Innovations in photoelectrochemical water splitting for hydrogen generation
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photoelectrochemical Hydrogen Evolution Background and Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, harnessing solar energy to directly convert water into hydrogen and oxygen. This technology emerged in the early 1970s when Fujishima and Honda demonstrated the photoelectrochemical decomposition of water using titanium dioxide electrodes under ultraviolet light. Since then, the field has witnessed significant advancements in materials science, electrode design, and system integration.
The evolution of PEC water splitting technology has been characterized by several key developments. Initially focused on simple semiconductor photoelectrodes, research has progressively shifted toward more complex architectures including tandem cells, heterojunction structures, and integrated photovoltaic-electrochemical systems. Material innovation has expanded from traditional metal oxides to include quantum dots, perovskites, and various nanostructured materials designed to enhance light absorption and charge separation efficiency.
Recent technological trends indicate a growing emphasis on earth-abundant materials to replace precious metal catalysts, development of protective layers to enhance stability, and exploration of Z-scheme systems that mimic natural photosynthesis. Additionally, there is increasing interest in integrating PEC systems with other renewable energy technologies to create hybrid solutions that maximize efficiency and overcome intermittency challenges.
The primary objective of current research in photoelectrochemical hydrogen evolution is to develop systems that achieve solar-to-hydrogen (STH) conversion efficiencies exceeding 10% with stability beyond 10,000 hours under real-world conditions. This represents a significant improvement over current laboratory demonstrations that typically achieve 5-8% efficiency with limited durability in controlled environments.
Additional technical goals include reducing the cost of hydrogen production to less than $2/kg to compete with fossil fuel-derived hydrogen, developing scalable manufacturing processes for large-area photoelectrodes, and designing integrated systems that can operate efficiently with minimal maintenance in diverse geographical locations and climatic conditions.
The long-term vision encompasses the creation of artificial photosynthetic systems that can match or exceed the efficiency of natural photosynthesis while maintaining economic viability. This involves addressing fundamental challenges in charge carrier dynamics, surface catalysis, and system integration. Success in this domain could revolutionize the global energy landscape by enabling large-scale, carbon-neutral hydrogen production as a versatile energy carrier for transportation, industrial processes, and grid-scale energy storage.
The evolution of PEC water splitting technology has been characterized by several key developments. Initially focused on simple semiconductor photoelectrodes, research has progressively shifted toward more complex architectures including tandem cells, heterojunction structures, and integrated photovoltaic-electrochemical systems. Material innovation has expanded from traditional metal oxides to include quantum dots, perovskites, and various nanostructured materials designed to enhance light absorption and charge separation efficiency.
Recent technological trends indicate a growing emphasis on earth-abundant materials to replace precious metal catalysts, development of protective layers to enhance stability, and exploration of Z-scheme systems that mimic natural photosynthesis. Additionally, there is increasing interest in integrating PEC systems with other renewable energy technologies to create hybrid solutions that maximize efficiency and overcome intermittency challenges.
The primary objective of current research in photoelectrochemical hydrogen evolution is to develop systems that achieve solar-to-hydrogen (STH) conversion efficiencies exceeding 10% with stability beyond 10,000 hours under real-world conditions. This represents a significant improvement over current laboratory demonstrations that typically achieve 5-8% efficiency with limited durability in controlled environments.
Additional technical goals include reducing the cost of hydrogen production to less than $2/kg to compete with fossil fuel-derived hydrogen, developing scalable manufacturing processes for large-area photoelectrodes, and designing integrated systems that can operate efficiently with minimal maintenance in diverse geographical locations and climatic conditions.
The long-term vision encompasses the creation of artificial photosynthetic systems that can match or exceed the efficiency of natural photosynthesis while maintaining economic viability. This involves addressing fundamental challenges in charge carrier dynamics, surface catalysis, and system integration. Success in this domain could revolutionize the global energy landscape by enabling large-scale, carbon-neutral hydrogen production as a versatile energy carrier for transportation, industrial processes, and grid-scale energy storage.
