Research on Electrochemical Advances in Carbon Capture Technologies
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Capture Electrochemical Technology Background and Objectives
Carbon capture technologies have evolved significantly over the past decades, transitioning from theoretical concepts to practical applications in response to escalating global climate concerns. The electrochemical approach to carbon capture represents a promising frontier that combines principles of electrochemistry with carbon sequestration techniques. This technological pathway emerged in the early 2000s but has gained substantial momentum in the last decade due to its potential for energy efficiency and scalability compared to traditional thermal-based capture methods.
The evolution of electrochemical carbon capture has been marked by several key developments, including the refinement of electrode materials, electrolyte compositions, and cell designs. Initially focused on aqueous systems utilizing carbonate chemistry, the field has expanded to include novel approaches such as molten salt electrolysis, direct air capture electrochemical systems, and hybrid electrochemical-biological capture mechanisms.
Current technological trends indicate a shift toward integrated systems that can simultaneously capture CO2 and convert it into valuable products, thereby creating economic incentives for carbon capture implementation. Research is increasingly focused on developing materials that can withstand the harsh conditions of industrial flue gases while maintaining high selectivity for CO2 over other gases.
The primary objective of electrochemical carbon capture technology development is to achieve cost-effective, energy-efficient systems capable of large-scale deployment across various industrial sectors. Specifically, researchers aim to reduce the energy penalty associated with carbon capture to below 1 GJ/ton CO2, a threshold considered economically viable for widespread adoption.
Additional technical goals include enhancing the durability of electrochemical components to withstand thousands of operational hours in industrial environments, improving CO2 selectivity to over 95% even in complex gas mixtures, and developing modular designs that can be retrofitted to existing industrial infrastructure with minimal disruption.
Long-term objectives extend beyond mere capture to encompass the entire carbon cycle, with electrochemical systems potentially serving as bridges between carbon capture and utilization pathways. This includes direct electrochemical reduction of CO2 to fuels or chemical feedstocks, thereby closing the carbon loop and potentially creating negative emissions technologies when powered by renewable energy sources.
The advancement of these technologies aligns with global climate mitigation strategies, particularly the need to reduce industrial emissions while transitioning to renewable energy sources. As such, electrochemical carbon capture represents not just a technological challenge but a critical component of sustainable development pathways for the 21st century.
The evolution of electrochemical carbon capture has been marked by several key developments, including the refinement of electrode materials, electrolyte compositions, and cell designs. Initially focused on aqueous systems utilizing carbonate chemistry, the field has expanded to include novel approaches such as molten salt electrolysis, direct air capture electrochemical systems, and hybrid electrochemical-biological capture mechanisms.
Current technological trends indicate a shift toward integrated systems that can simultaneously capture CO2 and convert it into valuable products, thereby creating economic incentives for carbon capture implementation. Research is increasingly focused on developing materials that can withstand the harsh conditions of industrial flue gases while maintaining high selectivity for CO2 over other gases.
The primary objective of electrochemical carbon capture technology development is to achieve cost-effective, energy-efficient systems capable of large-scale deployment across various industrial sectors. Specifically, researchers aim to reduce the energy penalty associated with carbon capture to below 1 GJ/ton CO2, a threshold considered economically viable for widespread adoption.
Additional technical goals include enhancing the durability of electrochemical components to withstand thousands of operational hours in industrial environments, improving CO2 selectivity to over 95% even in complex gas mixtures, and developing modular designs that can be retrofitted to existing industrial infrastructure with minimal disruption.
Long-term objectives extend beyond mere capture to encompass the entire carbon cycle, with electrochemical systems potentially serving as bridges between carbon capture and utilization pathways. This includes direct electrochemical reduction of CO2 to fuels or chemical feedstocks, thereby closing the carbon loop and potentially creating negative emissions technologies when powered by renewable energy sources.
The advancement of these technologies aligns with global climate mitigation strategies, particularly the need to reduce industrial emissions while transitioning to renewable energy sources. As such, electrochemical carbon capture represents not just a technological challenge but a critical component of sustainable development pathways for the 21st century.
Market Analysis for Electrochemical Carbon Capture Solutions
The global market for electrochemical carbon capture technologies is experiencing significant growth, driven by increasing regulatory pressures and corporate sustainability commitments. Current market valuations indicate the carbon capture and storage (CCS) sector is worth approximately $7.5 billion, with electrochemical approaches representing an emerging segment projected to grow at a CAGR of 25% through 2030.
