Cold Plasma Treatment in Biomedical Devices: Patent Analysis
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cold Plasma Technology Evolution and Objectives
Cold plasma technology has evolved significantly over the past century, with its origins dating back to the early 1900s when scientists first began exploring electrical discharges in gases. The fundamental understanding of plasma as the fourth state of matter emerged in the 1920s, but it wasn't until the 1970s that researchers began investigating its potential applications in biomedical fields. The technology has progressed from basic atmospheric pressure plasma jets to sophisticated cold atmospheric plasma (CAP) systems specifically designed for medical applications.
The evolution of cold plasma technology in biomedical devices has been characterized by several key milestones. In the 1990s, researchers demonstrated the antimicrobial properties of non-thermal plasma, opening doors to sterilization applications. The early 2000s witnessed the development of the first plasma-based wound healing devices, while the 2010s brought significant advancements in plasma medicine with targeted cancer treatment applications and precision plasma delivery systems.
Patent analysis reveals an accelerating trend in cold plasma biomedical innovations, with a notable increase in patent filings since 2010. The technology has evolved from broad applications to highly specialized medical devices addressing specific clinical needs. Early patents focused primarily on sterilization methods, while recent innovations emphasize precision treatment modalities, biocompatible plasma sources, and integration with existing medical technologies.
The primary objectives of current cold plasma technology development in biomedical applications include enhancing treatment efficacy while ensuring patient safety, miniaturizing devices for improved clinical usability, and developing specialized applications for challenging medical conditions. Patent trends indicate particular interest in developing plasma devices for chronic wound treatment, dental applications, cancer therapy, and antimicrobial surfaces for implantable devices.
Looking forward, the technological trajectory suggests continued refinement of plasma parameters for specific therapeutic outcomes, integration with diagnostic capabilities for real-time treatment monitoring, and development of personalized plasma medicine approaches. The convergence of cold plasma technology with other emerging fields such as nanotechnology, artificial intelligence, and regenerative medicine represents a promising frontier for innovation.
The ultimate goal of cold plasma technology in biomedical devices is to establish itself as a mainstream therapeutic modality with standardized protocols, regulatory approval pathways, and widespread clinical adoption. This requires addressing current challenges in reproducibility, dosimetry standardization, and long-term safety profiles, all of which are reflected in recent patent activities and research directions.
The evolution of cold plasma technology in biomedical devices has been characterized by several key milestones. In the 1990s, researchers demonstrated the antimicrobial properties of non-thermal plasma, opening doors to sterilization applications. The early 2000s witnessed the development of the first plasma-based wound healing devices, while the 2010s brought significant advancements in plasma medicine with targeted cancer treatment applications and precision plasma delivery systems.
Patent analysis reveals an accelerating trend in cold plasma biomedical innovations, with a notable increase in patent filings since 2010. The technology has evolved from broad applications to highly specialized medical devices addressing specific clinical needs. Early patents focused primarily on sterilization methods, while recent innovations emphasize precision treatment modalities, biocompatible plasma sources, and integration with existing medical technologies.
The primary objectives of current cold plasma technology development in biomedical applications include enhancing treatment efficacy while ensuring patient safety, miniaturizing devices for improved clinical usability, and developing specialized applications for challenging medical conditions. Patent trends indicate particular interest in developing plasma devices for chronic wound treatment, dental applications, cancer therapy, and antimicrobial surfaces for implantable devices.
Looking forward, the technological trajectory suggests continued refinement of plasma parameters for specific therapeutic outcomes, integration with diagnostic capabilities for real-time treatment monitoring, and development of personalized plasma medicine approaches. The convergence of cold plasma technology with other emerging fields such as nanotechnology, artificial intelligence, and regenerative medicine represents a promising frontier for innovation.
The ultimate goal of cold plasma technology in biomedical devices is to establish itself as a mainstream therapeutic modality with standardized protocols, regulatory approval pathways, and widespread clinical adoption. This requires addressing current challenges in reproducibility, dosimetry standardization, and long-term safety profiles, all of which are reflected in recent patent activities and research directions.
Biomedical Market Demand Analysis
The global biomedical device market is experiencing significant growth, with cold plasma treatment technologies emerging as a promising segment. Current market analysis indicates that the biomedical device sector is valued at approximately $470 billion globally, with projections showing consistent annual growth rates between 5-7% through 2030. Within this broader market, cold plasma applications represent a rapidly expanding niche, currently estimated at $1.5 billion with substantially higher growth rates of 15-20% annually.