Market Analysis for Green Hydrogen Production
The global green hydrogen market is experiencing unprecedented growth, driven by increasing environmental concerns and the push for decarbonization across industries. Current market valuations place the green hydrogen sector at approximately 2.5 billion USD in 2022, with projections indicating a compound annual growth rate (CAGR) of 39.5% through 2030. This exponential growth trajectory is particularly significant for photoelectrochemical (PEC) water splitting technologies, which represent an emerging segment within the broader hydrogen production landscape.
Demand for green hydrogen is primarily concentrated in industrial applications, with the chemical sector accounting for roughly 43% of current consumption. Transportation follows at 25%, with power generation and building heating comprising the remaining market share. Regionally, Europe leads in green hydrogen investments, allocating over 430 billion euros to hydrogen infrastructure development by 2030, followed by Asia-Pacific and North America.
Photoelectrochemical water splitting specifically addresses several critical market needs that conventional electrolysis cannot fully satisfy. The technology's ability to directly convert solar energy to hydrogen without intermediate electricity generation potentially offers cost advantages of 20-30% compared to traditional electrolysis paired with photovoltaics. Market analysis indicates that PEC systems could achieve hydrogen production costs of 2-4 USD/kg by 2030, approaching cost parity with fossil-derived hydrogen.
Industry stakeholders have identified several market drivers accelerating PEC water splitting adoption. These include increasingly stringent carbon regulations, volatile fossil fuel prices, and substantial government incentives for clean hydrogen production. The Inflation Reduction Act in the United States, for instance, provides production tax credits of up to 3 USD per kilogram for green hydrogen, significantly improving the economic case for PEC technologies.
Market barriers remain significant, however. Capital expenditure requirements for PEC systems currently exceed those of conventional electrolyzers by 40-60%, creating adoption hesitancy among potential industrial users. Additionally, the technology's relative immaturity compared to alkaline and PEM electrolysis presents market entry challenges, with concerns about long-term durability and scalability frequently cited by potential customers.
The competitive landscape is evolving rapidly, with over 30 companies actively developing hydrogen production technologies. While traditional electrolyzer manufacturers dominate current market share, several startups focused specifically on PEC water splitting have secured substantial venture funding, totaling over 450 million USD since 2020. This indicates growing investor confidence in the commercial viability of photoelectrochemical approaches to hydrogen generation.
Demand for green hydrogen is primarily concentrated in industrial applications, with the chemical sector accounting for roughly 43% of current consumption. Transportation follows at 25%, with power generation and building heating comprising the remaining market share. Regionally, Europe leads in green hydrogen investments, allocating over 430 billion euros to hydrogen infrastructure development by 2030, followed by Asia-Pacific and North America.
Photoelectrochemical water splitting specifically addresses several critical market needs that conventional electrolysis cannot fully satisfy. The technology's ability to directly convert solar energy to hydrogen without intermediate electricity generation potentially offers cost advantages of 20-30% compared to traditional electrolysis paired with photovoltaics. Market analysis indicates that PEC systems could achieve hydrogen production costs of 2-4 USD/kg by 2030, approaching cost parity with fossil-derived hydrogen.
Industry stakeholders have identified several market drivers accelerating PEC water splitting adoption. These include increasingly stringent carbon regulations, volatile fossil fuel prices, and substantial government incentives for clean hydrogen production. The Inflation Reduction Act in the United States, for instance, provides production tax credits of up to 3 USD per kilogram for green hydrogen, significantly improving the economic case for PEC technologies.
Market barriers remain significant, however. Capital expenditure requirements for PEC systems currently exceed those of conventional electrolyzers by 40-60%, creating adoption hesitancy among potential industrial users. Additionally, the technology's relative immaturity compared to alkaline and PEM electrolysis presents market entry challenges, with concerns about long-term durability and scalability frequently cited by potential customers.
The competitive landscape is evolving rapidly, with over 30 companies actively developing hydrogen production technologies. While traditional electrolyzer manufacturers dominate current market share, several startups focused specifically on PEC water splitting have secured substantial venture funding, totaling over 450 million USD since 2020. This indicates growing investor confidence in the commercial viability of photoelectrochemical approaches to hydrogen generation.
