Comparative energy efficiency of direct seawater electrolysis methods
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Seawater Electrolysis Background and Objectives
Seawater electrolysis represents a promising frontier in sustainable hydrogen production, offering a potential solution to the global energy transition challenges by utilizing Earth's most abundant resource - seawater. The evolution of this technology dates back to the early 20th century, but significant advancements have emerged only in recent decades as the urgency for carbon-neutral energy sources intensified. Traditional freshwater electrolysis has been well-established, but its dependence on scarce freshwater resources limits large-scale implementation, particularly in water-stressed regions.
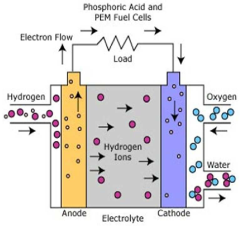
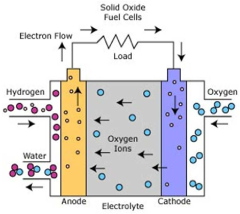
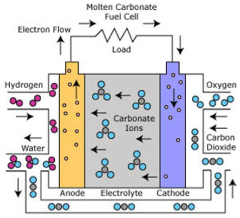
The technological trajectory has progressed from conventional alkaline electrolysis to more advanced methods including proton exchange membrane (PEM) electrolysis and solid oxide electrolysis cells (SOEC), each presenting unique advantages when adapted for seawater applications. Recent breakthroughs in catalyst development and membrane technology have accelerated the field's growth, with notable improvements in addressing chloride corrosion and scaling issues that previously hindered direct seawater utilization.
Current research trends focus on enhancing energy efficiency while minimizing pretreatment requirements. The comparative energy efficiency of various direct seawater electrolysis methods remains a critical metric, as it directly impacts economic viability and environmental sustainability. Conventional approaches typically require 4.5-6.0 kWh/m³ of hydrogen produced, while emerging technologies aim to reduce this to below 4.0 kWh/m³.
The primary technical objectives for advancing seawater electrolysis include developing corrosion-resistant electrodes capable of withstanding chloride-rich environments, designing selective membranes that prevent chlorine evolution reactions, and creating integrated systems that maintain high efficiency despite fluctuating seawater compositions. Additionally, researchers aim to minimize or eliminate energy-intensive pretreatment processes that currently diminish overall system efficiency.
From a strategic perspective, seawater electrolysis technology seeks to achieve cost parity with fossil fuel-derived hydrogen by 2030, requiring approximately 40% improvement in energy efficiency from current benchmarks. This ambitious target necessitates interdisciplinary collaboration across materials science, electrochemistry, and process engineering domains.
The ultimate goal extends beyond technical feasibility to commercial viability, with particular emphasis on coupling seawater electrolysis systems with renewable energy sources to create truly sustainable hydrogen production pathways. Success in this domain could fundamentally transform global energy systems by enabling decentralized, carbon-neutral hydrogen production in coastal regions worldwide, addressing both energy security and climate change mitigation objectives simultaneously.
The technological trajectory has progressed from conventional alkaline electrolysis to more advanced methods including proton exchange membrane (PEM) electrolysis and solid oxide electrolysis cells (SOEC), each presenting unique advantages when adapted for seawater applications. Recent breakthroughs in catalyst development and membrane technology have accelerated the field's growth, with notable improvements in addressing chloride corrosion and scaling issues that previously hindered direct seawater utilization.
Current research trends focus on enhancing energy efficiency while minimizing pretreatment requirements. The comparative energy efficiency of various direct seawater electrolysis methods remains a critical metric, as it directly impacts economic viability and environmental sustainability. Conventional approaches typically require 4.5-6.0 kWh/m³ of hydrogen produced, while emerging technologies aim to reduce this to below 4.0 kWh/m³.
The primary technical objectives for advancing seawater electrolysis include developing corrosion-resistant electrodes capable of withstanding chloride-rich environments, designing selective membranes that prevent chlorine evolution reactions, and creating integrated systems that maintain high efficiency despite fluctuating seawater compositions. Additionally, researchers aim to minimize or eliminate energy-intensive pretreatment processes that currently diminish overall system efficiency.
From a strategic perspective, seawater electrolysis technology seeks to achieve cost parity with fossil fuel-derived hydrogen by 2030, requiring approximately 40% improvement in energy efficiency from current benchmarks. This ambitious target necessitates interdisciplinary collaboration across materials science, electrochemistry, and process engineering domains.