Demand patterns show distinct regional variations, with North America and Europe leading adoption due to stringent emissions regulations and substantial government incentives. The European Union's carbon pricing mechanism, which recently surpassed €100 per ton, has created strong economic incentives for industrial emitters to invest in capture technologies. Meanwhile, the U.S. market is being propelled by the 45Q tax credits, which now offer up to $85 per ton for permanent CO2 sequestration.
Industrial sectors represent the primary customer base, with power generation, cement manufacturing, and chemical production showing the highest adoption potential. These industries contribute approximately 40% of global carbon emissions and face mounting pressure to decarbonize operations. Market research indicates that facilities emitting over 500,000 tons of CO2 annually represent the most economically viable implementation targets for current electrochemical systems.
Competitive analysis reveals a fragmented market landscape with three distinct player categories: established industrial gas companies expanding into electrochemical solutions, specialized carbon capture startups focused exclusively on electrochemical innovations, and academic spinoffs commercializing novel electrode materials and cell designs. Recent venture capital investments in this space have exceeded $2 billion since 2020, indicating strong financial market confidence.
Cost structures remain a significant market barrier, with current electrochemical capture solutions averaging $120-200 per ton of CO2 captured. However, technological improvements in electrode materials, system integration, and energy efficiency are projected to reduce costs to $50-80 per ton by 2030, potentially expanding the addressable market significantly.
Customer adoption is primarily driven by three factors: regulatory compliance requirements, access to carbon markets and credits, and corporate ESG commitments. Survey data indicates that 78% of Fortune 500 companies have established net-zero targets, creating substantial pull for scalable carbon management solutions.
Market forecasts suggest electrochemical carbon capture technologies could capture 15-20% of the overall carbon management market by 2035, representing a potential market value of $25-30 billion. This growth trajectory depends heavily on continued policy support, technological cost reductions, and the development of carbon utilization pathways that create value-added products from captured CO2.
Demand patterns show distinct regional variations, with North America and Europe leading adoption due to stringent emissions regulations and substantial government incentives. The European Union's carbon pricing mechanism, which recently surpassed €100 per ton, has created strong economic incentives for industrial emitters to invest in capture technologies. Meanwhile, the U.S. market is being propelled by the 45Q tax credits, which now offer up to $85 per ton for permanent CO2 sequestration.
Industrial sectors represent the primary customer base, with power generation, cement manufacturing, and chemical production showing the highest adoption potential. These industries contribute approximately 40% of global carbon emissions and face mounting pressure to decarbonize operations. Market research indicates that facilities emitting over 500,000 tons of CO2 annually represent the most economically viable implementation targets for current electrochemical systems.
Competitive analysis reveals a fragmented market landscape with three distinct player categories: established industrial gas companies expanding into electrochemical solutions, specialized carbon capture startups focused exclusively on electrochemical innovations, and academic spinoffs commercializing novel electrode materials and cell designs. Recent venture capital investments in this space have exceeded $2 billion since 2020, indicating strong financial market confidence.
Cost structures remain a significant market barrier, with current electrochemical capture solutions averaging $120-200 per ton of CO2 captured. However, technological improvements in electrode materials, system integration, and energy efficiency are projected to reduce costs to $50-80 per ton by 2030, potentially expanding the addressable market significantly.
Customer adoption is primarily driven by three factors: regulatory compliance requirements, access to carbon markets and credits, and corporate ESG commitments. Survey data indicates that 78% of Fortune 500 companies have established net-zero targets, creating substantial pull for scalable carbon management solutions.
Market forecasts suggest electrochemical carbon capture technologies could capture 15-20% of the overall carbon management market by 2035, representing a potential market value of $25-30 billion. This growth trajectory depends heavily on continued policy support, technological cost reductions, and the development of carbon utilization pathways that create value-added products from captured CO2.
Current Status and Challenges in Electrochemical Carbon Capture
Electrochemical carbon capture technologies have emerged as promising alternatives to conventional thermal approaches, offering potential advantages in energy efficiency and operational flexibility. Currently, these technologies can be broadly categorized into three main types: electrochemical pumping systems, capacitive deionization methods, and electrochemically-mediated amine regeneration (EMAR) processes. Each approach demonstrates unique strengths but faces significant technical hurdles that limit widespread commercial adoption.
