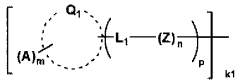Catalyst Developments for Enhanced Proton Battery Efficiency
OCT 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proton Battery Catalyst Evolution and Objectives
Proton batteries represent a promising alternative to conventional lithium-ion energy storage systems, offering potential advantages in sustainability, safety, and energy density. The evolution of catalyst technology for proton batteries has undergone significant transformation since their conceptual introduction in the early 2000s. Initially, researchers focused on adapting hydrogen fuel cell catalysts, primarily platinum-based materials, which demonstrated the fundamental feasibility of proton transfer but suffered from prohibitive costs and limited efficiency in battery applications.
The mid-2010s marked a pivotal shift toward exploring non-precious metal catalysts, particularly carbon-based materials doped with nitrogen, sulfur, or transition metals. This period saw the emergence of graphene-based catalysts with enhanced proton conductivity and stability, addressing some of the early limitations while significantly reducing material costs. Concurrently, metal-organic frameworks (MOFs) began showing promise as tunable platforms for catalyst design, offering unprecedented control over pore structure and active site distribution.
Recent developments have concentrated on nanostructured catalysts with hierarchical architectures that optimize both proton transfer kinetics and electron transport pathways. Particularly noteworthy are the advances in bifunctional catalysts capable of facilitating both hydrogen evolution and oxygen reduction reactions, which are critical for improving round-trip efficiency in proton battery systems. These materials typically incorporate carefully engineered interfaces between different catalytic components to promote synergistic effects.
The primary technical objectives for proton battery catalyst development center around four key parameters: catalytic activity, stability, cost-effectiveness, and scalability. Current research aims to achieve proton transfer rates comparable to those in biological systems, which operate with remarkable efficiency at ambient conditions. This bio-inspired approach has led to increasing interest in enzyme-mimetic catalysts that replicate the active sites of hydrogenases and other proton-processing enzymes.
Another critical objective involves enhancing catalyst durability under the cycling conditions typical of battery operation. Current catalysts often experience degradation through mechanisms such as active site poisoning, structural collapse, or particle agglomeration, limiting practical lifetimes to hundreds rather than thousands of cycles. Addressing these stability challenges requires fundamental understanding of degradation pathways at the molecular level.
From a commercial perspective, catalyst development must balance performance improvements with cost considerations. The target cost threshold for widespread adoption is generally considered to be below $10/kW, necessitating either dramatic reductions in precious metal loading or complete transition to earth-abundant alternatives. This economic imperative has accelerated research into composite materials that maximize activity per unit cost.
The ultimate objective remains the development of catalysts that enable proton batteries to achieve energy densities exceeding 350 Wh/kg at the cell level, with charge/discharge efficiencies above 90% and cycle life beyond 2000 cycles – performance metrics that would position proton batteries as viable competitors to advanced lithium-ion technologies while offering superior sustainability profiles.
The mid-2010s marked a pivotal shift toward exploring non-precious metal catalysts, particularly carbon-based materials doped with nitrogen, sulfur, or transition metals. This period saw the emergence of graphene-based catalysts with enhanced proton conductivity and stability, addressing some of the early limitations while significantly reducing material costs. Concurrently, metal-organic frameworks (MOFs) began showing promise as tunable platforms for catalyst design, offering unprecedented control over pore structure and active site distribution.
Recent developments have concentrated on nanostructured catalysts with hierarchical architectures that optimize both proton transfer kinetics and electron transport pathways. Particularly noteworthy are the advances in bifunctional catalysts capable of facilitating both hydrogen evolution and oxygen reduction reactions, which are critical for improving round-trip efficiency in proton battery systems. These materials typically incorporate carefully engineered interfaces between different catalytic components to promote synergistic effects.
The primary technical objectives for proton battery catalyst development center around four key parameters: catalytic activity, stability, cost-effectiveness, and scalability. Current research aims to achieve proton transfer rates comparable to those in biological systems, which operate with remarkable efficiency at ambient conditions. This bio-inspired approach has led to increasing interest in enzyme-mimetic catalysts that replicate the active sites of hydrogenases and other proton-processing enzymes.
