC) technology using innovative adsorbent materials. Their proprietary filter material consists of porous granulates functionalized with amines that selectively bind CO2 when air passes through collectors. The material can be regenerated at relatively low temperatures (80-100°C) compared to traditional sorbents, reducing energy requirements[2]. Their latest generation materials can capture CO2 for less than $600 per ton, representing a significant cost reduction from earlier iterations[4]. Climeworks' modular collectors are arranged in stackable units, with each collector containing multiple layers of the specialized sorbent material. The company has demonstrated long-term stability of their materials, with minimal degradation after thousands of adsorption-desorption cycles in field conditions. Their Orca plant in Iceland combines these advanced materials with geothermal energy for regeneration and permanent storage through mineralization in basaltic rock formations.
Strengths: Purpose-built for atmospheric carbon removal with proven commercial-scale implementation. Their materials work effectively at atmospheric CO2 concentrations and can be powered by renewable energy. Weaknesses: Current materials still face high costs compared to point-source capture technologies. The energy requirements for material regeneration remain significant despite improvements.
Key Innovations in Carbon Capture Material Science
Materials for gas capture, methods of making materials for gas capture, and methods of capturing gas
PatentActiveUS20160129386A1
Innovation
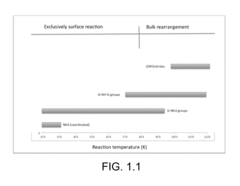
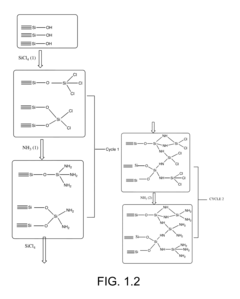
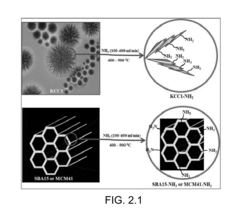
- The development of ammonolyated/nitridated materials, such as silica, metal oxides, and non-metal oxides, which are dehydroxylated and exposed to ammonia at various temperatures to form materials with enhanced CO2 capture capabilities, thermal stability, and clean synthesis processes.
Selective carbon binding on carbon quantum dots
PatentPendingUS20240075453A1
Innovation
- Carbon quantum dots (CQDs) are synthesized from lignin and modified with nitrogen doping or amine functionalization to enhance selective CO2 adsorption over N2 and O2, optimizing their size and composition for improved selectivity and adsorption capacity.
Environmental Impact Assessment of Carbon Capture Materials
The environmental impact assessment of carbon capture materials is a critical component in evaluating the sustainability of carbon capture technologies. While these materials aim to mitigate climate change by reducing atmospheric CO2, their production, deployment, and end-of-life management carry environmental implications that must be thoroughly examined.
Life cycle assessment (LCA) studies reveal that traditional carbon capture materials such as monoethanolamine (MEA) and other amine-based sorbents often require energy-intensive manufacturing processes. The production of these materials involves chemical synthesis routes that may generate hazardous byproducts and consume significant water resources. For instance, the synthesis of zeolites and metal-organic frameworks (MOFs) typically requires organic solvents that can contribute to air and water pollution if not properly managed.
During operational phases, the environmental footprint of carbon capture materials extends beyond their carbon reduction benefits. Amine degradation in post-combustion capture systems can lead to emissions of ammonia and other nitrogen compounds that contribute to air pollution and potential eutrophication of water bodies. Additionally, the regeneration of capture materials often demands substantial energy inputs, potentially offsetting a portion of the climate benefits if powered by fossil fuel sources.
Water consumption represents another significant environmental concern. Wet scrubbing technologies utilizing aqueous amine solutions require cooling water for absorption processes, with consumption rates estimated at 0.7-2.0 cubic meters per ton of CO2 captured. This water intensity becomes particularly problematic in water-stressed regions, where carbon capture deployment may compete with agricultural and municipal water needs.
Advanced materials such as porous polymers and functionalized nanomaterials present their own environmental challenges. While these materials often demonstrate superior capture performance, their nanoscale properties may pose ecotoxicological risks if released into the environment. Current research indicates potential bioaccumulation concerns for certain nanomaterials used in carbon capture applications, though long-term environmental fate studies remain limited.
End-of-life management of spent carbon capture materials constitutes an emerging environmental challenge. The disposal or regeneration of saturated sorbents may release captured carbon back to the atmosphere if not properly handled. Furthermore, specialized materials like ionic liquids and certain MOFs contain heavy metals or persistent organic components that require careful waste management protocols to prevent environmental contamination.
Comparative assessments indicate that biologically-derived capture materials, such as chitosan-based sorbents and enzyme-immobilized systems, generally demonstrate lower environmental impacts across production phases. However, these materials often face durability limitations that necessitate more frequent replacement, potentially increasing lifecycle resource consumption.
Life cycle assessment (LCA) studies reveal that traditional carbon capture materials such as monoethanolamine (MEA) and other amine-based sorbents often require energy-intensive manufacturing processes. The production of these materials involves chemical synthesis routes that may generate hazardous byproducts and consume significant water resources. For instance, the synthesis of zeolites and metal-organic frameworks (MOFs) typically requires organic solvents that can contribute to air and water pollution if not properly managed.
During operational phases, the environmental footprint of carbon capture materials extends beyond their carbon reduction benefits. Amine degradation in post-combustion capture systems can lead to emissions of ammonia and other nitrogen compounds that contribute to air pollution and potential eutrophication of water bodies. Additionally, the regeneration of capture materials often demands substantial energy inputs, potentially offsetting a portion of the climate benefits if powered by fossil fuel sources.
