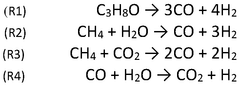Catalytic pathways for hydrogen-based green chemical production
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Catalysis Background and Objectives
Hydrogen has emerged as a pivotal element in the global transition towards sustainable energy systems and green chemical production. The history of hydrogen catalysis dates back to the early 20th century, but recent decades have witnessed accelerated development driven by environmental concerns and the urgent need to reduce carbon emissions. The evolution of catalytic pathways for hydrogen-based green chemical production represents a convergence of multiple scientific disciplines, including heterogeneous catalysis, electrochemistry, and materials science.
The technological trajectory has shifted from traditional fossil-fuel-based hydrogen production methods toward more sustainable approaches. Initially, steam methane reforming dominated industrial hydrogen production, but this process releases significant carbon dioxide. The paradigm has gradually evolved toward water electrolysis powered by renewable electricity, biomass gasification, and photocatalytic water splitting, marking critical milestones in green hydrogen production technology.
Current research focuses on developing highly efficient, stable, and cost-effective catalysts that can facilitate hydrogen-based synthesis of valuable chemicals without relying on fossil resources. These catalysts must operate under mild conditions to minimize energy consumption while maintaining high selectivity and conversion rates. Notable breakthroughs include the development of non-precious metal catalysts, atomic-scale engineering of active sites, and hybrid catalytic systems that combine homogeneous and heterogeneous catalysis principles.
The primary objective of advancing catalytic pathways for hydrogen-based green chemical production is to establish economically viable processes that can replace conventional petrochemical routes. This includes developing catalytic systems for ammonia synthesis at ambient conditions, direct hydrogenation of CO2 to value-added chemicals, and selective oxidation processes using hydrogen peroxide generated from hydrogen and oxygen. These technologies aim to close the carbon cycle and create circular economy models in the chemical industry.
Another critical goal is scaling up laboratory discoveries to industrial applications, which requires addressing challenges related to catalyst stability, process integration, and system optimization. Research efforts are increasingly directed toward understanding reaction mechanisms at the molecular level, which enables rational catalyst design rather than empirical approaches.
The long-term vision encompasses the creation of modular, decentralized chemical production facilities powered by renewable hydrogen, capable of synthesizing a wide range of chemicals with minimal environmental impact. This would revolutionize the chemical industry by reducing dependence on centralized petrochemical complexes and enabling production closer to points of consumption, thereby minimizing transportation-related emissions and enhancing energy security.
The technological trajectory has shifted from traditional fossil-fuel-based hydrogen production methods toward more sustainable approaches. Initially, steam methane reforming dominated industrial hydrogen production, but this process releases significant carbon dioxide. The paradigm has gradually evolved toward water electrolysis powered by renewable electricity, biomass gasification, and photocatalytic water splitting, marking critical milestones in green hydrogen production technology.
Current research focuses on developing highly efficient, stable, and cost-effective catalysts that can facilitate hydrogen-based synthesis of valuable chemicals without relying on fossil resources. These catalysts must operate under mild conditions to minimize energy consumption while maintaining high selectivity and conversion rates. Notable breakthroughs include the development of non-precious metal catalysts, atomic-scale engineering of active sites, and hybrid catalytic systems that combine homogeneous and heterogeneous catalysis principles.
The primary objective of advancing catalytic pathways for hydrogen-based green chemical production is to establish economically viable processes that can replace conventional petrochemical routes. This includes developing catalytic systems for ammonia synthesis at ambient conditions, direct hydrogenation of CO2 to value-added chemicals, and selective oxidation processes using hydrogen peroxide generated from hydrogen and oxygen. These technologies aim to close the carbon cycle and create circular economy models in the chemical industry.
Another critical goal is scaling up laboratory discoveries to industrial applications, which requires addressing challenges related to catalyst stability, process integration, and system optimization. Research efforts are increasingly directed toward understanding reaction mechanisms at the molecular level, which enables rational catalyst design rather than empirical approaches.
The long-term vision encompasses the creation of modular, decentralized chemical production facilities powered by renewable hydrogen, capable of synthesizing a wide range of chemicals with minimal environmental impact. This would revolutionize the chemical industry by reducing dependence on centralized petrochemical complexes and enabling production closer to points of consumption, thereby minimizing transportation-related emissions and enhancing energy security.