Another critical objective involves enhancing catalyst durability under the cycling conditions typical of battery operation. Current catalysts often experience degradation through mechanisms such as active site poisoning, structural collapse, or particle agglomeration, limiting practical lifetimes to hundreds rather than thousands of cycles. Addressing these stability challenges requires fundamental understanding of degradation pathways at the molecular level.
From a commercial perspective, catalyst development must balance performance improvements with cost considerations. The target cost threshold for widespread adoption is generally considered to be below $10/kW, necessitating either dramatic reductions in precious metal loading or complete transition to earth-abundant alternatives. This economic imperative has accelerated research into composite materials that maximize activity per unit cost.
The ultimate objective remains the development of catalysts that enable proton batteries to achieve energy densities exceeding 350 Wh/kg at the cell level, with charge/discharge efficiencies above 90% and cycle life beyond 2000 cycles – performance metrics that would position proton batteries as viable competitors to advanced lithium-ion technologies while offering superior sustainability profiles.
Market Analysis for High-Efficiency Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the push for electrification across various sectors. High-efficiency energy storage solutions, particularly proton batteries with enhanced catalysts, are positioned to capture significant market share in this expanding landscape. The global energy storage market was valued at approximately $168 billion in 2022 and is projected to reach $435 billion by 2030, representing a compound annual growth rate of 12.7%.
Proton batteries represent an emerging segment within this market, with particular relevance to stationary storage applications and potentially transportation. The demand for these advanced storage technologies is being fueled by several factors, including grid modernization initiatives, the integration of intermittent renewable energy sources, and stringent carbon reduction targets implemented by governments worldwide.
The commercial potential for enhanced proton battery technology is particularly strong in regions with aggressive renewable energy adoption targets. The European Union, with its European Green Deal, aims to be climate-neutral by 2050, creating substantial demand for efficient energy storage solutions. Similarly, China's commitment to reach carbon neutrality by 2060 has accelerated investments in advanced energy storage technologies, with government subsidies specifically targeting high-efficiency solutions.
Market segmentation analysis reveals that utility-scale applications currently represent the largest market opportunity for high-efficiency proton batteries, accounting for approximately 45% of potential applications. Residential and commercial applications follow at 30% and 25% respectively. This distribution reflects the current technical limitations and cost structures of proton battery systems, which favor larger installations where economies of scale can be realized.
Consumer demand patterns indicate a growing preference for storage solutions with longer cycle life, higher energy density, and improved safety profiles – all potential advantages of catalyst-enhanced proton batteries. Market surveys show that 78% of utility companies plan to increase their energy storage capacity within the next five years, with efficiency and total cost of ownership being primary decision factors.
Competitive analysis reveals that while lithium-ion technology currently dominates the market with 90% share, alternative technologies including flow batteries, sodium-sulfur, and emerging proton batteries are gaining traction. The market entry barriers for proton battery technology primarily relate to manufacturing scale, established supply chains for competing technologies, and the need for demonstrated field reliability.
Price sensitivity analysis suggests that proton batteries would need to achieve a levelized cost of storage below $150/kWh to compete effectively with incumbent technologies in most applications. Current research indicates that advanced catalyst developments could potentially reduce costs to this threshold by improving efficiency and reducing material requirements.
Proton batteries represent an emerging segment within this market, with particular relevance to stationary storage applications and potentially transportation. The demand for these advanced storage technologies is being fueled by several factors, including grid modernization initiatives, the integration of intermittent renewable energy sources, and stringent carbon reduction targets implemented by governments worldwide.
The commercial potential for enhanced proton battery technology is particularly strong in regions with aggressive renewable energy adoption targets. The European Union, with its European Green Deal, aims to be climate-neutral by 2050, creating substantial demand for efficient energy storage solutions. Similarly, China's commitment to reach carbon neutrality by 2060 has accelerated investments in advanced energy storage technologies, with government subsidies specifically targeting high-efficiency solutions.