Water consumption represents another significant environmental concern. Wet scrubbing technologies utilizing aqueous amine solutions require cooling water for absorption processes, with consumption rates estimated at 0.7-2.0 cubic meters per ton of CO2 captured. This water intensity becomes particularly problematic in water-stressed regions, where carbon capture deployment may compete with agricultural and municipal water needs.
Advanced materials such as porous polymers and functionalized nanomaterials present their own environmental challenges. While these materials often demonstrate superior capture performance, their nanoscale properties may pose ecotoxicological risks if released into the environment. Current research indicates potential bioaccumulation concerns for certain nanomaterials used in carbon capture applications, though long-term environmental fate studies remain limited.
End-of-life management of spent carbon capture materials constitutes an emerging environmental challenge. The disposal or regeneration of saturated sorbents may release captured carbon back to the atmosphere if not properly handled. Furthermore, specialized materials like ionic liquids and certain MOFs contain heavy metals or persistent organic components that require careful waste management protocols to prevent environmental contamination.
Comparative assessments indicate that biologically-derived capture materials, such as chitosan-based sorbents and enzyme-immobilized systems, generally demonstrate lower environmental impacts across production phases. However, these materials often face durability limitations that necessitate more frequent replacement, potentially increasing lifecycle resource consumption.
Cost-Benefit Analysis of Advanced Carbon Capture Materials
The economic viability of advanced carbon capture materials represents a critical factor in their widespread adoption. Current cost analyses indicate that traditional carbon capture technologies require significant capital investment, with estimates ranging from $40-$80 per ton of CO2 captured using conventional amine-based sorbents. Advanced materials such as metal-organic frameworks (MOFs), zeolites, and functionalized porous polymers demonstrate potential for reducing these costs by 30-45% through improved capture efficiency and reduced regeneration energy requirements.
When evaluating cost-benefit ratios, MOFs present particularly promising economics with theoretical capture capacities exceeding 1.5 g CO2/g sorbent under optimal conditions, compared to 0.4-0.5 g CO2/g for conventional materials. This translates to smaller equipment footprints and reduced capital expenditure. However, manufacturing scalability remains a challenge, with current production costs for advanced MOFs ranging from $100-$1000/kg depending on complexity and purity requirements.
Energy consumption during the regeneration phase constitutes approximately 70% of operational expenses in carbon capture systems. Advanced materials demonstrating lower regeneration temperatures (60-80°C versus 120-150°C for conventional amines) offer potential energy savings of 2-3 GJ/ton CO2, representing annual operational cost reductions of $15-25 per ton captured when implemented at industrial scale.
Lifecycle assessments reveal that while advanced materials may have higher initial production environmental footprints, their extended operational lifespans (3-5 years versus 1-2 years for conventional sorbents) and superior performance characteristics yield net positive environmental returns within 6-18 months of deployment. This improved durability directly impacts maintenance schedules and replacement costs, reducing total ownership expenses by an estimated 20-35%.
Market sensitivity analysis suggests that with carbon pricing mechanisms in the range of $50-100 per ton CO2, advanced materials become economically competitive across multiple industrial sectors. Early adopters in power generation and cement manufacturing report payback periods of 3-7 years, depending on facility scale and regional energy costs. Government incentives and carbon tax structures can further improve these economics, potentially reducing payback periods to 2-4 years in favorable regulatory environments.
Integration costs with existing infrastructure remain a significant consideration, with retrofitting expenses typically adding 15-30% to initial implementation costs. However, modular designs utilizing advanced materials can reduce these integration challenges while providing flexibility for capacity expansion as technologies mature and economies of scale improve manufacturing economics.
When evaluating cost-benefit ratios, MOFs present particularly promising economics with theoretical capture capacities exceeding 1.5 g CO2/g sorbent under optimal conditions, compared to 0.4-0.5 g CO2/g for conventional materials. This translates to smaller equipment footprints and reduced capital expenditure. However, manufacturing scalability remains a challenge, with current production costs for advanced MOFs ranging from $100-$1000/kg depending on complexity and purity requirements.
Energy consumption during the regeneration phase constitutes approximately 70% of operational expenses in carbon capture systems. Advanced materials demonstrating lower regeneration temperatures (60-80°C versus 120-150°C for conventional amines) offer potential energy savings of 2-3 GJ/ton CO2, representing annual operational cost reductions of $15-25 per ton captured when implemented at industrial scale.
Lifecycle assessments reveal that while advanced materials may have higher initial production environmental footprints, their extended operational lifespans (3-5 years versus 1-2 years for conventional sorbents) and superior performance characteristics yield net positive environmental returns within 6-18 months of deployment. This improved durability directly impacts maintenance schedules and replacement costs, reducing total ownership expenses by an estimated 20-35%.
Market sensitivity analysis suggests that with carbon pricing mechanisms in the range of $50-100 per ton CO2, advanced materials become economically competitive across multiple industrial sectors. Early adopters in power generation and cement manufacturing report payback periods of 3-7 years, depending on facility scale and regional energy costs. Government incentives and carbon tax structures can further improve these economics, potentially reducing payback periods to 2-4 years in favorable regulatory environments.
Integration costs with existing infrastructure remain a significant consideration, with retrofitting expenses typically adding 15-30% to initial implementation costs. However, modular designs utilizing advanced materials can reduce these integration challenges while providing flexibility for capacity expansion as technologies mature and economies of scale improve manufacturing economics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!