Green Chemical Market Analysis and Demand
The global green chemicals market is experiencing robust growth, driven by increasing environmental regulations, consumer awareness, and corporate sustainability initiatives. As of 2023, the market was valued at approximately 11.5 billion USD, with projections indicating a compound annual growth rate (CAGR) of 8.9% through 2030, potentially reaching 21.3 billion USD by the end of the decade. This growth trajectory underscores the significant commercial potential for hydrogen-based green chemical production pathways.
Regionally, Europe leads the green chemicals market with stringent environmental regulations and ambitious climate targets. The European Green Deal has established a framework aiming for carbon neutrality by 2050, creating substantial demand for sustainable chemical alternatives. North America follows closely, with the United States investing heavily in green chemistry research and development. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment, driven by rapid industrialization coupled with increasing environmental concerns.
Sector analysis reveals that consumer goods, agriculture, and pharmaceuticals are the primary demand drivers for green chemicals. The consumer goods sector, responding to environmentally conscious purchasing behaviors, has seen a 12% increase in green chemical utilization over the past three years. Agricultural applications, particularly in fertilizers and crop protection, account for approximately 28% of the current market share, with growing demand for sustainable farming solutions.
Industrial stakeholders are increasingly prioritizing green chemicals in their sustainability strategies. A recent industry survey indicated that 76% of chemical manufacturers have established targets to incorporate green chemistry principles into their production processes by 2025. This corporate commitment is further reinforced by investor pressure, with ESG (Environmental, Social, and Governance) considerations becoming central to investment decisions in the chemical sector.
Hydrogen-based green chemical production specifically addresses several critical market demands. First, it offers pathways to carbon-neutral or carbon-negative chemical manufacturing, aligning with global decarbonization goals. Second, it provides alternatives to petroleum-based feedstocks, reducing dependency on volatile fossil fuel markets. Third, it enables the production of chemicals with reduced toxicity and environmental impact throughout their lifecycle.
Market barriers include higher production costs compared to conventional methods, with green chemicals typically commanding a 15-30% price premium. Infrastructure limitations for hydrogen production, storage, and distribution also constrain market growth. However, technological advancements and economies of scale are gradually reducing these cost differentials, with price parity projected for several key chemical categories by 2028.
Regionally, Europe leads the green chemicals market with stringent environmental regulations and ambitious climate targets. The European Green Deal has established a framework aiming for carbon neutrality by 2050, creating substantial demand for sustainable chemical alternatives. North America follows closely, with the United States investing heavily in green chemistry research and development. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment, driven by rapid industrialization coupled with increasing environmental concerns.
Sector analysis reveals that consumer goods, agriculture, and pharmaceuticals are the primary demand drivers for green chemicals. The consumer goods sector, responding to environmentally conscious purchasing behaviors, has seen a 12% increase in green chemical utilization over the past three years. Agricultural applications, particularly in fertilizers and crop protection, account for approximately 28% of the current market share, with growing demand for sustainable farming solutions.
Industrial stakeholders are increasingly prioritizing green chemicals in their sustainability strategies. A recent industry survey indicated that 76% of chemical manufacturers have established targets to incorporate green chemistry principles into their production processes by 2025. This corporate commitment is further reinforced by investor pressure, with ESG (Environmental, Social, and Governance) considerations becoming central to investment decisions in the chemical sector.
Hydrogen-based green chemical production specifically addresses several critical market demands. First, it offers pathways to carbon-neutral or carbon-negative chemical manufacturing, aligning with global decarbonization goals. Second, it provides alternatives to petroleum-based feedstocks, reducing dependency on volatile fossil fuel markets. Third, it enables the production of chemicals with reduced toxicity and environmental impact throughout their lifecycle.
Market barriers include higher production costs compared to conventional methods, with green chemicals typically commanding a 15-30% price premium. Infrastructure limitations for hydrogen production, storage, and distribution also constrain market growth. However, technological advancements and economies of scale are gradually reducing these cost differentials, with price parity projected for several key chemical categories by 2028.