Market segmentation analysis reveals that utility-scale applications currently represent the largest market opportunity for high-efficiency proton batteries, accounting for approximately 45% of potential applications. Residential and commercial applications follow at 30% and 25% respectively. This distribution reflects the current technical limitations and cost structures of proton battery systems, which favor larger installations where economies of scale can be realized.
Consumer demand patterns indicate a growing preference for storage solutions with longer cycle life, higher energy density, and improved safety profiles – all potential advantages of catalyst-enhanced proton batteries. Market surveys show that 78% of utility companies plan to increase their energy storage capacity within the next five years, with efficiency and total cost of ownership being primary decision factors.
Competitive analysis reveals that while lithium-ion technology currently dominates the market with 90% share, alternative technologies including flow batteries, sodium-sulfur, and emerging proton batteries are gaining traction. The market entry barriers for proton battery technology primarily relate to manufacturing scale, established supply chains for competing technologies, and the need for demonstrated field reliability.
Price sensitivity analysis suggests that proton batteries would need to achieve a levelized cost of storage below $150/kWh to compete effectively with incumbent technologies in most applications. Current research indicates that advanced catalyst developments could potentially reduce costs to this threshold by improving efficiency and reducing material requirements.
Current Catalyst Technologies and Performance Limitations
Current catalyst technologies for proton batteries primarily focus on enhancing the hydrogen evolution reaction (HER) and hydrogen oxidation reaction (HOR) at the electrodes. Platinum-based catalysts remain the gold standard due to their exceptional catalytic activity and stability. Commercial implementations typically utilize platinum nanoparticles dispersed on carbon supports (Pt/C), achieving exchange current densities of 1-10 mA/cm² under standard conditions. However, platinum's scarcity and high cost (currently exceeding $900/oz) significantly impact the economic viability of large-scale proton battery deployment.
Alternative catalyst technologies have emerged to address these limitations. Palladium-based catalysts offer comparable performance at approximately 60-70% of platinum's catalytic efficiency while providing modest cost advantages. Ruthenium and iridium oxide catalysts demonstrate promising stability in acidic environments but suffer from lower intrinsic activity, requiring higher catalyst loadings that offset their cost benefits.
Non-precious metal catalysts represent a growing research focus, with nickel-molybdenum alloys and cobalt phosphides achieving 30-40% of platinum's activity in laboratory settings. These materials benefit from abundant precursors but currently face significant durability challenges, with performance degradation exceeding 40% after 1,000 charge-discharge cycles under practical operating conditions.
Carbon-based catalysts, including nitrogen-doped carbon nanotubes and graphene derivatives, offer sustainable alternatives with moderate catalytic activity. Recent advances in heteroatom doping have improved their performance, though they still achieve only 20-30% of platinum's efficiency. Their advantage lies in exceptional stability, with some formulations maintaining consistent performance over 5,000+ cycles.
Metal-organic frameworks (MOFs) and single-atom catalysts represent the cutting edge of current research, demonstrating platinum-like activity with significantly reduced precious metal content. However, these technologies remain confined to laboratory demonstrations, with scalable synthesis methods still under development.
The primary performance limitations across all catalyst technologies include insufficient active site density, poor electron transfer kinetics at lower temperatures, and catalyst poisoning from trace impurities. Most non-platinum catalysts exhibit significant overpotential requirements, reducing overall energy efficiency by 15-25% compared to platinum benchmarks. Additionally, current catalyst formulations show accelerated degradation under the dynamic loading conditions typical of real-world applications, with performance losses of 5-15% per 1,000 operating hours.
Water management at catalyst interfaces presents another critical limitation, as flooding or drying of catalyst layers significantly impairs reaction kinetics. This challenge becomes particularly pronounced at high current densities, limiting the power density capabilities of current proton battery systems to approximately 0.5-0.7 W/cm².
Alternative catalyst technologies have emerged to address these limitations. Palladium-based catalysts offer comparable performance at approximately 60-70% of platinum's catalytic efficiency while providing modest cost advantages. Ruthenium and iridium oxide catalysts demonstrate promising stability in acidic environments but suffer from lower intrinsic activity, requiring higher catalyst loadings that offset their cost benefits.