Market demand for cold plasma treatment in biomedical applications is primarily driven by several key factors. The rising prevalence of hospital-acquired infections (HAIs) has created urgent demand for advanced sterilization technologies, with healthcare facilities increasingly seeking alternatives to traditional chemical and heat-based methods. Cold plasma offers significant advantages in this context, providing effective microbial decontamination without the drawbacks of chemical residues or heat damage to sensitive materials.
Wound care represents another substantial market segment, valued at approximately $20 billion globally, where cold plasma treatments are gaining traction. Clinical studies demonstrating accelerated healing rates and effective pathogen elimination in chronic wounds have sparked interest among healthcare providers and patients alike. The aging global population and rising incidence of diabetes-related wounds further amplify this demand trajectory.
Dental applications constitute a growing market segment for cold plasma technology, with particular interest in periodontal treatments and implant sterilization. The dental device market, currently valued at around $30 billion, has shown receptivity to cold plasma innovations due to their precision and effectiveness in addressing biofilm-related challenges.
Regional analysis reveals varying adoption patterns, with North America currently leading market share at approximately 40%, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the highest growth potential, with annual increases projected at 18-22% due to expanding healthcare infrastructure and increasing healthcare expenditure in countries like China and India.
End-user segmentation shows hospitals as the primary adopters (45% market share), followed by specialized wound care centers (25%), dental clinics (15%), and research institutions (10%). Consumer-facing applications remain limited but represent a potential future growth avenue as miniaturization and cost reduction make home-use devices feasible.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions, and reimbursement limitations that currently restrict widespread adoption. Additionally, awareness gaps among healthcare practitioners regarding cold plasma benefits and applications represent a significant market development obstacle that requires targeted educational initiatives.
Market demand for cold plasma treatment in biomedical applications is primarily driven by several key factors. The rising prevalence of hospital-acquired infections (HAIs) has created urgent demand for advanced sterilization technologies, with healthcare facilities increasingly seeking alternatives to traditional chemical and heat-based methods. Cold plasma offers significant advantages in this context, providing effective microbial decontamination without the drawbacks of chemical residues or heat damage to sensitive materials.
Wound care represents another substantial market segment, valued at approximately $20 billion globally, where cold plasma treatments are gaining traction. Clinical studies demonstrating accelerated healing rates and effective pathogen elimination in chronic wounds have sparked interest among healthcare providers and patients alike. The aging global population and rising incidence of diabetes-related wounds further amplify this demand trajectory.
Dental applications constitute a growing market segment for cold plasma technology, with particular interest in periodontal treatments and implant sterilization. The dental device market, currently valued at around $30 billion, has shown receptivity to cold plasma innovations due to their precision and effectiveness in addressing biofilm-related challenges.
Regional analysis reveals varying adoption patterns, with North America currently leading market share at approximately 40%, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the highest growth potential, with annual increases projected at 18-22% due to expanding healthcare infrastructure and increasing healthcare expenditure in countries like China and India.
End-user segmentation shows hospitals as the primary adopters (45% market share), followed by specialized wound care centers (25%), dental clinics (15%), and research institutions (10%). Consumer-facing applications remain limited but represent a potential future growth avenue as miniaturization and cost reduction make home-use devices feasible.
Market challenges include regulatory hurdles, with approval processes varying significantly across regions, and reimbursement limitations that currently restrict widespread adoption. Additionally, awareness gaps among healthcare practitioners regarding cold plasma benefits and applications represent a significant market development obstacle that requires targeted educational initiatives.
Global Cold Plasma Technical Challenges
Despite significant advancements in cold plasma technology for biomedical applications, several critical technical challenges persist globally that impede its widespread clinical adoption. The primary challenge remains the precise control of plasma parameters, including electron temperature, ion density, and reactive species concentration, which directly impact treatment efficacy and safety. Current plasma generation systems struggle to maintain consistent output across varying environmental conditions and treatment durations, resulting in unpredictable biological responses.