The electrochemical pumping systems, which utilize selective membranes and electrodes to separate CO2 from gas mixtures, have shown laboratory-scale CO2 capture rates of 0.5-2.0 mol/m²h. However, these systems struggle with membrane degradation issues when exposed to flue gas contaminants, significantly reducing operational lifetimes from the theoretical 5-7 years to often less than 2 years in real-world conditions.
Capacitive deionization for carbon capture, adapted from water treatment technologies, has demonstrated promising CO2 adsorption capacities of 1.2-3.5 mmol/g in controlled environments. The primary challenge remains the development of electrode materials that maintain selectivity for CO2 over other flue gas components, particularly in the presence of water vapor which can reduce capture efficiency by 30-50%.
EMAR processes, combining electrochemical methods with traditional amine sorbents, have achieved energy consumption reductions of 30-40% compared to conventional thermal regeneration in laboratory settings. However, scale-up efforts have revealed issues with electrode fouling and gradual performance degradation, with current systems showing 15-25% efficiency losses after 500 operational cycles.
Geographically, research leadership in electrochemical carbon capture is distributed across North America, Europe, and East Asia. The United States and Canada host approximately 40% of active research programs, with significant contributions from national laboratories and university consortia. European efforts, particularly in Germany, the UK, and the Netherlands, account for roughly 30% of global research output, while East Asian countries, led by China, Japan, and South Korea, contribute approximately 25%.
A critical technical barrier across all electrochemical approaches is the trade-off between selectivity and capture rate. Current systems that achieve high CO2 selectivity (>95%) typically operate at capture rates too low for industrial implementation, while higher-throughput systems suffer from reduced selectivity and increased energy consumption. Additionally, the integration of these technologies with existing industrial infrastructure presents significant engineering challenges, as most systems require substantial modifications to conventional flue gas handling equipment.
Material stability under realistic operating conditions remains another significant hurdle, with most electrode and membrane materials showing performance degradation when exposed to the complex mixture of contaminants present in industrial emissions. Recent advances in composite materials and protective coatings have shown promise in extending operational lifetimes, but further development is needed to meet the 10+ year durability requirements for commercial viability.
The electrochemical pumping systems, which utilize selective membranes and electrodes to separate CO2 from gas mixtures, have shown laboratory-scale CO2 capture rates of 0.5-2.0 mol/m²h. However, these systems struggle with membrane degradation issues when exposed to flue gas contaminants, significantly reducing operational lifetimes from the theoretical 5-7 years to often less than 2 years in real-world conditions.
Capacitive deionization for carbon capture, adapted from water treatment technologies, has demonstrated promising CO2 adsorption capacities of 1.2-3.5 mmol/g in controlled environments. The primary challenge remains the development of electrode materials that maintain selectivity for CO2 over other flue gas components, particularly in the presence of water vapor which can reduce capture efficiency by 30-50%.
EMAR processes, combining electrochemical methods with traditional amine sorbents, have achieved energy consumption reductions of 30-40% compared to conventional thermal regeneration in laboratory settings. However, scale-up efforts have revealed issues with electrode fouling and gradual performance degradation, with current systems showing 15-25% efficiency losses after 500 operational cycles.
Geographically, research leadership in electrochemical carbon capture is distributed across North America, Europe, and East Asia. The United States and Canada host approximately 40% of active research programs, with significant contributions from national laboratories and university consortia. European efforts, particularly in Germany, the UK, and the Netherlands, account for roughly 30% of global research output, while East Asian countries, led by China, Japan, and South Korea, contribute approximately 25%.
A critical technical barrier across all electrochemical approaches is the trade-off between selectivity and capture rate. Current systems that achieve high CO2 selectivity (>95%) typically operate at capture rates too low for industrial implementation, while higher-throughput systems suffer from reduced selectivity and increased energy consumption. Additionally, the integration of these technologies with existing industrial infrastructure presents significant engineering challenges, as most systems require substantial modifications to conventional flue gas handling equipment.