Current Status and Challenges in PEC Water Splitting
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, leveraging solar energy to directly convert water into hydrogen and oxygen. Currently, the global research landscape shows significant advancements in this field, with research centers across North America, Europe, and East Asia leading development efforts. Despite these advancements, PEC water splitting technology remains primarily at the laboratory scale, with limited commercial deployment due to several persistent challenges.
The primary technical hurdle facing PEC water splitting is efficiency. Current benchmark systems typically achieve solar-to-hydrogen (STH) conversion efficiencies between 5-15%, falling short of the 20-25% threshold generally considered necessary for commercial viability. This efficiency gap stems from fundamental limitations in light absorption, charge separation, and catalytic performance of existing materials.
Material stability presents another significant challenge. Many high-performance photoelectrode materials suffer from photocorrosion or degradation during operation, particularly in the acidic or alkaline conditions required for efficient water splitting. Silicon-based photoelectrodes, for instance, demonstrate promising efficiency but require protective layers to prevent rapid degradation in aqueous environments.
Scalability issues further complicate commercial implementation. Laboratory-scale demonstrations often utilize expensive materials like III-V semiconductors or platinum-group metal catalysts that are economically prohibitive at industrial scales. Additionally, current manufacturing techniques for high-quality photoelectrodes typically involve energy-intensive processes that undermine the sustainability benefits of the technology.
System integration challenges also persist. Efficient PEC systems require careful engineering of multiple components—semiconductors, catalysts, membranes, and device architectures—to work synergistically. Current systems often suffer from interface losses and operational incompatibilities between components optimized separately.
From a geographical perspective, research leadership is distributed across several regions. The United States maintains strong positions through programs at NREL, Caltech, and Stanford. European efforts are concentrated in Germany, Switzerland, and the Netherlands, while East Asian research is dominated by Japan, China, and South Korea. This distribution has created distinct approaches to solving technical challenges, with some regions focusing on high-efficiency materials and others prioritizing scalable manufacturing techniques.
Recent benchmarking studies indicate that while laboratory demonstrations have achieved impressive milestones, the technology readiness level (TRL) of PEC water splitting remains between 3-5, indicating significant development is still required before widespread commercial implementation becomes feasible. The gap between laboratory performance and practical requirements continues to drive research toward more stable materials, earth-abundant catalysts, and innovative device architectures.
The primary technical hurdle facing PEC water splitting is efficiency. Current benchmark systems typically achieve solar-to-hydrogen (STH) conversion efficiencies between 5-15%, falling short of the 20-25% threshold generally considered necessary for commercial viability. This efficiency gap stems from fundamental limitations in light absorption, charge separation, and catalytic performance of existing materials.
Material stability presents another significant challenge. Many high-performance photoelectrode materials suffer from photocorrosion or degradation during operation, particularly in the acidic or alkaline conditions required for efficient water splitting. Silicon-based photoelectrodes, for instance, demonstrate promising efficiency but require protective layers to prevent rapid degradation in aqueous environments.
Scalability issues further complicate commercial implementation. Laboratory-scale demonstrations often utilize expensive materials like III-V semiconductors or platinum-group metal catalysts that are economically prohibitive at industrial scales. Additionally, current manufacturing techniques for high-quality photoelectrodes typically involve energy-intensive processes that undermine the sustainability benefits of the technology.
System integration challenges also persist. Efficient PEC systems require careful engineering of multiple components—semiconductors, catalysts, membranes, and device architectures—to work synergistically. Current systems often suffer from interface losses and operational incompatibilities between components optimized separately.
From a geographical perspective, research leadership is distributed across several regions. The United States maintains strong positions through programs at NREL, Caltech, and Stanford. European efforts are concentrated in Germany, Switzerland, and the Netherlands, while East Asian research is dominated by Japan, China, and South Korea. This distribution has created distinct approaches to solving technical challenges, with some regions focusing on high-efficiency materials and others prioritizing scalable manufacturing techniques.
Recent benchmarking studies indicate that while laboratory demonstrations have achieved impressive milestones, the technology readiness level (TRL) of PEC water splitting remains between 3-5, indicating significant development is still required before widespread commercial implementation becomes feasible. The gap between laboratory performance and practical requirements continues to drive research toward more stable materials, earth-abundant catalysts, and innovative device architectures.
