Comparative analysis of proton-conducting ceramics for hydrogen electrolysis
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proton-Conducting Ceramics Background and Objectives
Proton-conducting ceramics have emerged as a critical technology in the field of hydrogen production through electrolysis, representing a significant advancement over traditional methods. The evolution of these materials spans several decades, beginning with the discovery of proton conduction in perovskite oxides in the 1980s. Since then, research has progressed through various material systems including perovskites (BaCeO₃, BaZrO₃), brownmillerites, and more complex structures, each offering unique advantages for hydrogen electrolysis applications.
The technological trajectory has been characterized by continuous improvements in ionic conductivity, chemical stability, and mechanical properties. Early proton conductors suffered from poor stability in CO₂ and H₂O-containing environments, leading to the development of more robust compositions. The field has witnessed a paradigm shift from purely academic research to industrial implementation, with significant breakthroughs in material synthesis, processing techniques, and device fabrication.
Current research trends focus on developing ceramics that operate efficiently at intermediate temperatures (400-700°C), representing a sweet spot between high ionic conductivity and reasonable system costs. This temperature range allows for faster kinetics than low-temperature electrolysis while avoiding the materials degradation issues associated with high-temperature operation.
The primary technical objectives in this field include enhancing proton conductivity to exceed 10⁻² S/cm at operating temperatures, improving chemical stability in steam and CO₂-rich environments, developing cost-effective fabrication methods for thin electrolytes, and achieving long-term operational stability exceeding 40,000 hours. Additionally, there is a growing emphasis on understanding and optimizing the complex interfaces between the electrolyte and electrodes, which often limit overall system performance.
Global interest in hydrogen as a clean energy carrier has accelerated research in proton-conducting ceramics, with particular momentum gained after the Paris Climate Agreement. The technology is positioned at the intersection of renewable energy integration and industrial decarbonization efforts, with potential applications extending beyond electrolysis to include fuel cells, gas separation membranes, and chemical synthesis.
The comparative analysis of different proton-conducting ceramic systems is essential for identifying optimal materials for specific applications and operating conditions. This analysis must consider not only intrinsic properties like conductivity and stability but also practical aspects such as manufacturability, cost, and compatibility with other system components. Understanding these trade-offs is crucial for advancing the technology toward commercial viability and addressing the growing demand for green hydrogen production methods.
The technological trajectory has been characterized by continuous improvements in ionic conductivity, chemical stability, and mechanical properties. Early proton conductors suffered from poor stability in CO₂ and H₂O-containing environments, leading to the development of more robust compositions. The field has witnessed a paradigm shift from purely academic research to industrial implementation, with significant breakthroughs in material synthesis, processing techniques, and device fabrication.
Current research trends focus on developing ceramics that operate efficiently at intermediate temperatures (400-700°C), representing a sweet spot between high ionic conductivity and reasonable system costs. This temperature range allows for faster kinetics than low-temperature electrolysis while avoiding the materials degradation issues associated with high-temperature operation.
The primary technical objectives in this field include enhancing proton conductivity to exceed 10⁻² S/cm at operating temperatures, improving chemical stability in steam and CO₂-rich environments, developing cost-effective fabrication methods for thin electrolytes, and achieving long-term operational stability exceeding 40,000 hours. Additionally, there is a growing emphasis on understanding and optimizing the complex interfaces between the electrolyte and electrodes, which often limit overall system performance.
Global interest in hydrogen as a clean energy carrier has accelerated research in proton-conducting ceramics, with particular momentum gained after the Paris Climate Agreement. The technology is positioned at the intersection of renewable energy integration and industrial decarbonization efforts, with potential applications extending beyond electrolysis to include fuel cells, gas separation membranes, and chemical synthesis.
The comparative analysis of different proton-conducting ceramic systems is essential for identifying optimal materials for specific applications and operating conditions. This analysis must consider not only intrinsic properties like conductivity and stability but also practical aspects such as manufacturability, cost, and compatibility with other system components. Understanding these trade-offs is crucial for advancing the technology toward commercial viability and addressing the growing demand for green hydrogen production methods.
Market Analysis for Hydrogen Electrolysis Technologies
The global hydrogen electrolysis market is experiencing unprecedented growth, driven by increasing focus on decarbonization and renewable energy integration. Current market valuations place the hydrogen electrolysis technology sector at approximately 280 million USD in 2022, with projections indicating a compound annual growth rate of 24.5% through 2030. This remarkable expansion is primarily fueled by government initiatives worldwide to reduce carbon emissions and establish hydrogen-based economies.
Proton-conducting ceramic electrolysis cells (PCECs) represent a significant segment within this market, offering distinct advantages over conventional alkaline and PEM electrolyzers. The market for ceramic-based electrolysis technologies is currently smaller but growing at an accelerated rate due to their superior performance at intermediate-to-high temperatures and enhanced durability characteristics.