The ultimate goal extends beyond technical feasibility to commercial viability, with particular emphasis on coupling seawater electrolysis systems with renewable energy sources to create truly sustainable hydrogen production pathways. Success in this domain could fundamentally transform global energy systems by enabling decentralized, carbon-neutral hydrogen production in coastal regions worldwide, addressing both energy security and climate change mitigation objectives simultaneously.
Market Analysis for Hydrogen Production from Seawater
The global hydrogen market is experiencing unprecedented growth, driven by the increasing focus on decarbonization and clean energy transitions. Current estimates value the hydrogen market at approximately $130 billion, with projections suggesting expansion to $500 billion by 2030. Hydrogen derived from seawater electrolysis represents a particularly promising segment within this market, addressing both environmental concerns and resource scarcity issues associated with traditional hydrogen production methods.
Demand for green hydrogen produced via seawater electrolysis is emerging across multiple sectors. The industrial sector, particularly refining and chemical production, constitutes the largest current market, accounting for roughly 70% of hydrogen consumption. Transportation represents the fastest-growing segment, with hydrogen fuel cell vehicles gaining traction in commercial fleets, heavy-duty transport, and maritime applications where battery electrification faces limitations.
Energy storage applications present another significant market opportunity, with hydrogen serving as a medium for long-duration storage to complement intermittent renewable energy sources. This application is especially relevant for coastal regions and island communities where seawater is abundant and renewable energy potential is high.
Geographically, the Asia-Pacific region leads in hydrogen demand, with Japan and South Korea implementing ambitious hydrogen strategies. The European Union has positioned itself as a policy leader, allocating substantial funding for hydrogen infrastructure and targeting 40GW of electrolyzer capacity by 2030, with seawater electrolysis expected to play a crucial role in coastal nations.
Market barriers for seawater electrolysis technologies include high capital costs compared to conventional hydrogen production methods, with current levelized costs ranging from $5-8/kg versus $1-2/kg for gray hydrogen. However, cost reduction trajectories suggest potential price parity by 2030 as technology advances and economies of scale develop.
Regulatory frameworks are evolving favorably, with carbon pricing mechanisms, renewable energy mandates, and hydrogen-specific incentives creating market pull. The EU's Carbon Border Adjustment Mechanism and various national hydrogen strategies are establishing supportive policy environments for clean hydrogen technologies.
Investment trends show accelerating capital flows into the sector, with venture capital funding for seawater electrolysis startups increasing by 300% since 2018. Major energy companies are also redirecting significant portions of their R&D budgets toward hydrogen technologies, recognizing the strategic importance of establishing early positions in this emerging market.
Demand for green hydrogen produced via seawater electrolysis is emerging across multiple sectors. The industrial sector, particularly refining and chemical production, constitutes the largest current market, accounting for roughly 70% of hydrogen consumption. Transportation represents the fastest-growing segment, with hydrogen fuel cell vehicles gaining traction in commercial fleets, heavy-duty transport, and maritime applications where battery electrification faces limitations.
Energy storage applications present another significant market opportunity, with hydrogen serving as a medium for long-duration storage to complement intermittent renewable energy sources. This application is especially relevant for coastal regions and island communities where seawater is abundant and renewable energy potential is high.
Geographically, the Asia-Pacific region leads in hydrogen demand, with Japan and South Korea implementing ambitious hydrogen strategies. The European Union has positioned itself as a policy leader, allocating substantial funding for hydrogen infrastructure and targeting 40GW of electrolyzer capacity by 2030, with seawater electrolysis expected to play a crucial role in coastal nations.
Market barriers for seawater electrolysis technologies include high capital costs compared to conventional hydrogen production methods, with current levelized costs ranging from $5-8/kg versus $1-2/kg for gray hydrogen. However, cost reduction trajectories suggest potential price parity by 2030 as technology advances and economies of scale develop.
Regulatory frameworks are evolving favorably, with carbon pricing mechanisms, renewable energy mandates, and hydrogen-specific incentives creating market pull. The EU's Carbon Border Adjustment Mechanism and various national hydrogen strategies are establishing supportive policy environments for clean hydrogen technologies.
Investment trends show accelerating capital flows into the sector, with venture capital funding for seawater electrolysis startups increasing by 300% since 2018. Major energy companies are also redirecting significant portions of their R&D budgets toward hydrogen technologies, recognizing the strategic importance of establishing early positions in this emerging market.