Non-precious metal catalysts represent a growing research focus, with nickel-molybdenum alloys and cobalt phosphides achieving 30-40% of platinum's activity in laboratory settings. These materials benefit from abundant precursors but currently face significant durability challenges, with performance degradation exceeding 40% after 1,000 charge-discharge cycles under practical operating conditions.
Carbon-based catalysts, including nitrogen-doped carbon nanotubes and graphene derivatives, offer sustainable alternatives with moderate catalytic activity. Recent advances in heteroatom doping have improved their performance, though they still achieve only 20-30% of platinum's efficiency. Their advantage lies in exceptional stability, with some formulations maintaining consistent performance over 5,000+ cycles.
Metal-organic frameworks (MOFs) and single-atom catalysts represent the cutting edge of current research, demonstrating platinum-like activity with significantly reduced precious metal content. However, these technologies remain confined to laboratory demonstrations, with scalable synthesis methods still under development.
The primary performance limitations across all catalyst technologies include insufficient active site density, poor electron transfer kinetics at lower temperatures, and catalyst poisoning from trace impurities. Most non-platinum catalysts exhibit significant overpotential requirements, reducing overall energy efficiency by 15-25% compared to platinum benchmarks. Additionally, current catalyst formulations show accelerated degradation under the dynamic loading conditions typical of real-world applications, with performance losses of 5-15% per 1,000 operating hours.
Water management at catalyst interfaces presents another critical limitation, as flooding or drying of catalyst layers significantly impairs reaction kinetics. This challenge becomes particularly pronounced at high current densities, limiting the power density capabilities of current proton battery systems to approximately 0.5-0.7 W/cm².
Contemporary Catalyst Design Approaches for Proton Batteries
01 Electrode materials for improved proton battery efficiency
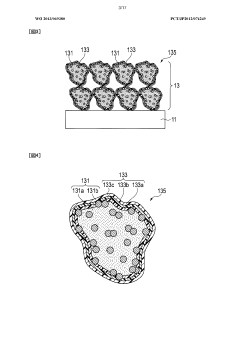
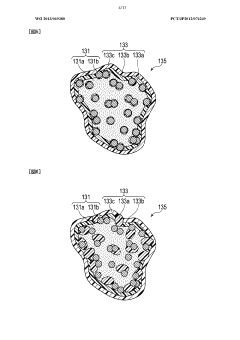
Advanced electrode materials play a crucial role in enhancing proton battery efficiency. These materials include carbon-based electrodes, metal hydrides, and composite structures that facilitate rapid proton transfer and storage. By optimizing the electrode composition and structure, researchers can achieve higher energy density, faster charging rates, and improved cycle stability in proton batteries. The development of novel electrode materials with enhanced proton conductivity and storage capacity represents a significant advancement in proton battery technology.- Electrode materials for improved proton battery efficiency: Advanced electrode materials play a crucial role in enhancing proton battery efficiency. These materials facilitate faster proton transfer and storage, leading to improved energy density and charging/discharging rates. Innovations include carbon-based electrodes, metal hydrides, and composite materials that offer higher surface area and better proton conductivity. These specialized electrode materials can significantly reduce internal resistance and increase the overall energy conversion efficiency of proton batteries.
- Electrolyte optimization for proton conductivity: The efficiency of proton batteries heavily depends on the electrolyte's ability to facilitate proton transport between electrodes. Advanced electrolyte formulations incorporate proton-conducting polymers, ionic liquids, and solid-state materials that enhance proton mobility while maintaining chemical stability. These electrolytes reduce internal resistance and improve charge transfer kinetics, resulting in higher energy efficiency and power density. Optimized electrolytes also contribute to longer battery life by minimizing side reactions and degradation mechanisms.