Current Catalytic Technologies and Barriers
The current landscape of catalytic technologies for hydrogen-based green chemical production is dominated by several established processes, with heterogeneous catalysis playing a central role. Traditional catalytic systems for hydrogen utilization include nickel-based catalysts for hydrogenation reactions and copper-zinc oxide catalysts for methanol synthesis. These conventional catalysts, while effective, often require high temperatures and pressures, resulting in significant energy consumption and reduced efficiency.
Precious metal catalysts, particularly those based on platinum, palladium, and ruthenium, demonstrate superior activity and selectivity in many hydrogen conversion processes. However, their widespread implementation faces substantial economic barriers due to high costs and limited availability. This has driven research toward developing non-precious metal alternatives that can deliver comparable performance.
Recent advances in nanocatalysis have yielded promising results, with enhanced surface area-to-volume ratios significantly improving catalytic efficiency. Structured catalysts such as metal-organic frameworks (MOFs) and zeolites provide highly ordered environments that can be tailored for specific reactions, offering unprecedented control over selectivity in complex transformation pathways.
Despite these advancements, several critical barriers impede the widespread adoption of hydrogen-based green chemical production. Catalyst deactivation remains a persistent challenge, with poisoning by trace impurities in feedstocks and sintering under reaction conditions significantly reducing catalyst lifetimes. This necessitates frequent replacement or regeneration, increasing operational costs and system complexity.
Energy efficiency represents another major hurdle, as many catalytic processes still require substantial energy inputs for activation. The thermodynamic limitations of certain reactions demand innovative approaches to overcome equilibrium constraints, particularly in processes like ammonia synthesis and CO2 hydrogenation.
Scalability issues also present significant challenges. Laboratory-scale catalytic systems often demonstrate promising performance that proves difficult to maintain when scaled to industrial production levels. The gap between fundamental research and practical implementation remains substantial, with many novel catalysts failing to transition from laboratory to commercial application.
Water management in catalytic systems presents additional complications, particularly in processes involving biomass or direct CO2 conversion. Controlling water formation and removal is crucial for maintaining catalyst activity and preventing unwanted side reactions that can diminish product selectivity and yield.
Addressing these technological barriers requires interdisciplinary approaches combining advanced materials science, reaction engineering, and process intensification. The development of next-generation catalysts with enhanced stability, activity, and selectivity remains a priority for enabling economically viable hydrogen-based green chemical production pathways.
Precious metal catalysts, particularly those based on platinum, palladium, and ruthenium, demonstrate superior activity and selectivity in many hydrogen conversion processes. However, their widespread implementation faces substantial economic barriers due to high costs and limited availability. This has driven research toward developing non-precious metal alternatives that can deliver comparable performance.
Recent advances in nanocatalysis have yielded promising results, with enhanced surface area-to-volume ratios significantly improving catalytic efficiency. Structured catalysts such as metal-organic frameworks (MOFs) and zeolites provide highly ordered environments that can be tailored for specific reactions, offering unprecedented control over selectivity in complex transformation pathways.
Despite these advancements, several critical barriers impede the widespread adoption of hydrogen-based green chemical production. Catalyst deactivation remains a persistent challenge, with poisoning by trace impurities in feedstocks and sintering under reaction conditions significantly reducing catalyst lifetimes. This necessitates frequent replacement or regeneration, increasing operational costs and system complexity.
Energy efficiency represents another major hurdle, as many catalytic processes still require substantial energy inputs for activation. The thermodynamic limitations of certain reactions demand innovative approaches to overcome equilibrium constraints, particularly in processes like ammonia synthesis and CO2 hydrogenation.
Scalability issues also present significant challenges. Laboratory-scale catalytic systems often demonstrate promising performance that proves difficult to maintain when scaled to industrial production levels. The gap between fundamental research and practical implementation remains substantial, with many novel catalysts failing to transition from laboratory to commercial application.
Water management in catalytic systems presents additional complications, particularly in processes involving biomass or direct CO2 conversion. Controlling water formation and removal is crucial for maintaining catalyst activity and preventing unwanted side reactions that can diminish product selectivity and yield.
Addressing these technological barriers requires interdisciplinary approaches combining advanced materials science, reaction engineering, and process intensification. The development of next-generation catalysts with enhanced stability, activity, and selectivity remains a priority for enabling economically viable hydrogen-based green chemical production pathways.