Regional analysis reveals that Europe leads the hydrogen electrolysis market, accounting for roughly 40% of global installations, followed by Asia-Pacific at 30% and North America at 20%. Within the proton-conducting ceramics segment specifically, Japan, Germany, and the United States dominate research and commercial development activities, collectively holding over 65% of related patents.
End-user segmentation shows increasing adoption across multiple sectors. Industrial applications currently represent the largest market share at 45%, followed by energy storage applications at 30%, and transportation at 15%. The remaining 10% encompasses emerging applications including building heat and power systems. For proton-conducting ceramic technologies specifically, industrial applications requiring high-temperature steam electrolysis show the strongest market potential.
Market barriers for proton-conducting ceramic technologies include high manufacturing costs, estimated at 2-3 times those of conventional electrolyzers, and limited production scale. However, cost reduction trajectories suggest a potential 60% decrease in manufacturing expenses by 2030 as production volumes increase and material innovations emerge.
Customer demand analysis indicates growing interest from industrial hydrogen users seeking higher efficiency and lower operational costs. Survey data shows that 72% of potential industrial adopters prioritize efficiency over initial capital expenditure, creating a favorable environment for premium ceramic-based solutions despite their higher upfront costs.
Competitive pricing analysis reveals that while proton-conducting ceramic electrolyzers currently command a premium price point, their total cost of ownership over a 10-year operational period may be lower than alternatives when considering efficiency gains, reduced maintenance requirements, and longer operational lifespans.
Proton-conducting ceramic electrolysis cells (PCECs) represent a significant segment within this market, offering distinct advantages over conventional alkaline and PEM electrolyzers. The market for ceramic-based electrolysis technologies is currently smaller but growing at an accelerated rate due to their superior performance at intermediate-to-high temperatures and enhanced durability characteristics.
Regional analysis reveals that Europe leads the hydrogen electrolysis market, accounting for roughly 40% of global installations, followed by Asia-Pacific at 30% and North America at 20%. Within the proton-conducting ceramics segment specifically, Japan, Germany, and the United States dominate research and commercial development activities, collectively holding over 65% of related patents.
End-user segmentation shows increasing adoption across multiple sectors. Industrial applications currently represent the largest market share at 45%, followed by energy storage applications at 30%, and transportation at 15%. The remaining 10% encompasses emerging applications including building heat and power systems. For proton-conducting ceramic technologies specifically, industrial applications requiring high-temperature steam electrolysis show the strongest market potential.
Market barriers for proton-conducting ceramic technologies include high manufacturing costs, estimated at 2-3 times those of conventional electrolyzers, and limited production scale. However, cost reduction trajectories suggest a potential 60% decrease in manufacturing expenses by 2030 as production volumes increase and material innovations emerge.
Customer demand analysis indicates growing interest from industrial hydrogen users seeking higher efficiency and lower operational costs. Survey data shows that 72% of potential industrial adopters prioritize efficiency over initial capital expenditure, creating a favorable environment for premium ceramic-based solutions despite their higher upfront costs.
Competitive pricing analysis reveals that while proton-conducting ceramic electrolyzers currently command a premium price point, their total cost of ownership over a 10-year operational period may be lower than alternatives when considering efficiency gains, reduced maintenance requirements, and longer operational lifespans.
Current Status and Challenges in Proton-Conducting Ceramics
Proton-conducting ceramics have emerged as promising materials for hydrogen electrolysis applications, with significant research advancements globally. Currently, the field is dominated by several key material systems, including BaZrO₃-based perovskites (particularly BaZr₀.₈Y₀.₂O₃₋δ or BZY), BaCeO₃-based materials, and various composite structures. These materials have demonstrated proton conductivities in the range of 10⁻² to 10⁻³ S/cm at intermediate temperatures (400-700°C), making them viable candidates for efficient hydrogen production.
Despite these promising developments, several significant challenges persist in the field. Chemical stability remains a critical issue, particularly for cerium-based materials which exhibit excellent conductivity but poor stability in CO₂ and H₂O-containing atmospheres. Zirconium-based alternatives offer superior chemical stability but suffer from lower conductivity and challenging sinterability, often requiring extreme processing conditions (>1700°C) or sintering aids that may compromise performance.
The mechanical integrity of these ceramic materials presents another substantial challenge. Thermal cycling during operation induces stress that can lead to microcracking and eventual failure. This is particularly problematic at the electrolyte-electrode interfaces where thermal expansion coefficient mismatches exacerbate mechanical stress. Current research efforts focus on developing composite structures and gradient materials to mitigate these issues.
Manufacturing scalability represents a significant bottleneck for widespread implementation. Laboratory-scale synthesis methods often fail to translate effectively to industrial production, resulting in inconsistent material properties and performance. The high processing temperatures required for densification further complicate mass production efforts and increase manufacturing costs.