Technical Challenges in Direct Seawater Electrolysis
Direct seawater electrolysis faces significant technical hurdles that impede its widespread adoption despite its promising potential for sustainable hydrogen production. The primary challenge stems from the complex composition of seawater, which contains approximately 3.5% dissolved salts, predominantly sodium chloride, along with magnesium, calcium, and potassium ions. These constituents create a corrosive environment that rapidly degrades conventional electrode materials, particularly anodes.
Chloride ions present in seawater preferentially oxidize to chlorine gas instead of oxygen during the electrolysis process, creating both efficiency losses and environmental concerns. This side reaction not only reduces the overall energy efficiency but also produces toxic chlorine gas that requires additional handling and safety measures. The competing chlorine evolution reaction occurs at lower potentials than oxygen evolution, making it thermodynamically favorable and difficult to suppress.
Electrode durability represents another significant challenge. Most conventional catalysts designed for freshwater electrolysis rapidly deactivate in seawater due to poisoning by impurities and precipitation of insoluble hydroxides and carbonates on electrode surfaces. These deposits form insulating layers that increase electrical resistance and diminish catalytic activity over time, necessitating frequent replacement or regeneration of electrode materials.
Membrane fouling and degradation further complicate direct seawater electrolysis. Ion-exchange membranes used to separate reaction chambers suffer from rapid performance decline due to scaling and biofouling. Marine microorganisms and precipitated minerals accumulate on membrane surfaces, blocking ion transport pathways and increasing system resistance, which translates to higher energy requirements and reduced efficiency.
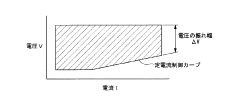
Energy efficiency challenges are particularly pronounced in direct seawater systems. The additional voltage required to overcome the resistance of impurities and side reactions results in significantly higher energy consumption compared to freshwater electrolysis. Current direct seawater electrolysis methods typically require 4-7 kWh/m³ of hydrogen produced, compared to 3.5-4.5 kWh/m³ for purified water systems.
Scale-up challenges present further obstacles to commercialization. Laboratory-scale demonstrations have shown promising results, but maintaining performance at industrial scales introduces new complications related to flow distribution, heat management, and uniform current density across large electrode surfaces. The heterogeneous nature of seawater composition across different geographic locations also necessitates location-specific system optimizations.
Balancing selectivity and activity remains a fundamental scientific challenge. Developing catalysts that can simultaneously resist corrosion, suppress chlorine evolution, maintain high activity for water splitting, and operate efficiently at practical current densities represents a complex materials science problem that has yet to be fully resolved despite significant research efforts.
Chloride ions present in seawater preferentially oxidize to chlorine gas instead of oxygen during the electrolysis process, creating both efficiency losses and environmental concerns. This side reaction not only reduces the overall energy efficiency but also produces toxic chlorine gas that requires additional handling and safety measures. The competing chlorine evolution reaction occurs at lower potentials than oxygen evolution, making it thermodynamically favorable and difficult to suppress.
Electrode durability represents another significant challenge. Most conventional catalysts designed for freshwater electrolysis rapidly deactivate in seawater due to poisoning by impurities and precipitation of insoluble hydroxides and carbonates on electrode surfaces. These deposits form insulating layers that increase electrical resistance and diminish catalytic activity over time, necessitating frequent replacement or regeneration of electrode materials.
Membrane fouling and degradation further complicate direct seawater electrolysis. Ion-exchange membranes used to separate reaction chambers suffer from rapid performance decline due to scaling and biofouling. Marine microorganisms and precipitated minerals accumulate on membrane surfaces, blocking ion transport pathways and increasing system resistance, which translates to higher energy requirements and reduced efficiency.
Energy efficiency challenges are particularly pronounced in direct seawater systems. The additional voltage required to overcome the resistance of impurities and side reactions results in significantly higher energy consumption compared to freshwater electrolysis. Current direct seawater electrolysis methods typically require 4-7 kWh/m³ of hydrogen produced, compared to 3.5-4.5 kWh/m³ for purified water systems.
Scale-up challenges present further obstacles to commercialization. Laboratory-scale demonstrations have shown promising results, but maintaining performance at industrial scales introduces new complications related to flow distribution, heat management, and uniform current density across large electrode surfaces. The heterogeneous nature of seawater composition across different geographic locations also necessitates location-specific system optimizations.