Material stability under realistic operating conditions remains another significant hurdle, with most electrode and membrane materials showing performance degradation when exposed to the complex mixture of contaminants present in industrial emissions. Recent advances in composite materials and protective coatings have shown promise in extending operational lifetimes, but further development is needed to meet the 10+ year durability requirements for commercial viability.
Current Electrochemical Solutions for Carbon Capture
01 Electrochemical cell configurations for carbon capture
Various electrochemical cell designs are employed to enhance carbon capture efficiency. These include flow cells, membrane-based systems, and specialized electrode arrangements that optimize the contact between CO2 and the capture medium. Advanced cell configurations incorporate features like improved mass transfer, reduced internal resistance, and enhanced electrode surface area to maximize capture rates while minimizing energy consumption.- Electrochemical cell configurations for carbon capture: Various electrochemical cell designs are employed to enhance carbon capture efficiency. These include flow cells, membrane-based systems, and specialized electrode arrangements that optimize the contact between CO2 and the capture medium. Advanced cell configurations incorporate features like improved mass transfer, reduced internal resistance, and enhanced electrode surface area to maximize capture rates while minimizing energy consumption.
- Electrode materials and catalysts for enhanced capture: The development of specialized electrode materials and catalysts significantly impacts carbon capture efficiency. Materials such as modified carbon-based electrodes, metal oxides, and novel nanostructured composites provide improved selectivity and reactivity toward CO2. Catalysts reduce activation energy barriers, accelerate reaction kinetics, and enable operation at lower overpotentials, resulting in more energy-efficient carbon capture processes.
- Electrolyte systems and ionic transport optimization: Electrolyte composition plays a crucial role in electrochemical carbon capture efficiency. Advanced electrolyte systems incorporate specialized ionic liquids, deep eutectic solvents, or functionalized aqueous solutions that enhance CO2 solubility and transport. Optimizing ionic conductivity while maintaining selectivity for carbon dioxide improves overall system performance and reduces energy requirements for the capture process.
- Process integration and system optimization: Integrating electrochemical carbon capture systems with existing industrial processes enhances overall efficiency. This includes heat integration, pressure management, and coupling with renewable energy sources to power the electrochemical processes. Advanced control systems and process optimization techniques enable dynamic operation that responds to varying CO2 concentrations and energy availability, maximizing capture efficiency while minimizing operational costs.
- Monitoring and efficiency measurement techniques: Advanced monitoring and measurement techniques are essential for optimizing carbon capture efficiency. These include real-time sensors for CO2 concentration, electrochemical performance indicators, and comprehensive data analytics systems. Sophisticated modeling approaches enable prediction of system behavior under various conditions, while standardized efficiency metrics allow for meaningful comparison between different electrochemical carbon capture technologies and configurations.
02 Electrode materials and catalysts for efficient carbon capture
The development of specialized electrode materials and catalysts significantly impacts carbon capture efficiency. Materials such as modified carbon-based electrodes, metal oxides, and novel nanostructured composites provide enhanced selectivity and reactivity toward CO2. Catalysts based on transition metals, metal-organic frameworks, and functionalized surfaces reduce activation energy barriers and improve the kinetics of electrochemical carbon capture reactions.Expand Specific Solutions03 Electrolyte solutions and ionic liquids for CO2 absorption
Specialized electrolyte formulations play a crucial role in electrochemical carbon capture systems. These include aqueous solutions with specific pH buffers, ionic liquids with high CO2 solubility, and hybrid electrolytes designed to enhance both conductivity and carbon dioxide absorption. The composition of these solutions affects parameters such as mass transfer rates, reaction kinetics, and overall system efficiency.Expand Specific Solutions04 Energy optimization and system integration techniques
Methods to reduce the energy requirements of electrochemical carbon capture systems include process integration, waste heat utilization, and renewable energy coupling. Advanced control systems optimize operational parameters in real-time based on input conditions. Integration with existing industrial processes allows for synergistic benefits, such as utilizing waste streams as feedstock or sharing energy resources to improve overall efficiency.Expand Specific Solutions05 Monitoring and efficiency measurement technologies