State-of-the-Art PEC Water Splitting Systems
01 Photocatalyst materials for water splitting
Various photocatalyst materials can be used in photoelectrochemical water splitting for hydrogen generation. These materials absorb light energy and facilitate the water splitting reaction. Different types of photocatalysts include metal oxides, semiconductors, and composite materials that can be optimized for better light absorption and charge separation efficiency, leading to improved hydrogen generation rates.- Photocatalyst materials for water splitting: Various photocatalyst materials can be used in photoelectrochemical water splitting for hydrogen generation. These materials, including metal oxides, semiconductors, and composite structures, absorb light and generate electron-hole pairs that facilitate the water splitting reaction. The efficiency of hydrogen generation depends on the properties of these photocatalysts, such as band gap, light absorption range, and charge separation efficiency.
- Electrode design and modification techniques: The design and modification of electrodes play a crucial role in enhancing the efficiency of photoelectrochemical water splitting. Various techniques include surface modification, nanostructuring, and incorporation of co-catalysts to improve charge transfer and reduce recombination losses. Optimized electrode architectures can significantly increase hydrogen generation rates by providing more active sites and facilitating efficient charge separation.
- System configuration and reactor design: The configuration of photoelectrochemical systems and reactor design significantly impact hydrogen generation efficiency. Factors such as light distribution, mass transport, and electrode arrangement affect the overall performance. Advanced reactor designs incorporate features that maximize light utilization, optimize electrolyte flow, and enhance gas separation, leading to improved hydrogen production rates and system stability.
- Electrolyte composition and additives: The composition of electrolytes and incorporation of additives can enhance the efficiency of photoelectrochemical water splitting. Optimized electrolyte solutions with appropriate pH levels, ionic strength, and sacrificial agents can improve charge transport and reduce overpotential. Certain additives can act as hole scavengers or electron donors, facilitating more efficient hydrogen generation by suppressing recombination processes.
- Integration with renewable energy systems: Integration of photoelectrochemical water splitting systems with other renewable energy sources can enhance overall hydrogen production efficiency. Hybrid systems combining solar cells, wind energy, or biomass with photoelectrochemical cells allow for continuous hydrogen generation even under varying light conditions. These integrated approaches offer more sustainable and economically viable solutions for large-scale hydrogen production.
02 Electrode design and modification
The design and modification of electrodes play a crucial role in photoelectrochemical water splitting. Techniques include surface modification, nanostructuring, and incorporation of co-catalysts to enhance charge transfer and reduce recombination losses. Optimized electrode architectures can significantly improve the efficiency and stability of hydrogen generation systems.Expand Specific Solutions03 System configuration and reactor design
The overall system configuration and reactor design are important factors in photoelectrochemical water splitting. This includes the arrangement of photoelectrodes, membrane separators, and light management systems. Innovative reactor designs can improve light utilization, mass transport, and gas separation, leading to more efficient hydrogen production processes.Expand Specific Solutions04 Integration with renewable energy sources
Photoelectrochemical water splitting systems can be integrated with other renewable energy sources to enhance hydrogen production. These hybrid systems may combine solar photovoltaics, wind energy, or biomass with photoelectrochemical cells to provide continuous operation and improved efficiency. Such integration allows for more sustainable and economically viable hydrogen generation.Expand Specific Solutions05 Scale-up and industrial applications
Scaling up photoelectrochemical water splitting systems from laboratory to industrial scale presents various challenges and opportunities. This includes considerations for large-scale manufacturing, system durability, cost reduction, and process optimization. Innovations in materials, fabrication techniques, and system engineering are essential for commercial viability of hydrogen generation through photoelectrochemical water splitting.Expand Specific Solutions
Leading Entities in Photoelectrochemical Research and Development
Photoelectrochemical water splitting for hydrogen generation is currently in the early commercialization phase, with a growing global market projected to reach significant scale as hydrogen economies develop. The technology maturity varies across different approaches, with key players demonstrating diverse levels of advancement. Research institutions like King Abdullah University of Science & Technology, Dalian Institute of Chemical Physics, and University of Tokyo are pioneering fundamental breakthroughs, while corporate entities such as Toyota Motor Corp. and SABIC Global Technologies are scaling up practical applications. National laboratories including Korea Institute of Energy Research and Alliance for Sustainable Energy are bridging the gap between academic research and industrial implementation. The competitive landscape shows geographic diversity with strong representation from Asia (particularly China and Japan), North America, and Europe, indicating global recognition of this technology's strategic importance for clean energy transition.