Balancing selectivity and activity remains a fundamental scientific challenge. Developing catalysts that can simultaneously resist corrosion, suppress chlorine evolution, maintain high activity for water splitting, and operate efficiently at practical current densities represents a complex materials science problem that has yet to be fully resolved despite significant research efforts.
Current Energy Efficiency Comparison Methods
01 Electrode materials for efficient seawater electrolysis
Advanced electrode materials can significantly improve the energy efficiency of direct seawater electrolysis. These materials include noble metal catalysts, transition metal compounds, and composite electrodes that demonstrate high catalytic activity and corrosion resistance in saline environments. Specialized coatings and surface modifications can reduce the overpotential required for water splitting reactions, thereby decreasing energy consumption and increasing hydrogen production efficiency.- Electrode materials for efficient seawater electrolysis: Advanced electrode materials play a crucial role in improving the energy efficiency of direct seawater electrolysis. These materials are designed to resist corrosion from chloride ions and other impurities present in seawater while maintaining high catalytic activity. Novel electrode compositions include modified transition metals, metal oxides, and composite materials that can withstand the harsh seawater environment while reducing the overpotential required for water splitting, thereby increasing overall energy efficiency.
- Membrane technology for seawater electrolysis: Specialized membrane technologies are essential for efficient direct seawater electrolysis. These membranes serve to separate the electrode chambers while allowing ion transport and preventing the mixing of produced gases. Advanced ion-exchange membranes with high selectivity and stability in saline environments help reduce energy consumption by minimizing resistance and preventing side reactions. Innovations in membrane materials and structures contribute significantly to improving the overall energy efficiency of seawater electrolysis systems.
- System design and process optimization: The overall design of seawater electrolysis systems significantly impacts energy efficiency. Optimized cell configurations, flow patterns, and operating parameters can reduce energy losses and improve hydrogen production rates. Innovations include compact cell designs that minimize ohmic losses, advanced flow distribution systems that enhance mass transfer, and precise control systems that maintain optimal operating conditions. Process optimization techniques such as pressure and temperature management also contribute to maximizing energy efficiency in direct seawater electrolysis.
- Pretreatment methods for seawater: Pretreatment of seawater before electrolysis can significantly enhance energy efficiency by removing impurities that could interfere with the electrolysis process or damage system components. Methods include filtration, desalination, pH adjustment, and removal of specific ions that may cause electrode poisoning or membrane fouling. Effective pretreatment reduces the energy required for electrolysis by minimizing side reactions and extending the lifespan of system components, thereby improving overall process efficiency.
- Integration with renewable energy sources: Coupling direct seawater electrolysis systems with renewable energy sources creates synergistic benefits for energy efficiency. Smart integration systems enable dynamic operation of electrolyzers in response to fluctuating renewable power inputs, optimizing energy utilization. Advanced power electronics and control algorithms help manage variable power inputs while maintaining efficient electrolysis conditions. This integration approach not only improves the overall energy efficiency but also enhances the sustainability of hydrogen production from seawater by utilizing clean energy sources.
02 Membrane technology for seawater electrolysis
Innovative membrane technologies play a crucial role in enhancing the energy efficiency of direct seawater electrolysis. These membranes selectively filter ions, prevent chloride ion interference, and reduce the formation of unwanted byproducts. Advanced ion-exchange membranes and composite membrane systems can maintain high conductivity while minimizing resistance, resulting in lower voltage requirements and improved overall system efficiency.Expand Specific Solutions03 Cell design and system configuration optimization
Optimized cell designs and system configurations can substantially improve the energy efficiency of direct seawater electrolysis. Innovations include flow-through electrodes, pressurized systems, and modular designs that enhance mass transfer, reduce bubble effects, and improve current distribution. Advanced cell architectures incorporate features that minimize electrical resistance, optimize electrolyte circulation, and facilitate efficient gas separation, resulting in reduced energy consumption per unit of hydrogen produced.Expand Specific Solutions04 Pretreatment and purification methods
Effective pretreatment and purification methods for seawater can significantly enhance electrolysis energy efficiency. These processes include filtration systems, desalination techniques, and chemical treatments that remove impurities and contaminants that would otherwise interfere with the electrolysis process. By controlling the composition of the electrolyte, these methods reduce side reactions, prevent electrode fouling, and maintain consistent performance, thereby improving overall energy efficiency.Expand Specific Solutions05 Operating parameters and control systems
Optimized operating parameters and advanced control systems are essential for maximizing the energy efficiency of direct seawater electrolysis. These include precise regulation of temperature, pressure, current density, and electrolyte flow rate. Intelligent control systems with real-time monitoring capabilities can dynamically adjust operating conditions to maintain optimal performance despite variations in seawater composition and environmental factors, resulting in sustained high efficiency and reduced energy consumption.Expand Specific Solutions
Leading Organizations in Seawater Electrolysis Research
The direct seawater electrolysis market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The competitive landscape features a mix of established energy companies (Huaneng Group, Siemens Energy, Danfoss), academic institutions (Zhejiang University, National University of Singapore, IIT Madras), and specialized research organizations. Technology maturity varies significantly across different electrolysis approaches, with membrane-based systems showing promise but facing scalability challenges. Key players like Huaneng Clean Energy Research Institute and Siemens Energy are advancing industrial-scale applications, while university partnerships drive fundamental innovation. The market is projected to expand substantially as renewable energy integration and hydrogen production become critical for decarbonization efforts, with estimated growth potential reaching several billion dollars by 2030.