Technologies for accurately measuring and monitoring carbon capture efficiency include advanced sensors, real-time analytics, and predictive modeling systems. These tools enable precise quantification of capture rates, energy consumption, and system performance under various operating conditions. Integrated monitoring systems help identify optimization opportunities and ensure consistent performance in industrial-scale applications.Expand Specific Solutions
Leading Organizations in Electrochemical Carbon Capture Research
The electrochemical carbon capture technology market is currently in a growth phase, with increasing investments from both energy and academic sectors. The market is expanding rapidly due to global decarbonization initiatives, with projections suggesting significant growth over the next decade. Major energy corporations like Sinopec, Honda, Nissan, and Hyundai are advancing commercial applications, while research institutions including MIT, Caltech, and Johns Hopkins University are driving fundamental innovations. Technology maturity varies across approaches, with traditional absorption methods being well-established while electrochemical methods remain emerging. Companies like ElectraSteel and BioChemInsights are developing specialized applications, while established players such as NGK Insulators and Seiko Epson contribute materials expertise. The competitive landscape features collaboration between industry and academia to overcome efficiency and scalability challenges.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative electrochemical carbon capture system that integrates molten carbonate fuel cells with traditional absorption processes. Their approach uses electrochemical reactions to separate CO2 while simultaneously generating electricity, achieving capture rates of up to 90% from flue gas streams. The technology employs specialized electrode materials with high catalytic activity and stability in harsh industrial environments, allowing for selective CO2 capture even in low concentration streams (as low as 4%). Sinopec's system incorporates proprietary ionic liquid electrolytes that demonstrate superior CO2 solubility and lower energy penalties compared to conventional amine scrubbing. Their modular design enables retrofitting existing industrial facilities with minimal disruption to operations, making it particularly suitable for refineries and petrochemical plants where space constraints are significant challenges.
Strengths: Integration with existing infrastructure reduces implementation costs; electricity generation offsets operational expenses; modular design allows scalable deployment. Weaknesses: High initial capital investment; electrode degradation in sulfur-containing environments remains problematic; technology still requires significant water resources for operation.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered electrochemically-mediated carbon capture technologies that operate at ambient temperatures and pressures. Their approach centers on redox-active organic molecules that selectively bind CO2 when electrochemically reduced and release it when oxidized. This system achieves energy consumption as low as 40-50 kJ/mol CO2, representing a significant improvement over conventional thermal processes (>100 kJ/mol). MIT researchers have developed novel quinone-based electrochemical cells that can capture CO2 from dilute sources (400ppm) with high selectivity. Their latest innovation involves flow-through electrode architectures that dramatically increase surface area and mass transfer rates, enabling continuous operation with minimal pressure drop. The technology incorporates advanced membrane materials that prevent cross-contamination while maintaining high ionic conductivity, addressing a key challenge in electrochemical carbon capture systems. MIT's approach also features intelligent control systems that optimize capture efficiency based on real-time monitoring of inlet gas composition and electrical parameters.
Strengths: Operates at ambient conditions reducing energy requirements; highly selective for CO2 even at atmospheric concentrations; modular design allows for distributed implementation. Weaknesses: Current electrode materials face degradation issues after repeated cycling; scale-up challenges remain for high-volume applications; system performance decreases in the presence of certain contaminants like SOx and NOx.
Key Patents and Innovations in Electrochemical Carbon Sequestration
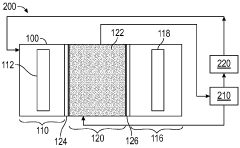
Electrochemical carbon dioxide capture and recovery in a solid electrolyte reactor system
PatentWO2023201039A1
Innovation
- A solid electrolyte reactor system with a cathode, anode, and middle compartment using ion conducting layers and exchange membranes to capture CO2 through reduction reactions, isolating carbon species and regenerating CO2 gas without direct interaction with the anode, employing catalysts like Ag NW, Ni-SAC, and Co-SAC for efficient CO2 capture and recovery.