Material compatibility presents another significant hurdle, as plasma interaction with biomedical device materials can lead to degradation, altered surface properties, or release of potentially harmful byproducts. This is particularly problematic for implantable devices where long-term stability is essential. The development of plasma-resistant materials or protective coatings remains an active but unresolved research area.
Scalability challenges also persist across the industry. While laboratory-scale plasma systems demonstrate promising results, scaling these technologies for industrial production or hospital settings introduces complications in maintaining uniform plasma distribution over larger treatment areas. This non-uniformity can lead to inconsistent treatment outcomes and quality control issues in mass production scenarios.
The biological mechanisms of plasma-cell interactions remain incompletely understood, creating barriers to optimizing treatment protocols. The complex interplay between various plasma-generated reactive species (ROS, RNS) and biological tissues creates unpredictable outcomes that vary between tissue types and pathological conditions. This knowledge gap hampers the development of standardized treatment protocols necessary for regulatory approval.
Safety concerns constitute a major technical challenge, particularly regarding the potential generation of harmful byproducts during plasma treatment. Ozone, nitrogen oxides, and other reactive species produced during plasma generation may pose toxicity risks if not properly controlled or neutralized. Current plasma systems lack sophisticated real-time monitoring capabilities to ensure treatment remains within safe parameters.
Energy efficiency limitations affect portable or point-of-care applications, as most effective plasma generation systems require substantial power input. This restricts the development of battery-operated or field-deployable biomedical plasma devices, limiting their use in resource-constrained settings or emergency situations.
Regulatory hurdles compound these technical challenges, as the complex and variable nature of plasma treatments makes it difficult to establish standardized testing protocols and safety parameters that satisfy regulatory requirements across different jurisdictions. The absence of universally accepted standards for plasma device characterization further complicates the path to clinical implementation.
Material compatibility presents another significant hurdle, as plasma interaction with biomedical device materials can lead to degradation, altered surface properties, or release of potentially harmful byproducts. This is particularly problematic for implantable devices where long-term stability is essential. The development of plasma-resistant materials or protective coatings remains an active but unresolved research area.
Scalability challenges also persist across the industry. While laboratory-scale plasma systems demonstrate promising results, scaling these technologies for industrial production or hospital settings introduces complications in maintaining uniform plasma distribution over larger treatment areas. This non-uniformity can lead to inconsistent treatment outcomes and quality control issues in mass production scenarios.
The biological mechanisms of plasma-cell interactions remain incompletely understood, creating barriers to optimizing treatment protocols. The complex interplay between various plasma-generated reactive species (ROS, RNS) and biological tissues creates unpredictable outcomes that vary between tissue types and pathological conditions. This knowledge gap hampers the development of standardized treatment protocols necessary for regulatory approval.
Safety concerns constitute a major technical challenge, particularly regarding the potential generation of harmful byproducts during plasma treatment. Ozone, nitrogen oxides, and other reactive species produced during plasma generation may pose toxicity risks if not properly controlled or neutralized. Current plasma systems lack sophisticated real-time monitoring capabilities to ensure treatment remains within safe parameters.
Energy efficiency limitations affect portable or point-of-care applications, as most effective plasma generation systems require substantial power input. This restricts the development of battery-operated or field-deployable biomedical plasma devices, limiting their use in resource-constrained settings or emergency situations.
Regulatory hurdles compound these technical challenges, as the complex and variable nature of plasma treatments makes it difficult to establish standardized testing protocols and safety parameters that satisfy regulatory requirements across different jurisdictions. The absence of universally accepted standards for plasma device characterization further complicates the path to clinical implementation.
Current Cold Plasma Treatment Solutions
01 Medical applications of cold plasma treatment
Cold plasma treatment has significant applications in medicine, particularly for wound healing, tissue regeneration, and treating various skin conditions. The technology uses non-thermal plasma to deliver reactive species that can promote healing while minimizing damage to surrounding healthy tissue. These treatments can be administered through specialized devices that generate controlled plasma at temperatures safe for biological tissues, offering non-invasive therapeutic options for various medical conditions.- Medical applications of cold plasma treatment: Cold plasma technology is being utilized in various medical applications for treatment of tissues and wounds. The non-thermal plasma can effectively kill bacteria and promote healing without damaging surrounding healthy tissue. These treatments can be applied to chronic wounds, dermatological conditions, and even in surgical settings where precision is required. The technology allows for controlled application of plasma to target specific areas while minimizing side effects.