Regionally, research leadership in this field shows distinct patterns. Japan and South Korea maintain strong positions in fundamental materials development, while European research institutions focus heavily on system integration and long-term stability. China has rapidly expanded its research output, particularly in novel synthesis methods and composite materials, while North American institutions lead in advanced characterization techniques and theoretical modeling.
The interface between the electrolyte and electrodes remains particularly challenging, with issues of chemical compatibility, interfacial resistance, and long-term stability under operating conditions. Recent advances in atomic layer deposition and interface engineering show promise in addressing these challenges, but significant work remains to optimize these critical regions for commercial viability.
Durability under real-world operating conditions continues to be insufficiently addressed, with most studies reporting performance data for relatively short periods (hundreds of hours) rather than the thousands of hours required for commercial applications. Degradation mechanisms, particularly those related to impurity segregation and phase transformations during extended operation, require further investigation.
Despite these promising developments, several significant challenges persist in the field. Chemical stability remains a critical issue, particularly for cerium-based materials which exhibit excellent conductivity but poor stability in CO₂ and H₂O-containing atmospheres. Zirconium-based alternatives offer superior chemical stability but suffer from lower conductivity and challenging sinterability, often requiring extreme processing conditions (>1700°C) or sintering aids that may compromise performance.
The mechanical integrity of these ceramic materials presents another substantial challenge. Thermal cycling during operation induces stress that can lead to microcracking and eventual failure. This is particularly problematic at the electrolyte-electrode interfaces where thermal expansion coefficient mismatches exacerbate mechanical stress. Current research efforts focus on developing composite structures and gradient materials to mitigate these issues.
Manufacturing scalability represents a significant bottleneck for widespread implementation. Laboratory-scale synthesis methods often fail to translate effectively to industrial production, resulting in inconsistent material properties and performance. The high processing temperatures required for densification further complicate mass production efforts and increase manufacturing costs.
Regionally, research leadership in this field shows distinct patterns. Japan and South Korea maintain strong positions in fundamental materials development, while European research institutions focus heavily on system integration and long-term stability. China has rapidly expanded its research output, particularly in novel synthesis methods and composite materials, while North American institutions lead in advanced characterization techniques and theoretical modeling.
The interface between the electrolyte and electrodes remains particularly challenging, with issues of chemical compatibility, interfacial resistance, and long-term stability under operating conditions. Recent advances in atomic layer deposition and interface engineering show promise in addressing these challenges, but significant work remains to optimize these critical regions for commercial viability.
Durability under real-world operating conditions continues to be insufficiently addressed, with most studies reporting performance data for relatively short periods (hundreds of hours) rather than the thousands of hours required for commercial applications. Degradation mechanisms, particularly those related to impurity segregation and phase transformations during extended operation, require further investigation.
Existing Ceramic Electrolyte Solutions
01 Perovskite-based proton conducting ceramics
Perovskite-structured ceramics, particularly those based on barium zirconate and barium cerate compositions, demonstrate excellent proton conductivity at intermediate temperatures. These materials can be doped with various elements such as yttrium, gadolinium, or indium to enhance their conductivity by creating oxygen vacancies that facilitate proton transport. The perovskite structure provides stable pathways for proton conduction, making these ceramics suitable for applications in solid oxide fuel cells and hydrogen separation membranes.- Perovskite-type proton-conducting ceramics: Perovskite-type ceramics are widely used as proton conductors due to their high conductivity and stability. These materials, typically with ABO₃ structure, can be doped with various elements to enhance proton conductivity. The incorporation of elements like barium, strontium, cerium, and zirconium in the crystal structure creates oxygen vacancies that facilitate proton transport. These ceramics demonstrate excellent conductivity at intermediate temperatures (400-700°C) and are particularly suitable for applications in solid oxide fuel cells and hydrogen separation membranes.
- Doping strategies to enhance proton conductivity: Various doping strategies can significantly improve the proton conductivity of ceramic materials. Acceptor doping with elements such as yttrium, gadolinium, or indium creates oxygen vacancies that serve as proton transport sites. Co-doping approaches using multiple dopants can create synergistic effects that further enhance conductivity. The concentration and distribution of dopants play crucial roles in determining the final conductivity properties. Optimized doping can lower the activation energy for proton transport and increase the overall conductivity by orders of magnitude.
- Composite proton-conducting ceramics: Composite ceramic materials combine different phases to achieve enhanced proton conductivity. These composites often incorporate a highly conductive phase within a mechanically stable matrix. Common approaches include mixing proton-conducting ceramics with other ionic conductors or adding a second phase to create beneficial interfaces that enhance proton transport. Some composites utilize nanoscale effects at grain boundaries to improve conductivity. These materials often show improved thermal stability and mechanical properties compared to single-phase ceramics while maintaining high proton conductivity.