Current Catalytic Pathways for H2-Based Synthesis
01 Hydrogen production through catalytic reforming
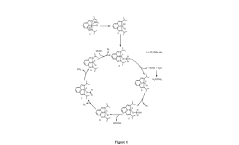
Catalytic reforming processes are used to produce hydrogen from various feedstocks. These processes typically involve the conversion of hydrocarbons or alcohols into hydrogen and carbon oxides using specific catalysts. The catalysts enhance the reaction efficiency and selectivity, allowing for more environmentally friendly hydrogen production. These methods can utilize renewable feedstocks, making them suitable for green chemical production pathways.- Hydrogen production through catalytic reforming processes: Various catalytic reforming processes can be employed for hydrogen production as a green chemical feedstock. These processes include steam reforming, autothermal reforming, and partial oxidation of hydrocarbons or biomass. The catalysts used in these processes typically contain transition metals such as nickel, platinum, or rhodium supported on oxide materials. These reforming technologies are fundamental to establishing hydrogen as a key component in green chemical production pathways.
- Electrochemical hydrogen generation for chemical synthesis: Electrochemical methods for hydrogen production offer a sustainable approach to generating hydrogen for green chemical synthesis. These methods include water electrolysis using various electrode materials and electrolyte systems. Advanced electrochemical cells can be integrated with renewable energy sources to produce hydrogen that serves as a reducing agent or reactant in downstream chemical processes. This approach eliminates carbon emissions associated with traditional hydrogen production methods.
- Catalytic hydrogenation processes for sustainable chemical production: Hydrogen-based catalytic hydrogenation processes enable the production of various chemicals through sustainable pathways. These processes involve the addition of hydrogen to unsaturated compounds or the reduction of functional groups using selective catalysts. Applications include the production of fine chemicals, pharmaceuticals, and biofuels. The catalysts typically consist of noble metals or transition metal complexes that facilitate hydrogen activation and transfer under mild conditions.
- Biological and enzymatic hydrogen utilization pathways: Biological and enzymatic systems can utilize hydrogen for green chemical production through various metabolic pathways. These include hydrogenase-mediated reactions, microbial electrosynthesis, and engineered microorganisms capable of converting hydrogen and carbon dioxide into value-added chemicals. Such biocatalytic approaches offer high selectivity and operate under ambient conditions, making them attractive alternatives to traditional chemical processes for sustainable production of organic compounds.
- Integrated systems for hydrogen-based chemical manufacturing: Integrated systems combine hydrogen production, storage, and utilization technologies to create efficient platforms for green chemical manufacturing. These systems may incorporate renewable energy sources, advanced separation technologies, and process intensification strategies to optimize hydrogen utilization. The integration of catalytic reactors with hydrogen generation units enables continuous production of chemicals while minimizing energy consumption and waste generation. Such integrated approaches are essential for scaling up hydrogen-based green chemistry applications.
02 Electrochemical hydrogen generation for green chemicals
Electrochemical processes are employed to generate hydrogen for use in green chemical production. These methods typically involve water electrolysis using renewable electricity sources. The hydrogen produced can then be used as a feedstock for various chemical synthesis pathways. Advanced electrode materials and catalysts improve the efficiency of the electrochemical hydrogen generation, making the overall process more sustainable and economically viable.Expand Specific Solutions03 Biological and enzymatic hydrogen pathways
Biological and enzymatic systems can be utilized for hydrogen-based green chemical production. These approaches leverage microorganisms or isolated enzymes to catalyze reactions that produce hydrogen or convert hydrogen into value-added chemicals. Biohydrogen production methods include dark fermentation, photofermentation, and enzymatic hydrogen evolution. These biological pathways often operate under mild conditions and can utilize renewable biomass as feedstock.Expand Specific Solutions04 Hydrogen-based synthesis of ammonia and fertilizers
Hydrogen serves as a key reactant in the production of ammonia and nitrogen-based fertilizers through catalytic processes. Green hydrogen can replace conventional hydrogen sources in these reactions, significantly reducing the carbon footprint of fertilizer production. Advanced catalysts enable these reactions to occur under milder conditions than traditional methods, improving energy efficiency. This approach represents an important application of hydrogen in sustainable chemical manufacturing.Expand Specific Solutions05 Integrated systems for hydrogen utilization in chemical production