- Cold plasma devices and delivery systems: Various devices and systems have been developed for the generation and delivery of cold plasma for treatment purposes. These include handheld applicators, specialized nozzles, and integrated treatment systems that can generate plasma at atmospheric pressure. The designs focus on controlled delivery of plasma to target areas, with adjustable parameters such as gas flow, power settings, and treatment duration to optimize therapeutic outcomes while ensuring safety.
- Cold plasma for surface modification and sterilization: Cold plasma treatment is effective for modifying surface properties of materials and sterilizing various objects. The technology can alter surface chemistry, improve wettability, and enhance adhesion properties of materials. In sterilization applications, cold plasma can inactivate microorganisms on heat-sensitive materials without causing thermal damage. This makes it particularly valuable in medical device manufacturing, food processing, and pharmaceutical industries where traditional heat sterilization methods may be unsuitable.
- Biological effects and mechanisms of cold plasma: Research has focused on understanding the biological mechanisms through which cold plasma exerts its therapeutic effects. Cold plasma generates reactive oxygen and nitrogen species that can interact with biological tissues, triggering cellular responses including antimicrobial action, modulation of inflammatory processes, and stimulation of tissue regeneration. These mechanisms are being studied to optimize treatment protocols and develop new therapeutic applications across various medical fields.
- Industrial and agricultural applications of cold plasma: Cold plasma technology has found applications beyond medicine in industrial processing and agriculture. In industrial settings, it is used for surface cleaning, activation of materials prior to coating or bonding, and modification of polymers and textiles. In agriculture, cold plasma can be used for seed treatment to improve germination rates, decontamination of produce, and enhancement of plant growth. These applications leverage the non-thermal nature of cold plasma to achieve desired effects without heat damage.
02 Cold plasma sterilization and disinfection systems
Cold plasma technology is utilized for sterilization and disinfection purposes in various settings including medical facilities, food processing, and industrial environments. These systems generate reactive oxygen and nitrogen species that effectively eliminate pathogens, bacteria, viruses, and fungi from surfaces and equipment. The non-thermal nature of cold plasma allows for sterilization of heat-sensitive materials without causing damage, providing advantages over traditional thermal or chemical sterilization methods.Expand Specific Solutions03 Cold plasma devices for therapeutic delivery
Specialized devices have been developed for the controlled delivery of cold plasma for therapeutic purposes. These devices incorporate various electrode configurations, gas flow systems, and power supply mechanisms to generate and apply plasma to target areas. The designs focus on safety, precision, and effectiveness, with features that allow for adjustable treatment parameters based on specific applications. Some devices are portable for clinical or home use, while others are designed for integration into existing medical equipment.Expand Specific Solutions04 Cold plasma for surface modification and material processing
Cold plasma treatment is employed for modifying surface properties of various materials including polymers, textiles, metals, and biomaterials. The process can alter surface energy, wettability, adhesion properties, and biocompatibility without affecting bulk material properties. This technology enables functionalization of surfaces with specific chemical groups, improving material performance for applications in industries such as electronics, packaging, textiles, and biomedical implants. The non-thermal nature of the process makes it suitable for treating heat-sensitive materials.Expand Specific Solutions05 Agricultural and food processing applications of cold plasma
Cold plasma technology has emerging applications in agriculture and food processing, including seed treatment to enhance germination, crop protection, food preservation, and decontamination of agricultural products. The technology can inactivate foodborne pathogens, extend shelf life, and reduce spoilage without affecting nutritional quality or sensory attributes. Cold plasma systems for these applications are designed to treat various food products and agricultural materials while maintaining safety and quality standards.Expand Specific Solutions
Key Industry Players and Competition
Cold plasma treatment in biomedical devices is currently in a growth phase, with the market expanding rapidly due to increasing applications in wound healing, sterilization, and surface modification. The global market size for cold plasma technology in healthcare is projected to reach $3.1 billion by 2025, growing at a CAGR of approximately 16%. Technologically, the field shows moderate maturity with established applications but significant ongoing innovation. Leading players include US Patent Innovations LLC specializing in plasma-based surgical tools, CAPS Medical developing cancer treatment solutions, and Plasmology4 focusing on wound care applications. Established corporations like L'Oréal and Koninklijke Philips are also entering this space, while research institutions such as Technion Research & Development Foundation and Technical Institute of Physics & Chemistry CAS are driving fundamental innovations, indicating a competitive landscape balanced between specialized startups and diversifying industry giants.