- Processing techniques for high-conductivity ceramics: Advanced processing techniques significantly impact the final conductivity of proton-conducting ceramics. Methods such as sol-gel synthesis, solid-state reaction, hydrothermal processing, and spark plasma sintering can be used to control microstructure development. The sintering conditions, including temperature, atmosphere, and duration, strongly influence grain size, density, and grain boundary properties. Optimized processing can minimize impurities at grain boundaries that block proton transport and create ceramics with controlled porosity or dense microstructures depending on the application requirements.
- Novel proton-conducting ceramic materials: Research has led to the development of novel ceramic materials with exceptional proton conductivity. These include rare-earth tungstates, phosphates, niobates, and complex oxides with unique crystal structures. Some materials feature specialized conduction pathways that facilitate rapid proton transport. Layered structures and materials with inherent oxygen vacancies show promising conductivity even at lower temperatures. These novel ceramics often demonstrate improved chemical stability in harsh environments and resistance to poisoning by contaminants, making them suitable for next-generation energy conversion and storage applications.
02 Composite proton conducting ceramics
Composite proton conducting ceramics combine multiple materials to achieve enhanced conductivity and stability. These composites often incorporate a primary proton conducting ceramic phase with secondary phases that can improve grain boundary conductivity, mechanical strength, or chemical stability. Examples include ceramic-ceramic composites and ceramic-polymer composites. The interfaces between different phases in these composites can create additional pathways for proton transport, resulting in higher overall conductivity compared to single-phase materials.Expand Specific Solutions03 Surface modification and interface engineering
Surface modification and interface engineering techniques can significantly enhance the proton conductivity of ceramic materials. These approaches include coating grain boundaries with conductive materials, creating core-shell structures, or introducing specific dopants at interfaces. By controlling the composition and structure at interfaces, the resistance to proton transport can be reduced, leading to improved overall conductivity. These techniques are particularly effective for addressing the common issue of high grain boundary resistance in proton conducting ceramics.Expand Specific Solutions04 Novel synthesis methods for high conductivity ceramics
Advanced synthesis methods can produce proton conducting ceramics with enhanced conductivity properties. These methods include sol-gel processing, hydrothermal synthesis, spark plasma sintering, and chemical vapor deposition. By controlling the particle size, crystallinity, density, and microstructure during synthesis, the proton transport properties can be optimized. These novel methods often result in ceramics with reduced grain boundary resistance, higher density, and more uniform distribution of dopants, all contributing to improved proton conductivity.Expand Specific Solutions05 Temperature and humidity effects on conductivity
The proton conductivity of ceramic materials is significantly influenced by operating temperature and humidity conditions. Most proton conducting ceramics show increased conductivity at elevated temperatures, with specific temperature ranges optimal for different material compositions. Humidity also plays a crucial role, as water vapor can be incorporated into the ceramic structure, providing additional proton carriers. Understanding and controlling these environmental factors is essential for optimizing the performance of proton conducting ceramics in practical applications such as fuel cells and electrolyzers.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The proton-conducting ceramics for hydrogen electrolysis market is currently in a growth phase, with increasing interest driven by clean energy transitions. The global market size is expanding rapidly, projected to reach significant value as hydrogen becomes central to decarbonization strategies. Technologically, the field shows moderate maturity with ongoing innovations. Leading research institutions like Centre National de la Recherche Scientifique and universities (Nagoya, Tianjin) are advancing fundamental science, while established companies including CoorsTek, Électricité de France, and Samsung Electronics are commercializing applications. Emerging players such as 1s1 Energy and Kyoto Fusioneering are introducing disruptive technologies. The competitive landscape features collaboration between academic institutions and industry partners, with significant activity in Japan, China, Europe, and North America, indicating a globally distributed innovation ecosystem.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced proton-conducting ceramic electrolyzers (PCCEs) based on BaZrO3-based perovskites doped with yttrium (BZY). Their technology focuses on optimizing the triple-phase boundary microstructure to enhance proton conductivity while maintaining mechanical stability. CNRS researchers have achieved significant breakthroughs in reducing the operating temperature of PCCEs to 400-600°C while maintaining high hydrogen production rates. Their proprietary ceramic processing techniques include reactive sintering methods that achieve relative densities exceeding 95% and grain boundary optimization that reduces proton transport resistance. Recent developments include composite electrolytes combining BZY with cerium-based materials to enhance both ionic conductivity and chemical stability in steam environments. CNRS has also pioneered electrode materials with mixed ionic-electronic conductivity to improve electrode kinetics and reduce polarization losses.
Strengths: Superior proton conductivity at intermediate temperatures (400-600°C); excellent chemical stability in steam environments; reduced grain boundary resistance through advanced processing techniques. Weaknesses: Manufacturing complexity requiring specialized equipment; higher production costs compared to conventional electrolyzers; challenges in scaling up laboratory prototypes to industrial scale.