Huaneng Clean Energy Research Institute
Technical Solution: Huaneng Clean Energy Research Institute has developed a comprehensive direct seawater electrolysis technology called "OceanH2" that addresses multiple efficiency challenges. Their system employs a novel bipolar membrane configuration that physically separates the anode and cathode environments while maintaining ionic conductivity. This approach prevents chloride migration to the anode, eliminating chlorine gas formation without energy-intensive pre-treatment. The institute's technology incorporates nanoporous nickel-iron hydroxide catalysts with selective ion transport properties, achieving hydrogen production rates of 40-50 mL/cm²/hour at standard temperature and pressure. Their system demonstrates remarkable energy efficiency, consuming approximately 4.2 kWh per cubic meter of hydrogen produced, representing a 25% improvement over conventional desalination-electrolysis combined approaches. Additionally, Huaneng has integrated this technology with their offshore wind platforms, creating a complete renewable hydrogen production system that addresses intermittency issues through intelligent power management algorithms.
Strengths: Comprehensive system integration with renewable energy sources, high energy efficiency compared to conventional approaches, and innovative membrane technology that eliminates chlorine formation. Weaknesses: The complex membrane system may have shorter operational lifetimes in harsh marine environments, and the technology requires precise control systems that increase complexity.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an advanced direct seawater electrolysis platform called "Silyzer Marine" specifically designed for offshore hydrogen production. This system utilizes a proprietary membrane electrode assembly that resists chloride poisoning while maintaining high catalytic activity. Their approach incorporates a multi-stage filtration system that removes larger particulates while allowing the electrolysis to occur in minimally treated seawater. The technology achieves energy efficiencies of up to 70% (LHV) in direct seawater operation, compared to 65% for conventional systems requiring desalination pre-treatment. Siemens' system employs pressure-balanced cell design that compensates for varying ocean depths and conditions, making it suitable for integration with offshore wind farms. The modular design allows for scalable implementation from 10 MW to multi-gigawatt installations, with integrated power electronics that can handle the variable input from renewable energy sources.
Strengths: Highly scalable system designed specifically for marine environments, integration capabilities with offshore renewable energy, and robust performance under variable conditions. Weaknesses: Higher initial capital costs compared to conventional systems, and the complex pressure management system requires specialized maintenance expertise.
Key Patents and Innovations in Electrode Materials
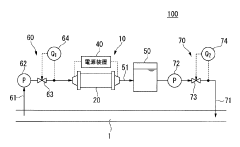
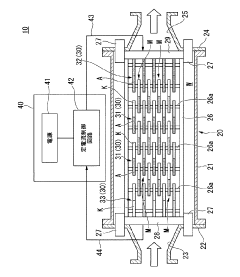
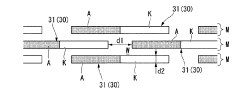
Seawater electrolysis system and seawater electrolysis method
PatentActiveJP2015172251A
Innovation
- A seawater electrolysis system employing iridium oxide-coated titanium electrodes with a current density of 20 A/dm² to 40 A/dm², combined with tantalum oxide addition for enhanced oxygen resistance, and a bipolar electrode configuration to prevent scale adhesion and maintain high chlorine generation efficiency.