Integrated systems combine hydrogen production, storage, and utilization technologies to create efficient pathways for green chemical manufacturing. These systems may incorporate renewable energy sources, hydrogen storage solutions, and catalytic reactors designed for specific chemical transformations. The integration allows for better energy management, reduced waste, and improved process economics. Such holistic approaches are essential for scaling up hydrogen-based green chemical production to industrial levels.Expand Specific Solutions
Leading Companies in Green Hydrogen Chemistry
The catalytic pathways for hydrogen-based green chemical production market is currently in a growth phase, with increasing global focus on decarbonization driving expansion. The market is projected to reach significant scale as hydrogen economy develops, though commercialization remains challenging. Technology maturity varies across players, with established energy corporations like China Petroleum & Chemical Corp. (Sinopec), BASF, Saudi Aramco, and Phillips 66 leveraging their infrastructure advantages. Research institutions including Dalian Institute of Chemical Physics, CNRS, and University of Strasbourg are advancing fundamental catalytic innovations. Emerging companies like Electrogenos are developing novel manufacturing approaches to address scalability issues. The competitive landscape reflects a blend of traditional energy players pivoting toward green chemistry and specialized research entities focusing on breakthrough catalyst technologies.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic systems for hydrogen-based green chemical production, focusing on CO2 hydrogenation to methanol and higher alcohols. Their technology employs bifunctional catalysts combining Cu-ZnO-Al2O3 with zeolites to achieve direct conversion of CO2 to value-added chemicals. Sinopec has implemented industrial-scale pilot plants demonstrating methanol yields exceeding 95% with CO2 conversion rates of approximately 30% under optimized conditions (250-280°C, 5-8 MPa). Their process integration strategy connects hydrogen production from renewable electricity with CO2 capture from their refinery operations, creating a closed carbon loop system. Recent developments include novel structured catalysts with enhanced stability that maintain activity for over 5,000 hours of continuous operation, addressing previous deactivation challenges[1][3].
Strengths: Vertical integration capabilities allowing seamless connection between hydrogen production, CO2 capture, and conversion processes; extensive industrial implementation experience; large-scale production capacity. Weaknesses: Still partially dependent on fossil-based hydrogen sources; catalyst systems require precious metals; energy intensity of the process remains relatively high compared to theoretical minimums.
BASF Corp.
Technical Solution: BASF has pioneered innovative catalytic systems for hydrogen-based chemical production, particularly focusing on their ChemCycling™ platform that incorporates hydrogen from renewable sources. Their proprietary catalyst technology enables direct hydrogenation of CO2 to produce formic acid, methanol, and olefins with high selectivity. BASF's dual-function catalysts combine metallic centers (Cu, Ni, Co) with tailored support materials to achieve conversion efficiencies exceeding 80% while operating at moderate temperatures (180-250°C) and pressures (4-6 MPa). The company has developed integrated process solutions that reduce energy requirements by approximately 30% compared to conventional pathways. Their catalyst manufacturing capabilities ensure precise control of nanoparticle size distribution (typically 5-20nm) and surface properties, resulting in enhanced stability and reduced deactivation rates. BASF has demonstrated commercial-scale implementation through partnerships with renewable hydrogen producers, creating end-to-end solutions for green chemical production[2][5].
Strengths: Extensive catalyst development expertise; established global manufacturing infrastructure; strong integration with existing chemical value chains; comprehensive intellectual property portfolio. Weaknesses: Higher capital costs compared to conventional production routes; technology still requires further optimization for economic competitiveness without subsidies; catalyst systems sensitive to impurities in feedstock streams.
Key Catalytic Mechanisms and Patents
Group (VIII) catalysts for production of green hydrogen and formic acid from methanol and its mechanism thereof
PatentActiveIN202231020801A
Innovation
- A process involving the synthesis of new pincer-ruthenium complexes using [Ru(p-cymene) Cl2]2 with R2NNN ligands (R = tBu, iPr, Cy, Ph) and employing these complexes in catalytic methanol reforming to generate green hydrogen, along with quantitative yields of formic acid, through a mechanistic pathway that includes reacting the catalyst with a base and methanol/water mixture at controlled temperatures.