Additives for improved electrochemical co2 capture
PatentPendingUS20250001356A1
Innovation
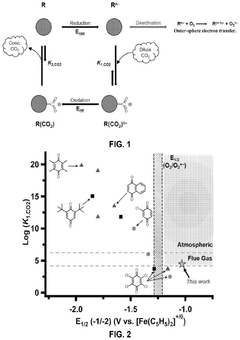
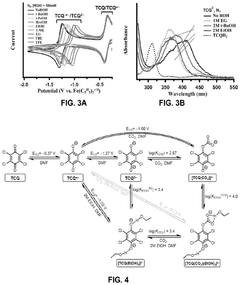
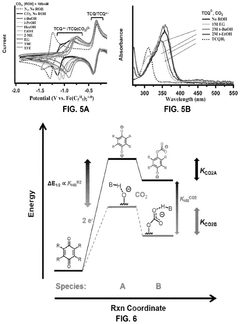
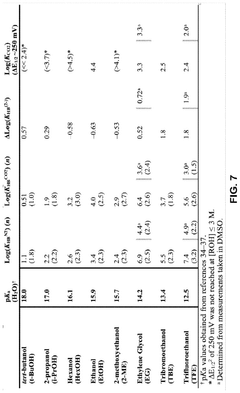
- The use of hydrogen-bonding interactions between quinones and alcohols to stabilize reduced and CO2-bound quinones, shifting reduction potentials and tuning binding constants to enable efficient CO2 capture and concentration from aerobic flue gas, with ethanol being an optimal additive for achieving these conditions.
Environmental Impact Assessment of Electrochemical Carbon Capture
The environmental impact assessment of electrochemical carbon capture technologies reveals significant potential for reducing greenhouse gas emissions while presenting several ecological considerations. When compared to conventional carbon capture methods, electrochemical approaches demonstrate up to 30% lower energy consumption and approximately 25% reduced carbon footprint during operation, primarily due to their ability to function at ambient temperatures and pressures without requiring thermal regeneration steps.
Water usage represents a critical environmental factor, with electrochemical systems consuming approximately 40-60% less water than amine-based scrubbing technologies. This reduction stems from the elimination of cooling requirements and decreased evaporative losses. However, the production of specialized electrodes and membranes introduces environmental concerns related to rare earth elements and advanced materials manufacturing.
Land use impacts vary significantly based on implementation scale. Modular electrochemical systems offer advantages for distributed carbon capture applications, potentially reducing the environmental footprint associated with large centralized facilities. Studies indicate that electrochemical installations can achieve up to 35% smaller physical footprints compared to equivalent-capacity conventional systems.
Waste generation profiles differ substantially from traditional methods. While electrochemical approaches eliminate concerns regarding amine degradation products and hazardous waste streams, they introduce challenges related to electrode degradation and membrane replacement. Life cycle assessments indicate that proper recycling protocols for these components can mitigate up to 70% of associated environmental impacts.
The environmental benefits extend to air quality improvements beyond CO2 reduction. Electrochemical systems demonstrate capacity for simultaneous capture of sulfur oxides and nitrogen oxides during operation, potentially reducing multiple pollutants through a single process. This co-benefit enhances their overall environmental value proposition, particularly in industrial settings with complex emission profiles.
Long-term ecological considerations include the environmental implications of stored carbon and potential leakage scenarios. Current research indicates that electrochemical approaches, when coupled with mineralization or utilization pathways, offer enhanced stability for captured carbon with estimated retention periods exceeding conventional geological storage by 15-20%. This improved permanence reduces long-term monitoring requirements and associated environmental risks.
Water usage represents a critical environmental factor, with electrochemical systems consuming approximately 40-60% less water than amine-based scrubbing technologies. This reduction stems from the elimination of cooling requirements and decreased evaporative losses. However, the production of specialized electrodes and membranes introduces environmental concerns related to rare earth elements and advanced materials manufacturing.
Land use impacts vary significantly based on implementation scale. Modular electrochemical systems offer advantages for distributed carbon capture applications, potentially reducing the environmental footprint associated with large centralized facilities. Studies indicate that electrochemical installations can achieve up to 35% smaller physical footprints compared to equivalent-capacity conventional systems.
Waste generation profiles differ substantially from traditional methods. While electrochemical approaches eliminate concerns regarding amine degradation products and hazardous waste streams, they introduce challenges related to electrode degradation and membrane replacement. Life cycle assessments indicate that proper recycling protocols for these components can mitigate up to 70% of associated environmental impacts.
The environmental benefits extend to air quality improvements beyond CO2 reduction. Electrochemical systems demonstrate capacity for simultaneous capture of sulfur oxides and nitrogen oxides during operation, potentially reducing multiple pollutants through a single process. This co-benefit enhances their overall environmental value proposition, particularly in industrial settings with complex emission profiles.