CoorsTek, Inc.
Technical Solution: CoorsTek has developed proprietary proton-conducting ceramic membranes based on doped barium zirconate-cerate (BZCY) compositions for hydrogen electrolysis applications. Their technology platform, known as CeraMem™, features a unique multi-layer ceramic structure with an ultra-thin (10-20 μm) dense electrolyte layer supported on a porous substrate to minimize ohmic resistance while maintaining mechanical integrity. CoorsTek's manufacturing process employs advanced tape casting and co-firing techniques that enable mass production of large-area ceramic cells with consistent performance. Their latest generation electrolyzers operate at 500-700°C and achieve hydrogen production rates of 6-8 Nml/min/cm² at 1.3V with faradaic efficiencies exceeding 95%. The company has also developed specialized glass-ceramic sealing materials that maintain hermeticity under thermal cycling conditions and resist degradation in steam-rich environments. CoorsTek's integrated stack design incorporates proprietary interconnect materials that minimize contact resistance and ensure uniform current distribution.
Strengths: Established large-scale manufacturing capabilities for ceramic components; proprietary materials with enhanced chemical stability; integrated system design expertise from materials to complete stacks. Weaknesses: Higher operating temperatures compared to polymer electrolyte systems; thermal cycling durability remains challenging; relatively high materials cost for specialized ceramic compositions.
Key Patents and Scientific Breakthroughs
Proton Conducting Ceramic Membranes For Hydrogen Separation
PatentInactiveUS20080032140A1
Innovation
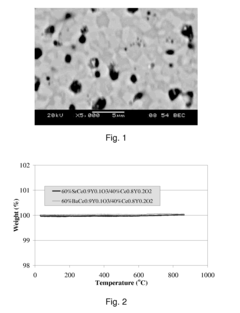
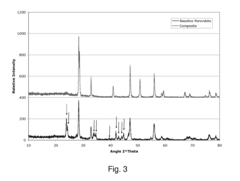
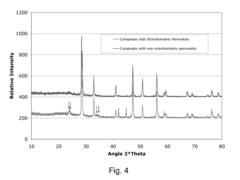
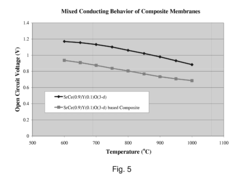
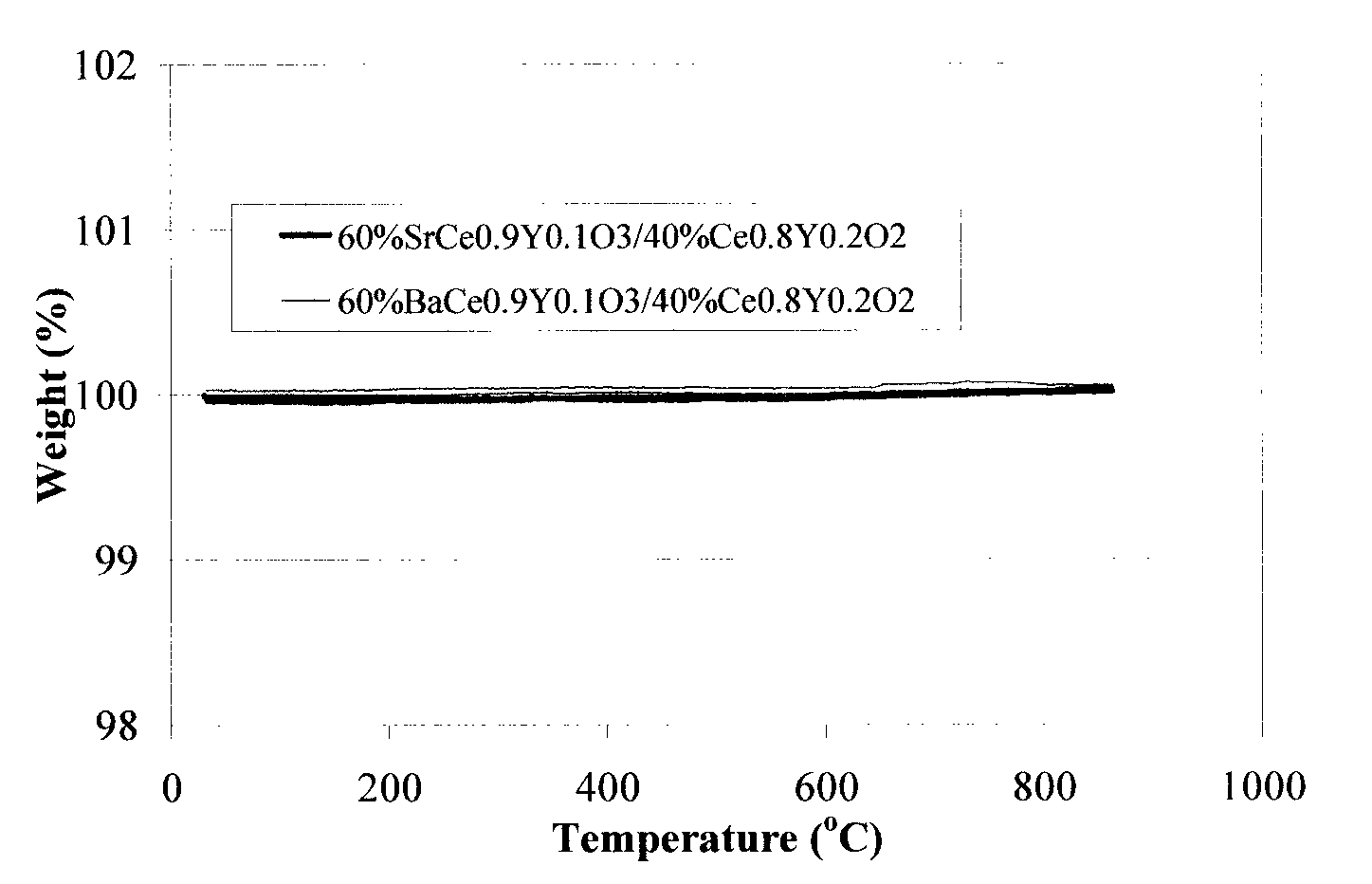
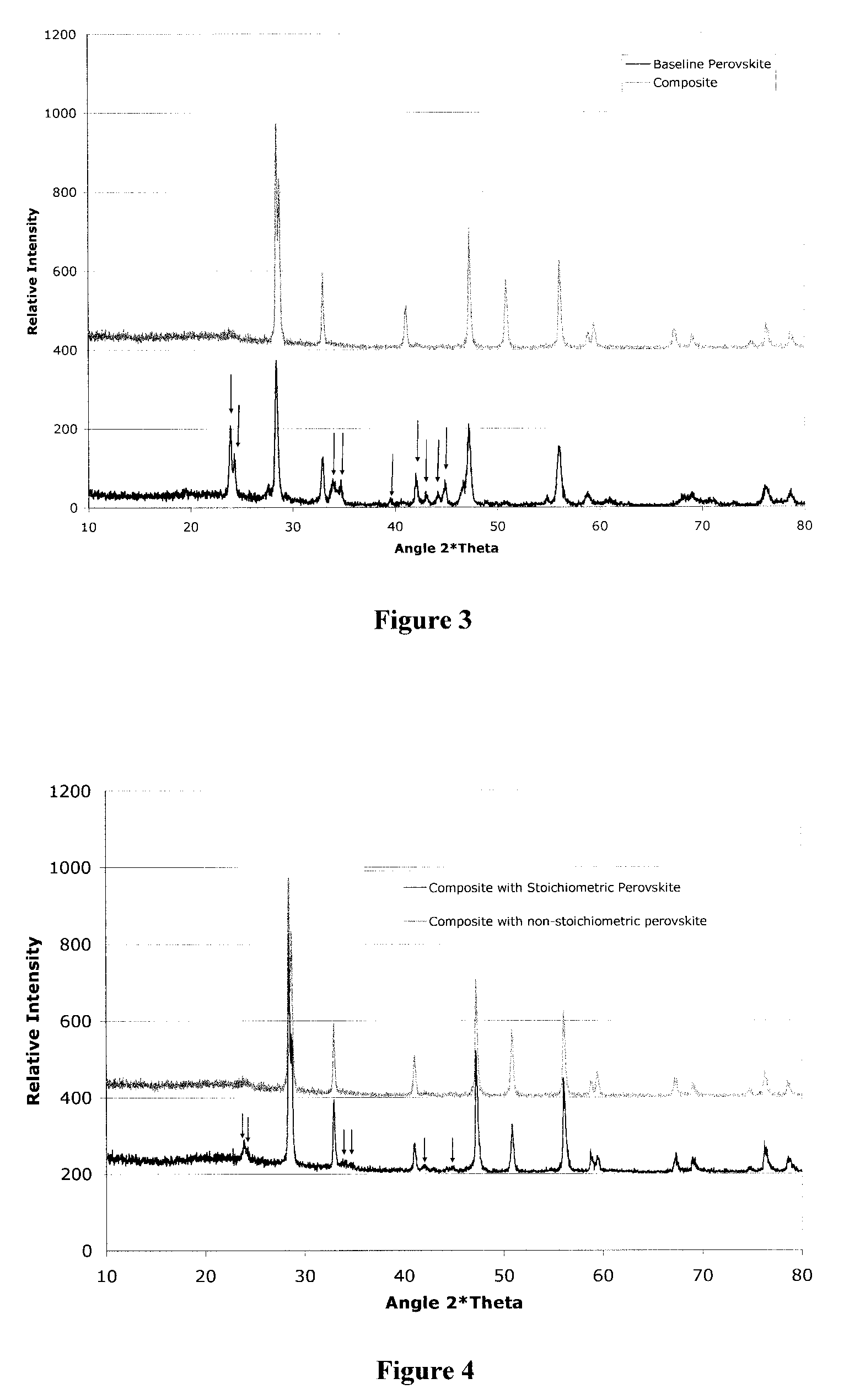
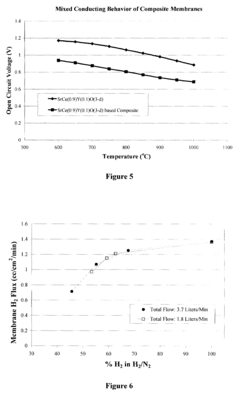
- A two-phase all-ceramic composite membrane is developed, comprising a proton-conducting ceramic phase and a stabilizing ceramic phase, with the addition of cerium oxide to enhance electronic conductivity and stability, and a non-stoichiometric perovskite phase to minimize chemical reactions with CO2, allowing for effective hydrogen separation in high-temperature syngas environments.