US Patent Innovations LLC
Technical Solution: US Patent Innovations LLC has developed a proprietary cold plasma technology called the Canady Helios Cold Plasma System specifically designed for biomedical applications. Their technology utilizes a unique ionization process that generates cold atmospheric plasma at temperatures below 40°C, making it suitable for direct contact with living tissues. The system employs a specialized delivery mechanism that combines inert gases (primarily helium or argon) with controlled electrical discharges to create a stable plasma field with antimicrobial and tissue regenerative properties. Their patents cover both the device architecture and specific treatment protocols for oncological applications, wound healing, and sterilization of medical devices. The company has demonstrated significant efficacy in selective cancer cell destruction while preserving healthy tissue integrity through controlled reactive oxygen and nitrogen species generation.
Strengths: Highly selective treatment capability that can target pathological tissues while preserving healthy cells; versatile platform technology adaptable to multiple clinical applications; demonstrated safety profile with minimal thermal damage. Weaknesses: Requires specialized gas delivery systems; treatment depth limitations compared to other modalities; relatively new technology with limited long-term clinical data.
Plasmology4, Inc.
Technical Solution: Plasmology4 has pioneered a portable cold plasma technology platform called PlasmaSol that operates at atmospheric pressure for biomedical applications. Their patented approach utilizes a dielectric barrier discharge (DBD) mechanism that generates non-thermal plasma between two electrodes with a dielectric material in between. This configuration allows for the production of reactive oxygen and nitrogen species (RONS) at biologically compatible temperatures (25-40°C). The company's innovation lies in their electrode design and power modulation system that enables precise control of plasma characteristics for different therapeutic applications. Their patents cover both the device architecture and specific treatment protocols for dermatological conditions, wound care, and dental applications. Clinical studies have shown their technology effectively reduces bacterial bioburden by over 99.9% within minutes of application while promoting tissue regeneration through controlled inflammation and growth factor stimulation.
Strengths: Highly portable design suitable for point-of-care applications; tunable plasma parameters allowing customization for different tissue types; demonstrated efficacy against antibiotic-resistant bacteria. Weaknesses: Limited penetration depth (typically <2mm); requires direct line-of-sight application; potential variability in treatment outcomes depending on tissue moisture content and composition.
Patent Landscape Analysis
Cold Plasma Treatment Devices and Associated Methods
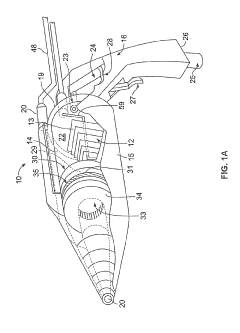
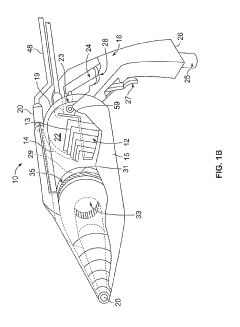
PatentInactiveUS20190254154A1
Innovation
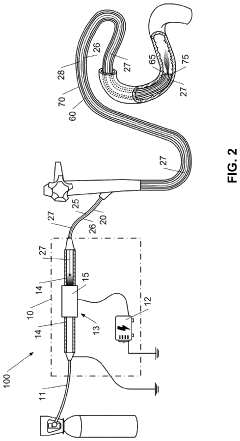
- A hand-held cold plasma device with a magnet-free gas compartment and a modular electrode configuration, coupled to a high voltage electrical inlet, generates cold plasma in the range of 65 to 120 degrees Fahrenheit, using a capacitor charging power supply and a double tuned RF transformer to produce a rich harmonic output voltage, allowing for adjustable settings and easy component insertion/removal.