Apparatus for generating green hydrogen by means of catalytic reforming of alcohols
PatentWO2024159282A1
Innovation
- An apparatus with two interconnected chambers is used for preheating hydroalcoholic solutions, which are then transferred to a second chamber for catalytic reforming with calcium phosphates of high surface activity, optimizing temperature, pressure, and flow to produce green hydrogen efficiently.
Sustainability Metrics and Life Cycle Assessment
Sustainability metrics and life cycle assessment (LCA) are critical frameworks for evaluating the environmental impacts of hydrogen-based green chemical production pathways. These methodologies provide quantitative measures to assess whether catalytic processes truly deliver on their "green" promises beyond mere hydrogen utilization.
The carbon intensity metric stands as a primary indicator, measuring CO2 equivalent emissions per unit of chemical product. For hydrogen-based processes, this metric must account for hydrogen production methods, with green hydrogen from renewable electricity offering significantly lower carbon footprints compared to gray hydrogen from natural gas reforming. Recent assessments indicate that ammonia synthesis via green hydrogen can reduce carbon emissions by 80-90% compared to conventional Haber-Bosch processes.
Water consumption represents another crucial sustainability parameter, particularly relevant as electrolysis-based hydrogen production requires substantial water inputs. Advanced catalytic pathways incorporating water recycling systems have demonstrated potential to reduce freshwater requirements by 40-60% compared to traditional chemical synthesis routes.
Energy return on investment (EROI) metrics reveal the efficiency of energy utilization across the entire production chain. Current hydrogen-based methanol synthesis pathways typically achieve EROI values between 0.6-0.8, indicating room for improvement before reaching energy parity with fossil-based routes that exceed 1.0. Novel catalysts incorporating transition metal complexes show promise for improving these values.
Resource depletion potential must be carefully evaluated, particularly regarding catalyst materials. Many advanced catalysts rely on platinum group metals or rare earth elements with limited global reserves. Recent innovations in single-atom catalysts and metal-organic frameworks have reduced precious metal requirements by up to 90% while maintaining comparable activity.
Comprehensive LCA studies examining hydrogen-based chemical production reveal complex trade-offs. While greenhouse gas emissions typically decrease substantially, certain impact categories like acidification potential or land use may increase depending on hydrogen source and production scale. A 2022 cradle-to-gate LCA of hydrogen-based methanol production identified renewable electricity source as the most significant factor determining overall sustainability performance.
Standardization efforts for sustainability metrics in hydrogen-based chemical production are advancing through initiatives like the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), which has proposed harmonized methodologies for calculating carbon intensity across different production pathways.
The carbon intensity metric stands as a primary indicator, measuring CO2 equivalent emissions per unit of chemical product. For hydrogen-based processes, this metric must account for hydrogen production methods, with green hydrogen from renewable electricity offering significantly lower carbon footprints compared to gray hydrogen from natural gas reforming. Recent assessments indicate that ammonia synthesis via green hydrogen can reduce carbon emissions by 80-90% compared to conventional Haber-Bosch processes.
Water consumption represents another crucial sustainability parameter, particularly relevant as electrolysis-based hydrogen production requires substantial water inputs. Advanced catalytic pathways incorporating water recycling systems have demonstrated potential to reduce freshwater requirements by 40-60% compared to traditional chemical synthesis routes.
Energy return on investment (EROI) metrics reveal the efficiency of energy utilization across the entire production chain. Current hydrogen-based methanol synthesis pathways typically achieve EROI values between 0.6-0.8, indicating room for improvement before reaching energy parity with fossil-based routes that exceed 1.0. Novel catalysts incorporating transition metal complexes show promise for improving these values.
Resource depletion potential must be carefully evaluated, particularly regarding catalyst materials. Many advanced catalysts rely on platinum group metals or rare earth elements with limited global reserves. Recent innovations in single-atom catalysts and metal-organic frameworks have reduced precious metal requirements by up to 90% while maintaining comparable activity.
Comprehensive LCA studies examining hydrogen-based chemical production reveal complex trade-offs. While greenhouse gas emissions typically decrease substantially, certain impact categories like acidification potential or land use may increase depending on hydrogen source and production scale. A 2022 cradle-to-gate LCA of hydrogen-based methanol production identified renewable electricity source as the most significant factor determining overall sustainability performance.