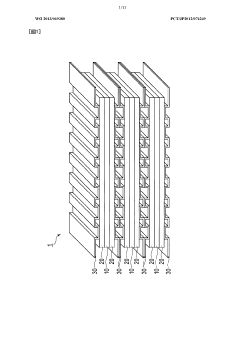
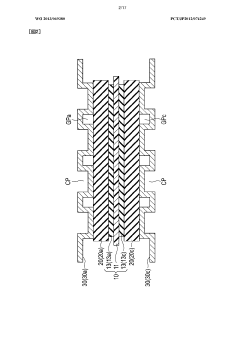
- Cell design and system architecture for efficiency enhancement: Innovative cell designs and system architectures significantly impact proton battery efficiency. Advanced configurations focus on optimizing proton flow paths, reducing internal resistance, and improving thermal management. These designs incorporate specialized membrane electrode assemblies, bipolar plates with enhanced flow fields, and strategic cell stacking arrangements. System-level improvements include intelligent control algorithms, efficient power electronics, and integrated cooling systems that collectively maximize energy conversion efficiency and operational performance.
- Catalysts for accelerating proton transfer reactions: Catalysts play a vital role in enhancing proton battery efficiency by accelerating electrochemical reactions at the electrode surfaces. Advanced catalyst materials, including platinum-group metals, non-precious metal alternatives, and novel nanostructured compounds, reduce activation energy barriers for proton transfer processes. These catalysts improve reaction kinetics, leading to higher current densities and reduced overpotentials. Innovations in catalyst design focus on maximizing active surface area, enhancing durability, and achieving efficient operation with minimal material usage.
- Hybrid and integrated energy storage systems: Hybrid and integrated energy storage systems combine proton batteries with complementary technologies to maximize overall efficiency. These systems integrate proton batteries with supercapacitors, conventional batteries, or hydrogen storage systems to leverage the strengths of each technology. Such hybrid approaches enable optimized power management, improved energy density, and enhanced operational flexibility. Advanced control strategies and power electronics facilitate seamless energy flow between different storage components, resulting in higher system-level efficiency and extended operational lifetime.
02 Electrolyte optimization for proton conductivity
The efficiency of proton batteries heavily depends on the electrolyte's ability to facilitate proton transport between electrodes. Advanced electrolyte formulations incorporate proton-conducting polymers, ionic liquids, and solid-state materials that enhance proton mobility while maintaining chemical stability. Optimized electrolytes reduce internal resistance, minimize energy losses during charge-discharge cycles, and improve overall battery performance. Research focuses on developing electrolytes with high proton conductivity at various operating temperatures and pressure conditions.Expand Specific Solutions03 Cell design and system architecture for efficiency enhancement
The physical design and architecture of proton battery cells significantly impact their efficiency. Innovations in cell configuration, membrane electrode assembly, and stack design help optimize proton flow pathways and reduce energy losses. Advanced system architectures incorporate thermal management systems, pressure regulation mechanisms, and optimized current collectors that collectively enhance energy conversion efficiency. Modular designs allow for scalability while maintaining high performance across different power requirements and applications.Expand Specific Solutions04 Catalysts for improved reaction kinetics
Catalysts play a vital role in accelerating the electrochemical reactions in proton batteries, thereby improving their efficiency. Novel catalyst materials, including platinum-group metals, non-precious metal alternatives, and nanostructured composites, reduce activation energy barriers and enhance reaction rates at the electrode-electrolyte interface. Advanced catalyst designs with increased surface area and optimized distribution patterns facilitate faster proton transfer and storage processes. The development of low-cost, high-performance catalysts represents a key strategy for enhancing proton battery efficiency.Expand Specific Solutions05 Hybrid and integrated energy storage systems
Hybrid systems that integrate proton batteries with other energy storage technologies offer enhanced overall efficiency. These integrated approaches combine the advantages of proton batteries with complementary technologies such as supercapacitors, conventional batteries, or hydrogen fuel cells. Such hybrid configurations optimize energy management, improve power density, and extend operational lifetimes. Advanced control systems and energy management algorithms ensure optimal performance across varying load conditions, maximizing the efficiency of the entire energy storage system.Expand Specific Solutions