Device for cold plasma treatment, cold plasma endoscopic system
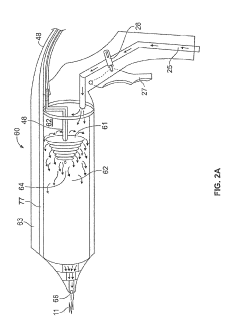
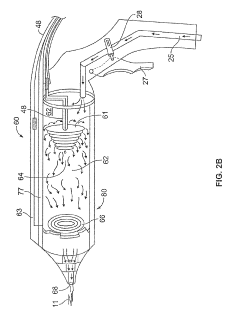
PatentPendingUS20220054183A1
Innovation
- A device for cold plasma treatment utilizing a pulsed plasma generation system with a flexible tube and deployable confinement means, which allows for the efficient transport and uniform application of cold plasma over long distances, minimizing collateral damage and ensuring complete treatment of target areas.
Regulatory Framework
The regulatory landscape for cold plasma technology in biomedical applications presents a complex framework that manufacturers and researchers must navigate carefully. In the United States, the FDA regulates cold plasma devices primarily under the medical device classification system, with most plasma-based therapeutic devices falling under Class II (moderate risk) or Class III (high risk) categories, requiring either 510(k) clearance or premarket approval (PMA). The regulatory pathway depends significantly on the intended use, with wound healing applications generally facing less stringent requirements than those targeting cancer treatment or sterilization of implantable devices.
European regulation follows the Medical Device Regulation (MDR) framework, which implemented stricter requirements in 2021, particularly regarding clinical evidence and post-market surveillance. Cold plasma devices must demonstrate compliance with essential requirements for safety and performance, with particular attention to electrical safety standards (IEC 60601) and biocompatibility testing (ISO 10993 series). The classification typically ranges from Class IIa to Class III depending on the invasiveness and duration of contact with the human body.
In Asia, regulatory approaches vary significantly. Japan's PMDA has established specific guidelines for plasma-based medical technologies, while China's NMPA requires extensive local testing even for devices approved elsewhere. South Korea has emerged as a relatively favorable regulatory environment for novel plasma technologies, with expedited pathways for innovative devices.
A critical regulatory consideration across all jurisdictions is the characterization of plasma-generated reactive species. Regulatory bodies increasingly require manufacturers to identify and quantify reactive oxygen and nitrogen species (RONS) produced during treatment, and to establish safety thresholds for patient exposure. This presents significant analytical challenges due to the short-lived nature of many plasma-generated species.
Patent analysis reveals that regulatory considerations have significantly influenced patent filing strategies, with many applications including specific claims addressing regulatory requirements. Companies with strong patent portfolios often include regulatory compliance methods within their protected intellectual property, creating potential barriers for new market entrants who must develop alternative approaches to meeting regulatory requirements while avoiding patent infringement.
The evolving nature of cold plasma technology presents ongoing regulatory challenges, as existing frameworks were not specifically designed for plasma-based treatments. Several jurisdictions are currently developing specialized guidance documents for plasma technologies, recognizing their unique risk profiles and mechanisms of action that don't fit neatly into traditional regulatory categories.
European regulation follows the Medical Device Regulation (MDR) framework, which implemented stricter requirements in 2021, particularly regarding clinical evidence and post-market surveillance. Cold plasma devices must demonstrate compliance with essential requirements for safety and performance, with particular attention to electrical safety standards (IEC 60601) and biocompatibility testing (ISO 10993 series). The classification typically ranges from Class IIa to Class III depending on the invasiveness and duration of contact with the human body.
In Asia, regulatory approaches vary significantly. Japan's PMDA has established specific guidelines for plasma-based medical technologies, while China's NMPA requires extensive local testing even for devices approved elsewhere. South Korea has emerged as a relatively favorable regulatory environment for novel plasma technologies, with expedited pathways for innovative devices.
A critical regulatory consideration across all jurisdictions is the characterization of plasma-generated reactive species. Regulatory bodies increasingly require manufacturers to identify and quantify reactive oxygen and nitrogen species (RONS) produced during treatment, and to establish safety thresholds for patient exposure. This presents significant analytical challenges due to the short-lived nature of many plasma-generated species.
Patent analysis reveals that regulatory considerations have significantly influenced patent filing strategies, with many applications including specific claims addressing regulatory requirements. Companies with strong patent portfolios often include regulatory compliance methods within their protected intellectual property, creating potential barriers for new market entrants who must develop alternative approaches to meeting regulatory requirements while avoiding patent infringement.
The evolving nature of cold plasma technology presents ongoing regulatory challenges, as existing frameworks were not specifically designed for plasma-based treatments. Several jurisdictions are currently developing specialized guidance documents for plasma technologies, recognizing their unique risk profiles and mechanisms of action that don't fit neatly into traditional regulatory categories.