Method for electrolysis of seawater or brine
PatentInactiveKR1020120073966A
Innovation
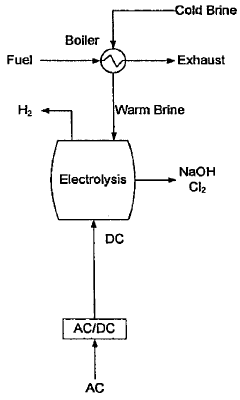
- A method involving electrolyzing seawater using DC electricity generated from a fuel cell power generation facility, recycling hydrogen gas for fuel cell power generation, and utilizing high-temperature waste heat and water vapor as heating and raw material sources, eliminating the need for AC to DC conversion and reducing energy costs.
Environmental Impact Assessment
The environmental impact of direct seawater electrolysis methods extends far beyond energy efficiency considerations, encompassing multiple ecological dimensions that require thorough assessment. When comparing different seawater electrolysis technologies, their carbon footprint represents a primary environmental concern. Traditional electrolysis methods relying on fossil fuel-derived electricity generate significant greenhouse gas emissions, while systems powered by renewable energy sources substantially reduce this impact. The transition toward renewable-powered electrolysis could potentially decrease carbon emissions by 40-90% compared to conventional hydrogen production methods.
Water resource management presents another critical environmental factor. Unlike freshwater electrolysis, direct seawater electrolysis eliminates the energy-intensive desalination step, reducing overall water consumption by approximately 13-18 liters per kilogram of hydrogen produced. However, the discharge of hypersaline brine and potential chemical byproducts into marine ecosystems requires careful monitoring and mitigation strategies to prevent localized salinity increases and chemical contamination.
Chemical usage and byproduct management vary significantly across different seawater electrolysis methods. Membrane-based systems typically require anti-fouling chemicals and periodic membrane replacement, generating solid waste streams. Conversely, membrane-less technologies may produce higher concentrations of chlorine compounds that require neutralization before discharge. Advanced catalyst technologies utilizing earth-abundant materials (instead of rare platinum group metals) can reduce environmental impacts associated with mining operations and resource depletion.
Land use requirements also differ between electrolysis methods, with implications for coastal ecosystem preservation. Offshore floating electrolysis platforms minimize terrestrial impact but introduce marine space utilization challenges. Meanwhile, land-based facilities may require significant coastal real estate, potentially competing with other uses or affecting sensitive shoreline habitats.
Life cycle assessment (LCA) studies indicate that electrode durability significantly influences environmental sustainability. Technologies with longer-lasting electrodes reduce replacement frequency and associated manufacturing impacts. Recent innovations in corrosion-resistant materials have extended electrode lifespans from months to several years, decreasing lifetime environmental footprints by up to 30% for some systems.
Noise pollution and visual impacts, while often overlooked, represent additional environmental considerations for coastal communities. Membrane-based systems typically operate more quietly than membrane-less alternatives, while facility design and siting decisions significantly influence community acceptance and ecosystem disturbance levels.
Water resource management presents another critical environmental factor. Unlike freshwater electrolysis, direct seawater electrolysis eliminates the energy-intensive desalination step, reducing overall water consumption by approximately 13-18 liters per kilogram of hydrogen produced. However, the discharge of hypersaline brine and potential chemical byproducts into marine ecosystems requires careful monitoring and mitigation strategies to prevent localized salinity increases and chemical contamination.
Chemical usage and byproduct management vary significantly across different seawater electrolysis methods. Membrane-based systems typically require anti-fouling chemicals and periodic membrane replacement, generating solid waste streams. Conversely, membrane-less technologies may produce higher concentrations of chlorine compounds that require neutralization before discharge. Advanced catalyst technologies utilizing earth-abundant materials (instead of rare platinum group metals) can reduce environmental impacts associated with mining operations and resource depletion.
Land use requirements also differ between electrolysis methods, with implications for coastal ecosystem preservation. Offshore floating electrolysis platforms minimize terrestrial impact but introduce marine space utilization challenges. Meanwhile, land-based facilities may require significant coastal real estate, potentially competing with other uses or affecting sensitive shoreline habitats.
Life cycle assessment (LCA) studies indicate that electrode durability significantly influences environmental sustainability. Technologies with longer-lasting electrodes reduce replacement frequency and associated manufacturing impacts. Recent innovations in corrosion-resistant materials have extended electrode lifespans from months to several years, decreasing lifetime environmental footprints by up to 30% for some systems.