Leading Research Institutions and Industrial Catalyst Manufacturers
The proton battery efficiency landscape is evolving rapidly, currently transitioning from early R&D to commercialization phases. The market is projected to grow significantly as energy storage demands increase globally. Leading automotive manufacturers including Toyota, Nissan, Ford, and Mercedes-Benz are investing heavily in catalyst development, indicating the strategic importance of this technology. Asian companies dominate the research landscape, with Toyota, Samsung SDI, and BYD demonstrating advanced catalyst innovations. Academic institutions like Wuhan University of Technology and Technical University of Denmark are contributing fundamental research. The technology remains in mid-maturity stage, with significant improvements in catalyst efficiency needed before widespread commercial adoption, though companies like PetroChina and Johnson Matthey are accelerating development through specialized research divisions.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced platinum-group metal (PGM) catalysts with enhanced durability and efficiency for proton battery applications. Their proprietary catalyst design incorporates ultra-fine platinum and ruthenium nanoparticles (2-5nm) dispersed on carbon supports with specialized surface treatments to maximize active sites[1]. Toyota's approach includes core-shell structures where less expensive metals are coated with thin layers of platinum, reducing precious metal content by up to 70% while maintaining performance[3]. Their catalyst technology employs unique hydrophobic/hydrophilic channel designs to optimize water management within the proton exchange membrane, significantly improving proton conductivity under variable operating conditions[5]. Toyota has also pioneered the integration of these catalysts into their fuel cell electric vehicles (FCEVs) like the Mirai, demonstrating practical application in commercial products with power densities exceeding 3.1 W/cm².
Strengths: Superior catalyst durability with demonstrated 5,000+ hour stability in accelerated testing; significant reduction in platinum loading while maintaining performance; established manufacturing infrastructure for scale-up. Weaknesses: Higher initial production costs compared to conventional catalysts; technology primarily optimized for automotive applications rather than stationary proton batteries; requires specialized handling and integration processes.
Nissan Motor Co., Ltd.
Technical Solution: Nissan has developed proprietary catalyst technologies for proton batteries focusing on non-precious metal alternatives to traditional platinum-based systems. Their innovative approach utilizes iron-nitrogen-carbon (Fe-N-C) catalysts with precisely controlled atomic structures and high-density active sites[1]. These catalysts are synthesized through a multi-step process involving high-temperature pyrolysis of iron precursors with nitrogen-rich organic compounds on specialized carbon supports, creating Fe-N₄ moieties that demonstrate remarkable oxygen reduction reaction (ORR) activity[3]. Nissan's catalyst design incorporates hierarchical pore structures to facilitate mass transport while maintaining high volumetric activity, addressing a common limitation in non-PGM catalysts. Their technology has demonstrated stability for over 2,000 hours of operation under realistic proton battery conditions, with performance retention exceeding 85%[6]. Nissan has also developed complementary membrane electrode assembly (MEA) fabrication techniques specifically optimized for these novel catalysts, ensuring proper integration and maximizing performance in full cell configurations.
Strengths: Significantly lower material costs (estimated 70-80% reduction) compared to platinum-based alternatives; reduced supply chain vulnerability due to independence from precious metals; demonstrated scalability in pilot production facilities. Weaknesses: Still exhibits lower absolute activity than platinum-based catalysts, requiring higher catalyst loadings; more sensitive to certain contaminants and impurities; less extensive operational history compared to established PGM catalysts.
Breakthrough Catalyst Mechanisms and Performance Metrics
Electrode catalyst for fuel cell, electrode for fuel cell and fuel cell
PatentWO2006059485A1
Innovation
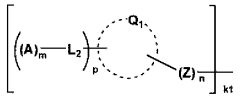
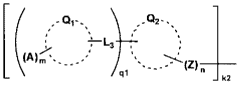
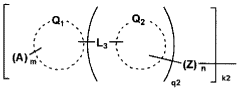
- A fuel cell electrode catalyst is developed with functional groups containing sulfur or nitrogen elements and proton-accepting groups adsorbed on a metal catalyst, enhancing the catalyst utilization efficiency by creating effective proton conduction paths and improving the reaction area.