Toyota Motor Corp.
Technical Solution: Toyota has developed a comprehensive photoelectrochemical water splitting technology platform focused on practical applications for hydrogen fuel production. Their approach integrates proprietary semiconductor photoelectrodes with specialized catalytic materials to achieve efficient solar-to-hydrogen conversion. Toyota's system employs a tandem cell architecture that utilizes multiple semiconductor junctions to harvest a broader spectrum of solar energy. Their photoelectrodes incorporate protective layers that significantly enhance stability in aqueous environments, addressing one of the key challenges in the field. Toyota has also pioneered advanced surface modification techniques that reduce interface recombination losses and improve charge transfer efficiency. Their latest systems incorporate earth-abundant materials to replace precious metal catalysts, making the technology more economically viable for large-scale deployment. Toyota's integrated systems have demonstrated solar-to-hydrogen efficiencies exceeding 10% in laboratory conditions with promising durability metrics.
Strengths: Exceptional system integration capabilities leveraging automotive manufacturing expertise, with robust designs suitable for real-world implementation. Their systems show excellent durability under variable operating conditions. Weaknesses: Higher initial capital costs compared to conventional hydrogen production and remaining challenges in achieving cost parity with fossil-derived hydrogen.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute has developed advanced photocatalytic systems for water splitting using innovative semiconductor materials. Their approach focuses on developing highly efficient photocatalysts through precise nanostructure engineering and surface modifications. They've pioneered work on g-C3N4-based photocatalysts with optimized band structures for visible light absorption and created novel Z-scheme heterojunction systems that effectively separate photogenerated charge carriers. Their research includes developing core-shell nanostructures with enhanced light harvesting capabilities and stability in aqueous environments. The institute has also made significant progress in co-catalyst loading strategies to reduce overpotentials and improve hydrogen evolution reaction kinetics. Their integrated systems demonstrate solar-to-hydrogen conversion efficiencies approaching 5-7% under simulated sunlight conditions.
Strengths: Superior photocatalyst design with exceptional charge separation efficiency and visible light utilization. Their systems show remarkable stability in long-term operation and scalable synthesis methods. Weaknesses: Higher production costs compared to conventional hydrogen production methods and potential challenges in large-scale implementation due to material complexity.
Key Innovations in Photocatalyst and Electrode Materials
Method of generating hydrogen from water splitting and a photoelectrochemical cell for performing water splitting
PatentInactiveUS20190047853A1
Innovation
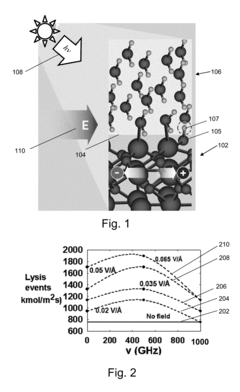
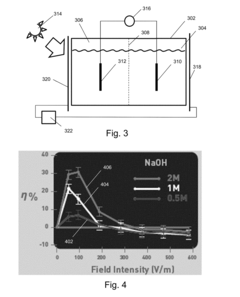
- The application of an external electric field at the semiconductor-water interface in a photoelectrochemical cell, using either static or dynamic electromagnetic fields, to increase the susceptibility of water molecules to break up, thereby enhancing hydrogen generation efficiency beyond zero-field setups, with the electric field acting as a quasi-catalyst to promote more efficient conversion of radiation into hydrogen.