Ceramic mixed protonic/electronic conducting membranes for hydrogen separation
PatentInactiveUS7258820B2
Innovation
- A two-phase all-ceramic composite membrane is developed, comprising a proton-conducting ceramic phase and an electronically conductive ceramic phase, which balances mixed conducting properties to achieve high hydrogen flux and stability at elevated temperatures, using perovskite-type compounds stabilized with cerium oxide and non-stoichiometric modifications to minimize chemical reactions with CO2.
Environmental Impact and Sustainability Assessment
The environmental impact of hydrogen production technologies has become increasingly critical as the world transitions toward sustainable energy systems. Proton-conducting ceramics for hydrogen electrolysis represent a promising pathway for clean hydrogen production, but their environmental footprint must be thoroughly assessed to ensure true sustainability.
When evaluating proton-conducting ceramic electrolyzers, lifecycle assessment (LCA) reveals significant advantages over conventional hydrogen production methods. These systems operate without precious metal catalysts like platinum, reducing resource depletion and associated mining impacts. Additionally, their high-temperature operation enables efficient integration with renewable heat sources, potentially decreasing the carbon footprint by 30-45% compared to low-temperature electrolysis technologies.
Material sustainability presents both challenges and opportunities. The rare earth elements often used in proton-conducting ceramics, such as yttrium and gadolinium, face supply constraints and geopolitical complications. However, recent research demonstrates promising alternatives using more abundant elements like calcium and strontium-doped materials, which maintain comparable performance while reducing supply chain vulnerabilities.
Water consumption metrics favor ceramic electrolyzers over traditional methods. While conventional electrolysis requires ultra-pure water inputs, high-temperature ceramic systems demonstrate greater tolerance for water quality variations, potentially reducing water purification energy by up to 25%. This advantage becomes particularly valuable in water-stressed regions where hydrogen production facilities might operate.
Energy efficiency considerations reveal that proton-conducting ceramic systems achieve higher overall system efficiency when waste heat recovery is implemented. Studies indicate that integrated systems can achieve energy conversion efficiencies exceeding 85% when coupled with industrial processes or renewable energy systems, substantially reducing the environmental burden per kilogram of hydrogen produced.
End-of-life management represents an emerging consideration. The ceramic materials used in these electrolyzers demonstrate excellent recyclability potential, with laboratory studies showing up to 80% recovery rates for critical elements. This circular economy approach significantly reduces the lifecycle environmental impact compared to technologies with limited material recovery options.
Carbon neutrality pathways become more achievable with proton-conducting ceramic technologies. When powered by renewable electricity and heat, these systems can achieve near-zero emissions hydrogen production. Comparative analysis indicates potential greenhouse gas reductions of 90-98% versus steam methane reforming, positioning these technologies as key enablers for decarbonization strategies across multiple sectors.
When evaluating proton-conducting ceramic electrolyzers, lifecycle assessment (LCA) reveals significant advantages over conventional hydrogen production methods. These systems operate without precious metal catalysts like platinum, reducing resource depletion and associated mining impacts. Additionally, their high-temperature operation enables efficient integration with renewable heat sources, potentially decreasing the carbon footprint by 30-45% compared to low-temperature electrolysis technologies.
Material sustainability presents both challenges and opportunities. The rare earth elements often used in proton-conducting ceramics, such as yttrium and gadolinium, face supply constraints and geopolitical complications. However, recent research demonstrates promising alternatives using more abundant elements like calcium and strontium-doped materials, which maintain comparable performance while reducing supply chain vulnerabilities.
Water consumption metrics favor ceramic electrolyzers over traditional methods. While conventional electrolysis requires ultra-pure water inputs, high-temperature ceramic systems demonstrate greater tolerance for water quality variations, potentially reducing water purification energy by up to 25%. This advantage becomes particularly valuable in water-stressed regions where hydrogen production facilities might operate.