Safety and Efficacy Assessment
The safety and efficacy assessment of cold plasma treatment in biomedical devices represents a critical component in the regulatory pathway and clinical adoption of this technology. Current patent analysis reveals a significant focus on establishing comprehensive safety protocols that address both direct and indirect risks associated with plasma-tissue interactions.
Primary safety considerations documented in patents include thermal effects, UV radiation exposure, and reactive oxygen and nitrogen species (RONS) generation. Leading patents have developed sophisticated monitoring systems that provide real-time feedback on plasma parameters, ensuring treatments remain within predetermined safety thresholds. Notable innovations include adaptive power control mechanisms that automatically adjust plasma intensity based on tissue response, significantly reducing the risk of thermal damage.
Efficacy assessment methodologies have evolved considerably, with patents revealing a trend toward quantifiable outcome measures. Early patents relied primarily on qualitative visual assessments, while contemporary innovations incorporate advanced imaging techniques and molecular biomarkers to objectively measure treatment efficacy. Several patents describe novel diagnostic components integrated within plasma devices that simultaneously deliver treatment and monitor therapeutic progress.
Dosimetry represents a particularly active area of patent activity, with multiple competing approaches to standardize plasma "dose" measurements. The most promising patents describe correlations between specific plasma parameters (electron temperature, gas composition, treatment duration) and biological outcomes, enabling more precise treatment protocols. These developments address a fundamental challenge in the field—establishing reproducible treatment parameters across different device architectures.
Biocompatibility testing frameworks feature prominently in recent patents, with innovations focusing on long-term safety assessments. Several patents describe novel in vitro models specifically designed to evaluate the unique interaction profile of cold plasma with biological tissues, moving beyond standard ISO 10993 testing paradigms to address plasma-specific concerns such as delayed cellular responses to oxidative stress.
Risk classification systems have emerged as a patent focus area, with several innovations proposing stratified approaches to safety assessment based on the intended application. These systems recognize that safety requirements differ substantially between external wound treatment applications and more invasive uses, allowing for appropriately tailored testing regimens.
The patent landscape indicates a clear trend toward integration of safety and efficacy parameters into unified assessment frameworks, reflecting the understanding that these aspects are inherently interconnected in plasma medicine applications.
Primary safety considerations documented in patents include thermal effects, UV radiation exposure, and reactive oxygen and nitrogen species (RONS) generation. Leading patents have developed sophisticated monitoring systems that provide real-time feedback on plasma parameters, ensuring treatments remain within predetermined safety thresholds. Notable innovations include adaptive power control mechanisms that automatically adjust plasma intensity based on tissue response, significantly reducing the risk of thermal damage.
Efficacy assessment methodologies have evolved considerably, with patents revealing a trend toward quantifiable outcome measures. Early patents relied primarily on qualitative visual assessments, while contemporary innovations incorporate advanced imaging techniques and molecular biomarkers to objectively measure treatment efficacy. Several patents describe novel diagnostic components integrated within plasma devices that simultaneously deliver treatment and monitor therapeutic progress.
Dosimetry represents a particularly active area of patent activity, with multiple competing approaches to standardize plasma "dose" measurements. The most promising patents describe correlations between specific plasma parameters (electron temperature, gas composition, treatment duration) and biological outcomes, enabling more precise treatment protocols. These developments address a fundamental challenge in the field—establishing reproducible treatment parameters across different device architectures.
Biocompatibility testing frameworks feature prominently in recent patents, with innovations focusing on long-term safety assessments. Several patents describe novel in vitro models specifically designed to evaluate the unique interaction profile of cold plasma with biological tissues, moving beyond standard ISO 10993 testing paradigms to address plasma-specific concerns such as delayed cellular responses to oxidative stress.
Risk classification systems have emerged as a patent focus area, with several innovations proposing stratified approaches to safety assessment based on the intended application. These systems recognize that safety requirements differ substantially between external wound treatment applications and more invasive uses, allowing for appropriately tailored testing regimens.
The patent landscape indicates a clear trend toward integration of safety and efficacy parameters into unified assessment frameworks, reflecting the understanding that these aspects are inherently interconnected in plasma medicine applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!