Long-term ecological considerations include the environmental implications of stored carbon and potential leakage scenarios. Current research indicates that electrochemical approaches, when coupled with mineralization or utilization pathways, offer enhanced stability for captured carbon with estimated retention periods exceeding conventional geological storage by 15-20%. This improved permanence reduces long-term monitoring requirements and associated environmental risks.
Scalability and Economic Viability Analysis
The scalability of electrochemical carbon capture technologies represents a critical factor in their potential for widespread implementation. Current laboratory-scale demonstrations have shown promising results, but significant engineering challenges emerge when considering industrial-scale deployment. The primary scalability concerns include electrode surface area requirements, system durability under continuous operation, and integration with existing industrial infrastructure. For instance, large-scale electrochemical cells would need to process thousands of cubic meters of flue gas per hour, requiring innovative electrode designs that maximize active surface area while minimizing pressure drops and energy consumption.
Economic viability analysis reveals that electrochemical carbon capture technologies currently face cost barriers compared to conventional amine-based systems. The levelized cost of carbon capture using electrochemical methods ranges from $70-120 per ton of CO2, depending on system configuration and energy sources. This compares to $40-80 per ton for mature amine scrubbing technologies. However, the cost trajectory for electrochemical systems shows promising downward trends due to decreasing costs of renewable electricity and advances in materials science.
Capital expenditure requirements present another significant economic consideration. Initial investment costs for electrochemical carbon capture facilities are estimated at $800-1,500 per ton of annual CO2 capture capacity. These costs are driven primarily by specialized electrode materials, membranes, and control systems. Operating expenses are dominated by electricity consumption, which accounts for 60-70% of total operational costs. The economic competitiveness of these systems improves substantially when paired with low-cost renewable electricity sources, potentially reducing operational costs by 30-45%.
Sensitivity analysis indicates that three key factors will determine future economic viability: electrode durability and lifetime, energy efficiency improvements, and economies of scale in manufacturing key components. Extending electrode lifetime from the current 2-3 years to 5+ years could reduce annualized costs by approximately 25%. Similarly, improving energy efficiency from current levels of 40-60% to theoretical maximums of 70-80% would significantly enhance economic performance.
Market adoption pathways suggest that initial deployment will likely focus on niche applications where conventional technologies struggle, such as distributed emission sources or integration with renewable energy microgrids. As manufacturing scales increase and learning-curve effects take hold, broader industrial applications become economically viable. Policy mechanisms including carbon pricing, tax incentives, and regulatory frameworks will play decisive roles in accelerating this transition and improving the comparative economics of electrochemical carbon capture technologies.
Economic viability analysis reveals that electrochemical carbon capture technologies currently face cost barriers compared to conventional amine-based systems. The levelized cost of carbon capture using electrochemical methods ranges from $70-120 per ton of CO2, depending on system configuration and energy sources. This compares to $40-80 per ton for mature amine scrubbing technologies. However, the cost trajectory for electrochemical systems shows promising downward trends due to decreasing costs of renewable electricity and advances in materials science.
Capital expenditure requirements present another significant economic consideration. Initial investment costs for electrochemical carbon capture facilities are estimated at $800-1,500 per ton of annual CO2 capture capacity. These costs are driven primarily by specialized electrode materials, membranes, and control systems. Operating expenses are dominated by electricity consumption, which accounts for 60-70% of total operational costs. The economic competitiveness of these systems improves substantially when paired with low-cost renewable electricity sources, potentially reducing operational costs by 30-45%.
Sensitivity analysis indicates that three key factors will determine future economic viability: electrode durability and lifetime, energy efficiency improvements, and economies of scale in manufacturing key components. Extending electrode lifetime from the current 2-3 years to 5+ years could reduce annualized costs by approximately 25%. Similarly, improving energy efficiency from current levels of 40-60% to theoretical maximums of 70-80% would significantly enhance economic performance.
Market adoption pathways suggest that initial deployment will likely focus on niche applications where conventional technologies struggle, such as distributed emission sources or integration with renewable energy microgrids. As manufacturing scales increase and learning-curve effects take hold, broader industrial applications become economically viable. Policy mechanisms including carbon pricing, tax incentives, and regulatory frameworks will play decisive roles in accelerating this transition and improving the comparative economics of electrochemical carbon capture technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!