Standardization efforts for sustainability metrics in hydrogen-based chemical production are advancing through initiatives like the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), which has proposed harmonized methodologies for calculating carbon intensity across different production pathways.
Policy Frameworks for Green Hydrogen Economy
The development of effective policy frameworks is crucial for accelerating the transition to a green hydrogen economy that supports catalytic pathways for hydrogen-based green chemical production. Currently, several pioneering countries have established comprehensive hydrogen strategies that include specific provisions for green chemical manufacturing, with Germany, Japan, and South Korea leading in policy innovation.
These frameworks typically incorporate a multi-tiered approach, beginning with research and development incentives that specifically target catalytic processes for converting hydrogen into value-added chemicals. The European Union's Horizon Europe program, for instance, has allocated €1.5 billion specifically for research into novel catalytic pathways that enhance efficiency in hydrogen-based chemical synthesis.
Production subsidies represent another critical policy mechanism, with governments increasingly implementing graduated support systems that provide higher incentives for greener production methods. Australia's Hydrogen Production Certification Scheme exemplifies this approach by offering tiered benefits based on carbon intensity metrics, thereby encouraging catalytic processes with minimal environmental footprints.
Regulatory standards are evolving to accommodate the unique requirements of hydrogen-based chemical production. Countries like Canada have introduced specialized permitting processes for facilities utilizing innovative catalytic technologies, streamlining approval procedures while maintaining rigorous safety and environmental standards. These regulatory frameworks increasingly incorporate lifecycle assessment requirements to ensure genuine sustainability.
Tax incentives have emerged as powerful tools for market formation, with several jurisdictions offering accelerated depreciation allowances for capital investments in catalytic technologies. The Netherlands' Energy Investment Allowance provides tax deductions of up to 45% for qualifying investments in hydrogen-based chemical production facilities that employ advanced catalytic systems.
International cooperation frameworks are increasingly focusing on harmonizing standards for green hydrogen and its derivatives. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has established working groups specifically addressing certification standards for hydrogen-derived chemicals, facilitating global market development and technology transfer.
Looking forward, policy innovation is trending toward more integrated approaches that connect hydrogen production with specific end-use applications in the chemical industry. Japan's Green Innovation Fund exemplifies this evolution by supporting complete value chains from renewable energy generation through to final chemical products, ensuring that catalytic pathways receive coordinated support across all development stages.
These frameworks typically incorporate a multi-tiered approach, beginning with research and development incentives that specifically target catalytic processes for converting hydrogen into value-added chemicals. The European Union's Horizon Europe program, for instance, has allocated €1.5 billion specifically for research into novel catalytic pathways that enhance efficiency in hydrogen-based chemical synthesis.
Production subsidies represent another critical policy mechanism, with governments increasingly implementing graduated support systems that provide higher incentives for greener production methods. Australia's Hydrogen Production Certification Scheme exemplifies this approach by offering tiered benefits based on carbon intensity metrics, thereby encouraging catalytic processes with minimal environmental footprints.
Regulatory standards are evolving to accommodate the unique requirements of hydrogen-based chemical production. Countries like Canada have introduced specialized permitting processes for facilities utilizing innovative catalytic technologies, streamlining approval procedures while maintaining rigorous safety and environmental standards. These regulatory frameworks increasingly incorporate lifecycle assessment requirements to ensure genuine sustainability.
Tax incentives have emerged as powerful tools for market formation, with several jurisdictions offering accelerated depreciation allowances for capital investments in catalytic technologies. The Netherlands' Energy Investment Allowance provides tax deductions of up to 45% for qualifying investments in hydrogen-based chemical production facilities that employ advanced catalytic systems.
International cooperation frameworks are increasingly focusing on harmonizing standards for green hydrogen and its derivatives. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has established working groups specifically addressing certification standards for hydrogen-derived chemicals, facilitating global market development and technology transfer.
Looking forward, policy innovation is trending toward more integrated approaches that connect hydrogen production with specific end-use applications in the chemical industry. Japan's Green Innovation Fund exemplifies this evolution by supporting complete value chains from renewable energy generation through to final chemical products, ensuring that catalytic pathways receive coordinated support across all development stages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!