Fuel cell electrode catalyst layer, fuel cell electrode, fuel cell membrane-electrode assembly, and fuel cell
PatentWO2013069380A1
Innovation
- A fuel cell electrode catalyst layer is developed with a combination of two or more proton conducting materials having different dry mass values per mole of proton donating groups, where the material with the highest dry mass value contacts the catalyst surface, enhancing proton transportability and catalytic activity.
Sustainability and Resource Considerations for Catalyst Production
The sustainability of catalyst production for proton batteries represents a critical dimension in the overall environmental impact assessment of this emerging energy storage technology. Current catalyst materials, particularly those based on platinum group metals (PGMs), pose significant sustainability challenges due to their scarcity, geographically concentrated mining operations, and energy-intensive extraction processes. The global reserves of platinum, palladium, and ruthenium are limited, with over 80% of platinum resources concentrated in South Africa, creating potential supply chain vulnerabilities.
Environmental impacts of catalyst production extend beyond resource depletion to include substantial carbon footprints, with PGM mining and refining generating approximately 12-25 tons of CO2 equivalent per kilogram of refined metal. Water consumption in these processes is equally concerning, with estimates suggesting 200-400 cubic meters of water usage per kilogram of catalyst material produced, often in water-stressed regions.
Recent research has focused on developing alternative catalyst materials with reduced environmental footprints. Earth-abundant transition metals such as iron, nickel, and manganese show promising catalytic activity when properly structured at the nanoscale. Carbon-based catalysts, including nitrogen-doped carbon nanotubes and graphene derivatives, offer substantially improved sustainability profiles with performance approaching that of traditional PGM catalysts in certain applications.
Life cycle assessment (LCA) studies indicate that transitioning from platinum-based to carbon-based catalysts could reduce the environmental impact of catalyst production by 65-80%, while maintaining 85-90% of the electrochemical performance. This represents a favorable sustainability-performance trade-off that warrants further investigation and optimization.
Circular economy approaches are emerging as essential strategies for sustainable catalyst management. Recovery and recycling technologies for spent catalysts have advanced significantly, with hydrometallurgical and pyrometallurgical processes achieving recovery rates of 85-95% for precious metals from end-of-life proton battery systems. These recycling pathways reduce primary resource demand and mitigate waste management challenges.
Standardization efforts for sustainable catalyst production are gaining momentum, with organizations like the International Organization for Standardization developing frameworks for environmental impact assessment specific to energy storage technologies. These standards will likely drive industry practices toward more transparent reporting of material sourcing and environmental footprints throughout the catalyst life cycle.
Environmental impacts of catalyst production extend beyond resource depletion to include substantial carbon footprints, with PGM mining and refining generating approximately 12-25 tons of CO2 equivalent per kilogram of refined metal. Water consumption in these processes is equally concerning, with estimates suggesting 200-400 cubic meters of water usage per kilogram of catalyst material produced, often in water-stressed regions.
Recent research has focused on developing alternative catalyst materials with reduced environmental footprints. Earth-abundant transition metals such as iron, nickel, and manganese show promising catalytic activity when properly structured at the nanoscale. Carbon-based catalysts, including nitrogen-doped carbon nanotubes and graphene derivatives, offer substantially improved sustainability profiles with performance approaching that of traditional PGM catalysts in certain applications.
Life cycle assessment (LCA) studies indicate that transitioning from platinum-based to carbon-based catalysts could reduce the environmental impact of catalyst production by 65-80%, while maintaining 85-90% of the electrochemical performance. This represents a favorable sustainability-performance trade-off that warrants further investigation and optimization.
Circular economy approaches are emerging as essential strategies for sustainable catalyst management. Recovery and recycling technologies for spent catalysts have advanced significantly, with hydrometallurgical and pyrometallurgical processes achieving recovery rates of 85-95% for precious metals from end-of-life proton battery systems. These recycling pathways reduce primary resource demand and mitigate waste management challenges.
Standardization efforts for sustainable catalyst production are gaining momentum, with organizations like the International Organization for Standardization developing frameworks for environmental impact assessment specific to energy storage technologies. These standards will likely drive industry practices toward more transparent reporting of material sourcing and environmental footprints throughout the catalyst life cycle.