Economic Viability and Cost Analysis
The economic viability of photoelectrochemical (PEC) water splitting for hydrogen generation remains a critical challenge despite significant technological advancements. Current cost analyses indicate that PEC hydrogen production ranges between $5-10 per kilogram, substantially higher than the U.S. Department of Energy's target of $2-3 per kilogram by 2030 for competitive market adoption. This cost gap represents one of the primary barriers to widespread commercialization.
Capital expenditure for PEC systems is dominated by semiconductor photoelectrode materials, which account for approximately 40-50% of total system costs. High-performance materials such as III-V semiconductors deliver superior efficiency but at prohibitive costs exceeding $10,000 per square meter. More economical alternatives like hematite (Fe2O3) and tungsten trioxide (WO3) offer reduced performance, creating a persistent efficiency-cost tradeoff that impacts economic feasibility.
System durability significantly affects lifetime operational costs. Current PEC devices typically demonstrate stability ranging from several hundred to a few thousand hours, falling short of the 50,000+ hours required for commercial viability. This limited lifespan necessitates frequent component replacement, substantially increasing the levelized cost of hydrogen (LCOH).
Energy conversion efficiency remains another economic bottleneck. Laboratory-scale systems have achieved solar-to-hydrogen efficiencies of 10-15%, but commercial-scale implementations typically operate at 5-8% efficiency. Economic modeling suggests that achieving market competitiveness requires minimum efficiencies of 15% combined with system lifetimes exceeding 10 years.
Recent techno-economic analyses reveal promising pathways toward cost reduction. Innovations in earth-abundant catalysts could potentially reduce noble metal dependency, decreasing catalyst costs by 60-70%. Advanced manufacturing techniques, including roll-to-roll processing and automated assembly, present opportunities to reduce production costs by an estimated 30-40% at scale.
Geographical factors significantly impact economic viability. Regions with high solar insolation (>2000 kWh/m²/year) and access to pure water sources can achieve up to 25% lower production costs compared to less favorable locations. Additionally, integration with existing renewable energy infrastructure could reduce system complexity and associated costs by 15-20%.
Policy mechanisms including carbon pricing, production tax credits, and renewable hydrogen standards will play crucial roles in bridging the near-term cost gap. Economic models suggest that a carbon price of $50-100 per ton CO2 equivalent could make PEC hydrogen competitive with conventional hydrogen production methods within specific market segments before broader cost parity is achieved through continued technological advancement.
Capital expenditure for PEC systems is dominated by semiconductor photoelectrode materials, which account for approximately 40-50% of total system costs. High-performance materials such as III-V semiconductors deliver superior efficiency but at prohibitive costs exceeding $10,000 per square meter. More economical alternatives like hematite (Fe2O3) and tungsten trioxide (WO3) offer reduced performance, creating a persistent efficiency-cost tradeoff that impacts economic feasibility.
System durability significantly affects lifetime operational costs. Current PEC devices typically demonstrate stability ranging from several hundred to a few thousand hours, falling short of the 50,000+ hours required for commercial viability. This limited lifespan necessitates frequent component replacement, substantially increasing the levelized cost of hydrogen (LCOH).
Energy conversion efficiency remains another economic bottleneck. Laboratory-scale systems have achieved solar-to-hydrogen efficiencies of 10-15%, but commercial-scale implementations typically operate at 5-8% efficiency. Economic modeling suggests that achieving market competitiveness requires minimum efficiencies of 15% combined with system lifetimes exceeding 10 years.
Recent techno-economic analyses reveal promising pathways toward cost reduction. Innovations in earth-abundant catalysts could potentially reduce noble metal dependency, decreasing catalyst costs by 60-70%. Advanced manufacturing techniques, including roll-to-roll processing and automated assembly, present opportunities to reduce production costs by an estimated 30-40% at scale.
Geographical factors significantly impact economic viability. Regions with high solar insolation (>2000 kWh/m²/year) and access to pure water sources can achieve up to 25% lower production costs compared to less favorable locations. Additionally, integration with existing renewable energy infrastructure could reduce system complexity and associated costs by 15-20%.
Policy mechanisms including carbon pricing, production tax credits, and renewable hydrogen standards will play crucial roles in bridging the near-term cost gap. Economic models suggest that a carbon price of $50-100 per ton CO2 equivalent could make PEC hydrogen competitive with conventional hydrogen production methods within specific market segments before broader cost parity is achieved through continued technological advancement.