Energy efficiency considerations reveal that proton-conducting ceramic systems achieve higher overall system efficiency when waste heat recovery is implemented. Studies indicate that integrated systems can achieve energy conversion efficiencies exceeding 85% when coupled with industrial processes or renewable energy systems, substantially reducing the environmental burden per kilogram of hydrogen produced.
End-of-life management represents an emerging consideration. The ceramic materials used in these electrolyzers demonstrate excellent recyclability potential, with laboratory studies showing up to 80% recovery rates for critical elements. This circular economy approach significantly reduces the lifecycle environmental impact compared to technologies with limited material recovery options.
Carbon neutrality pathways become more achievable with proton-conducting ceramic technologies. When powered by renewable electricity and heat, these systems can achieve near-zero emissions hydrogen production. Comparative analysis indicates potential greenhouse gas reductions of 90-98% versus steam methane reforming, positioning these technologies as key enablers for decarbonization strategies across multiple sectors.
Techno-Economic Analysis of Implementation Costs
The implementation costs of proton-conducting ceramics for hydrogen electrolysis systems represent a critical factor in determining their commercial viability. Current economic assessments indicate that material costs constitute approximately 40-60% of the total system expenses, with proton-conducting ceramics being among the most expensive components due to their complex manufacturing processes and high-purity requirements.
Manufacturing scalability presents significant challenges, as most advanced proton-conducting ceramics require specialized production techniques including high-temperature sintering (1400-1600°C) and precise atmosphere control. These requirements translate to substantial capital investments for production facilities, estimated at $15-25 million for a medium-scale manufacturing plant with an annual production capacity of 500-1000 m² of ceramic membranes.
Energy consumption during manufacturing represents another substantial cost factor. The high-temperature processing of ceramics such as BaZrO₃-based materials consumes approximately 75-120 kWh per kilogram of finished product, significantly higher than conventional ceramic processing. This energy intensity directly impacts production costs and carbon footprint, affecting the overall sustainability profile of the technology.
Installation and integration expenses vary significantly based on system scale and application environment. Small laboratory-scale systems (1-5 kW) typically require $2,000-5,000 per kW for installation, while industrial-scale implementations (100+ kW) benefit from economies of scale, reducing installation costs to $800-1,500 per kW. These figures exclude balance-of-plant components which can add 30-50% to total system costs.
Maintenance requirements and operational expenses must also be factored into long-term economic assessments. Proton-conducting ceramic systems generally require replacement of sealing materials every 2-3 years and potential electrode regeneration every 4-5 years. Annual maintenance costs typically range from 3-7% of the initial capital investment, depending on operating conditions and system complexity.
When comparing different ceramic materials, Y-doped BaZrO₃ systems currently demonstrate the highest implementation costs due to challenging sintering requirements, while BaCeO₃-based materials offer more favorable economics but with compromised chemical stability. Emerging composite systems incorporating both materials show promising cost-performance balance, potentially reducing implementation costs by 15-25% compared to pure BaZrO₃ systems.
Manufacturing scalability presents significant challenges, as most advanced proton-conducting ceramics require specialized production techniques including high-temperature sintering (1400-1600°C) and precise atmosphere control. These requirements translate to substantial capital investments for production facilities, estimated at $15-25 million for a medium-scale manufacturing plant with an annual production capacity of 500-1000 m² of ceramic membranes.
Energy consumption during manufacturing represents another substantial cost factor. The high-temperature processing of ceramics such as BaZrO₃-based materials consumes approximately 75-120 kWh per kilogram of finished product, significantly higher than conventional ceramic processing. This energy intensity directly impacts production costs and carbon footprint, affecting the overall sustainability profile of the technology.
Installation and integration expenses vary significantly based on system scale and application environment. Small laboratory-scale systems (1-5 kW) typically require $2,000-5,000 per kW for installation, while industrial-scale implementations (100+ kW) benefit from economies of scale, reducing installation costs to $800-1,500 per kW. These figures exclude balance-of-plant components which can add 30-50% to total system costs.
Maintenance requirements and operational expenses must also be factored into long-term economic assessments. Proton-conducting ceramic systems generally require replacement of sealing materials every 2-3 years and potential electrode regeneration every 4-5 years. Annual maintenance costs typically range from 3-7% of the initial capital investment, depending on operating conditions and system complexity.
When comparing different ceramic materials, Y-doped BaZrO₃ systems currently demonstrate the highest implementation costs due to challenging sintering requirements, while BaCeO₃-based materials offer more favorable economics but with compromised chemical stability. Emerging composite systems incorporating both materials show promising cost-performance balance, potentially reducing implementation costs by 15-25% compared to pure BaZrO₃ systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!