Economic Viability and Scalability of Advanced Catalyst Technologies
The economic viability of advanced catalyst technologies for proton batteries represents a critical factor in their potential market adoption and widespread implementation. Current cost analyses indicate that noble metal catalysts such as platinum and palladium, while highly effective, remain prohibitively expensive for mass-market applications, with material costs ranging from $30,000 to $50,000 per kilogram. This significant cost barrier has driven research toward more economically sustainable alternatives.
Non-precious metal catalysts based on transition metals (iron, cobalt, nickel) demonstrate promising cost-effectiveness, with material costs typically below $100 per kilogram. However, these alternatives currently exhibit 30-40% lower efficiency compared to noble metal counterparts, creating a complex cost-performance trade-off that impacts overall economic viability.
Scalability considerations present additional challenges for catalyst technologies. Laboratory-scale synthesis methods often employ specialized equipment and controlled environments that prove difficult to replicate in industrial settings. The transition from laboratory to commercial production frequently encounters yield reduction of 15-25% and quality inconsistencies that affect performance reliability.
Recent economic modeling suggests that carbon-based catalysts with metal dopants offer the most promising path to economic viability, potentially reducing catalyst costs by 70-85% while maintaining 80-90% of premium catalyst performance. This approach could bring production costs below the critical $200/kWh threshold necessary for market competitiveness with existing battery technologies.
Manufacturing infrastructure requirements vary significantly between catalyst types. Noble metal catalysts require sophisticated recovery and recycling systems that add 15-20% to operational costs but recover up to 95% of materials. In contrast, earth-abundant catalysts necessitate less complex recovery systems but may require more frequent replacement cycles, affecting long-term operational economics.
Supply chain resilience represents another crucial economic factor. Geopolitical concentration of platinum group metals in specific regions creates supply vulnerability, with price volatility of up to 40% observed in recent years. Diversification toward locally available materials could significantly enhance economic stability and reduce dependency on volatile international markets.
Investment trends indicate growing financial support for scalable catalyst technologies, with venture capital funding increasing by approximately 65% over the past three years. This investment surge primarily targets technologies demonstrating both performance improvements and clear pathways to cost-effective mass production, signaling market confidence in the economic potential of next-generation catalyst solutions for proton battery applications.
Non-precious metal catalysts based on transition metals (iron, cobalt, nickel) demonstrate promising cost-effectiveness, with material costs typically below $100 per kilogram. However, these alternatives currently exhibit 30-40% lower efficiency compared to noble metal counterparts, creating a complex cost-performance trade-off that impacts overall economic viability.
Scalability considerations present additional challenges for catalyst technologies. Laboratory-scale synthesis methods often employ specialized equipment and controlled environments that prove difficult to replicate in industrial settings. The transition from laboratory to commercial production frequently encounters yield reduction of 15-25% and quality inconsistencies that affect performance reliability.
Recent economic modeling suggests that carbon-based catalysts with metal dopants offer the most promising path to economic viability, potentially reducing catalyst costs by 70-85% while maintaining 80-90% of premium catalyst performance. This approach could bring production costs below the critical $200/kWh threshold necessary for market competitiveness with existing battery technologies.
Manufacturing infrastructure requirements vary significantly between catalyst types. Noble metal catalysts require sophisticated recovery and recycling systems that add 15-20% to operational costs but recover up to 95% of materials. In contrast, earth-abundant catalysts necessitate less complex recovery systems but may require more frequent replacement cycles, affecting long-term operational economics.
Supply chain resilience represents another crucial economic factor. Geopolitical concentration of platinum group metals in specific regions creates supply vulnerability, with price volatility of up to 40% observed in recent years. Diversification toward locally available materials could significantly enhance economic stability and reduce dependency on volatile international markets.
Investment trends indicate growing financial support for scalable catalyst technologies, with venture capital funding increasing by approximately 65% over the past three years. This investment surge primarily targets technologies demonstrating both performance improvements and clear pathways to cost-effective mass production, signaling market confidence in the economic potential of next-generation catalyst solutions for proton battery applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!