Environmental Impact and Sustainability Assessment
Photoelectrochemical (PEC) water splitting for hydrogen generation represents a promising pathway toward sustainable energy production, yet its environmental implications require thorough assessment. The life cycle analysis of PEC systems reveals significantly lower carbon emissions compared to conventional hydrogen production methods such as steam methane reforming. Studies indicate that PEC hydrogen generation can reduce greenhouse gas emissions by up to 90% when utilizing renewable energy sources for system manufacturing and operation.
Water consumption presents both advantages and challenges for PEC technology. While water serves as the primary feedstock, the technology requires high-purity water to prevent catalyst poisoning and system degradation. This necessitates water purification processes that may increase the overall environmental footprint, particularly in water-scarce regions. However, innovations in catalyst design have improved tolerance to impurities, potentially enabling the use of non-potable water sources including seawater.
Material sustainability constitutes a critical consideration for widespread PEC implementation. Current high-efficiency systems often incorporate rare earth elements and precious metals like platinum and iridium, raising concerns about resource depletion and geopolitical supply risks. Recent advances in earth-abundant catalysts based on nickel, iron, and cobalt compounds demonstrate promising performance while significantly reducing material criticality concerns.
Land use impacts of large-scale PEC deployment remain relatively modest compared to biofuel production. Theoretical calculations suggest that a 1 GW PEC hydrogen facility would require approximately 10-15 km² of land area, substantially less than equivalent bioenergy systems. Furthermore, PEC installations can potentially utilize non-arable land, minimizing competition with food production.
End-of-life management presents emerging challenges as PEC technologies approach commercialization. The recyclability of semiconductor photoelectrodes, catalysts, and system components varies considerably. Research into design-for-recycling approaches has demonstrated recovery rates exceeding 85% for critical materials in laboratory settings, though industrial-scale recycling infrastructure remains underdeveloped.
The environmental benefits of PEC hydrogen extend beyond production to end-use applications. When utilized in fuel cells, PEC-derived hydrogen produces only water as a byproduct, eliminating local air pollutants associated with combustion technologies. This characteristic makes PEC hydrogen particularly valuable for decarbonizing sectors with limited electrification potential, such as heavy transport, industrial heating, and chemical manufacturing.
Water consumption presents both advantages and challenges for PEC technology. While water serves as the primary feedstock, the technology requires high-purity water to prevent catalyst poisoning and system degradation. This necessitates water purification processes that may increase the overall environmental footprint, particularly in water-scarce regions. However, innovations in catalyst design have improved tolerance to impurities, potentially enabling the use of non-potable water sources including seawater.
Material sustainability constitutes a critical consideration for widespread PEC implementation. Current high-efficiency systems often incorporate rare earth elements and precious metals like platinum and iridium, raising concerns about resource depletion and geopolitical supply risks. Recent advances in earth-abundant catalysts based on nickel, iron, and cobalt compounds demonstrate promising performance while significantly reducing material criticality concerns.
Land use impacts of large-scale PEC deployment remain relatively modest compared to biofuel production. Theoretical calculations suggest that a 1 GW PEC hydrogen facility would require approximately 10-15 km² of land area, substantially less than equivalent bioenergy systems. Furthermore, PEC installations can potentially utilize non-arable land, minimizing competition with food production.
End-of-life management presents emerging challenges as PEC technologies approach commercialization. The recyclability of semiconductor photoelectrodes, catalysts, and system components varies considerably. Research into design-for-recycling approaches has demonstrated recovery rates exceeding 85% for critical materials in laboratory settings, though industrial-scale recycling infrastructure remains underdeveloped.
The environmental benefits of PEC hydrogen extend beyond production to end-use applications. When utilized in fuel cells, PEC-derived hydrogen produces only water as a byproduct, eliminating local air pollutants associated with combustion technologies. This characteristic makes PEC hydrogen particularly valuable for decarbonizing sectors with limited electrification potential, such as heavy transport, industrial heating, and chemical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!