Noise pollution and visual impacts, while often overlooked, represent additional environmental considerations for coastal communities. Membrane-based systems typically operate more quietly than membrane-less alternatives, while facility design and siting decisions significantly influence community acceptance and ecosystem disturbance levels.
Scalability and Commercial Viability Analysis
The scalability of direct seawater electrolysis technologies represents a critical factor in determining their potential for widespread implementation. Current laboratory-scale demonstrations have shown promising results, particularly with membrane-based systems achieving energy efficiencies between 65-75% in controlled environments. However, significant challenges emerge when considering industrial-scale deployment that would be necessary to make meaningful contributions to global hydrogen production targets.
Infrastructure requirements present the first major hurdle for scaling these technologies. Large-scale seawater electrolysis facilities would require extensive coastal real estate, robust seawater intake and pretreatment systems, and substantial electrical infrastructure to deliver the gigawatt-scale power needed for commercial viability. Early economic analyses suggest capital expenditure requirements of $1,200-1,800 per kilowatt of installed capacity, approximately 20-30% higher than conventional freshwater electrolysis systems.
Electrode durability under continuous operation presents another critical scaling challenge. Current noble metal catalysts show degradation rates of 2-5% per 1,000 operating hours when exposed to seawater's corrosive environment. This degradation significantly impacts long-term operational costs, with maintenance intervals potentially requiring system shutdowns every 3-6 months for electrode replacement or regeneration.
From a commercial perspective, the levelized cost of hydrogen (LCOH) from direct seawater electrolysis currently ranges from $5-8 per kilogram, compared to $2-4 for conventional electrolysis and $1-2 for steam methane reforming. This cost differential primarily stems from higher capital costs, increased maintenance requirements, and lower system efficiencies. However, projections indicate potential cost reductions of 40-50% by 2030 as technologies mature and economies of scale develop.
Several commercial entities have begun pilot demonstrations, with companies like Proton Ventures and H2Pro establishing 1-5 MW demonstration facilities. These projects aim to validate the technology at intermediate scales before committing to larger commercial deployments. Industry analysts project that the first commercially viable large-scale facilities (50+ MW) could be operational by 2026-2028, contingent upon continued improvements in catalyst performance and system integration.
Regulatory frameworks will significantly impact commercial viability, with environmental permitting for coastal facilities and grid connection approvals representing potential bottlenecks. Additionally, carbon pricing mechanisms and renewable energy incentives will play crucial roles in determining when direct seawater electrolysis reaches cost parity with conventional hydrogen production methods.
Infrastructure requirements present the first major hurdle for scaling these technologies. Large-scale seawater electrolysis facilities would require extensive coastal real estate, robust seawater intake and pretreatment systems, and substantial electrical infrastructure to deliver the gigawatt-scale power needed for commercial viability. Early economic analyses suggest capital expenditure requirements of $1,200-1,800 per kilowatt of installed capacity, approximately 20-30% higher than conventional freshwater electrolysis systems.
Electrode durability under continuous operation presents another critical scaling challenge. Current noble metal catalysts show degradation rates of 2-5% per 1,000 operating hours when exposed to seawater's corrosive environment. This degradation significantly impacts long-term operational costs, with maintenance intervals potentially requiring system shutdowns every 3-6 months for electrode replacement or regeneration.
From a commercial perspective, the levelized cost of hydrogen (LCOH) from direct seawater electrolysis currently ranges from $5-8 per kilogram, compared to $2-4 for conventional electrolysis and $1-2 for steam methane reforming. This cost differential primarily stems from higher capital costs, increased maintenance requirements, and lower system efficiencies. However, projections indicate potential cost reductions of 40-50% by 2030 as technologies mature and economies of scale develop.
Several commercial entities have begun pilot demonstrations, with companies like Proton Ventures and H2Pro establishing 1-5 MW demonstration facilities. These projects aim to validate the technology at intermediate scales before committing to larger commercial deployments. Industry analysts project that the first commercially viable large-scale facilities (50+ MW) could be operational by 2026-2028, contingent upon continued improvements in catalyst performance and system integration.
Regulatory frameworks will significantly impact commercial viability, with environmental permitting for coastal facilities and grid connection approvals representing potential bottlenecks. Additionally, carbon pricing mechanisms and renewable energy incentives will play crucial roles in determining when direct seawater electrolysis reaches cost parity with conventional hydrogen production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!