Standards Governing Cold Plasma Treatment in Medical Devices
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cold Plasma Technology Evolution and Objectives
Cold plasma technology has evolved significantly over the past century, with its medical applications gaining substantial attention in recent decades. The concept of plasma as the fourth state of matter was first introduced by Irving Langmuir in the 1920s, but it wasn't until the late 1990s that cold atmospheric plasma began to be seriously explored for biomedical applications. This technological evolution has been driven by the need for non-thermal sterilization methods that can effectively decontaminate heat-sensitive medical devices without causing damage.
The development trajectory of cold plasma technology has seen several key advancements. Initially, plasma systems operated primarily under vacuum conditions, limiting their practical applications in medical settings. The breakthrough came with the development of atmospheric pressure cold plasma systems in the early 2000s, which eliminated the need for vacuum chambers and made the technology more accessible for medical device manufacturing and treatment.
Recent technological innovations have focused on creating more precise, controlled, and customizable cold plasma systems. These include the development of dielectric barrier discharge (DBD) systems, plasma jets, and microplasma arrays that can deliver targeted treatment to specific areas of medical devices with complex geometries. The miniaturization of plasma generation equipment has further expanded potential applications in medical device manufacturing and treatment processes.
The primary objectives of cold plasma technology in medical device applications center around achieving effective microbial decontamination while maintaining material integrity. Cold plasma offers a unique advantage in this regard as it can inactivate a broad spectrum of pathogens including bacteria, viruses, fungi, and spores, without the thermal damage associated with conventional sterilization methods. Additionally, cold plasma treatment aims to enhance surface properties of medical devices, improving biocompatibility, cell adhesion, and integration with biological tissues.
Looking forward, the technological goals for cold plasma in medical device applications include developing standardized treatment protocols that ensure consistent antimicrobial efficacy across different device types and materials. There is also a push toward creating more energy-efficient plasma generation systems that can be easily integrated into existing medical device manufacturing lines. The ultimate objective is to establish cold plasma as a recognized and regulated sterilization method that complies with international standards for medical device safety and efficacy.
The evolution of this technology continues to be shaped by interdisciplinary collaboration between plasma physicists, materials scientists, microbiologists, and regulatory experts, all working toward harnessing the unique properties of cold plasma for advancing medical device safety and performance.
The development trajectory of cold plasma technology has seen several key advancements. Initially, plasma systems operated primarily under vacuum conditions, limiting their practical applications in medical settings. The breakthrough came with the development of atmospheric pressure cold plasma systems in the early 2000s, which eliminated the need for vacuum chambers and made the technology more accessible for medical device manufacturing and treatment.
Recent technological innovations have focused on creating more precise, controlled, and customizable cold plasma systems. These include the development of dielectric barrier discharge (DBD) systems, plasma jets, and microplasma arrays that can deliver targeted treatment to specific areas of medical devices with complex geometries. The miniaturization of plasma generation equipment has further expanded potential applications in medical device manufacturing and treatment processes.
The primary objectives of cold plasma technology in medical device applications center around achieving effective microbial decontamination while maintaining material integrity. Cold plasma offers a unique advantage in this regard as it can inactivate a broad spectrum of pathogens including bacteria, viruses, fungi, and spores, without the thermal damage associated with conventional sterilization methods. Additionally, cold plasma treatment aims to enhance surface properties of medical devices, improving biocompatibility, cell adhesion, and integration with biological tissues.
Looking forward, the technological goals for cold plasma in medical device applications include developing standardized treatment protocols that ensure consistent antimicrobial efficacy across different device types and materials. There is also a push toward creating more energy-efficient plasma generation systems that can be easily integrated into existing medical device manufacturing lines. The ultimate objective is to establish cold plasma as a recognized and regulated sterilization method that complies with international standards for medical device safety and efficacy.
The evolution of this technology continues to be shaped by interdisciplinary collaboration between plasma physicists, materials scientists, microbiologists, and regulatory experts, all working toward harnessing the unique properties of cold plasma for advancing medical device safety and performance.
Medical Device Market Demand Analysis
The global medical device market has witnessed a significant surge in demand for advanced treatment technologies, with cold plasma treatment emerging as a promising frontier. The market for cold plasma medical devices is projected to grow at a compound annual growth rate of 16.2% from 2021 to 2028, reaching a market value of 3.1 billion USD by 2028. This growth is primarily driven by the increasing prevalence of chronic wounds, hospital-acquired infections, and the rising demand for minimally invasive surgical procedures.
Healthcare facilities worldwide are seeking innovative solutions to address the growing challenge of antimicrobial resistance, which affects approximately 2.8 million patients annually. Cold plasma technology offers a compelling alternative to traditional antimicrobial treatments, demonstrating efficacy against multi-drug resistant pathogens without contributing to resistance development. Market research indicates that 78% of healthcare providers express interest in adopting plasma-based technologies for wound management and sterilization applications.
The wound care segment represents the largest application area for cold plasma devices, accounting for approximately 42% of the market share. This is attributed to the rising incidence of diabetic foot ulcers, pressure ulcers, and surgical site infections. With over 8.2 million patients requiring advanced wound care treatments annually, the demand for effective, non-pharmaceutical interventions continues to escalate.
Dermatological applications constitute another rapidly growing segment, with a 22% market share. The ability of cold plasma to treat various skin conditions, including acne, eczema, and psoriasis, has garnered significant attention from dermatologists and aesthetic medicine practitioners. Consumer surveys indicate that 65% of patients prefer non-pharmaceutical treatment options for chronic skin conditions, creating a substantial market opportunity for cold plasma technologies.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to exhibit the highest growth rate due to increasing healthcare expenditure, growing awareness about advanced medical technologies, and the rising burden of chronic diseases in countries like China, India, and Japan.
Market penetration challenges persist, primarily due to the high initial investment costs associated with cold plasma equipment and the need for specialized training. Healthcare facilities report that implementation costs and reimbursement uncertainties represent significant barriers to adoption, with 56% of potential buyers citing these factors as primary concerns. Nevertheless, the demonstrable clinical benefits and long-term cost savings potential continue to drive market expansion.
Healthcare facilities worldwide are seeking innovative solutions to address the growing challenge of antimicrobial resistance, which affects approximately 2.8 million patients annually. Cold plasma technology offers a compelling alternative to traditional antimicrobial treatments, demonstrating efficacy against multi-drug resistant pathogens without contributing to resistance development. Market research indicates that 78% of healthcare providers express interest in adopting plasma-based technologies for wound management and sterilization applications.
The wound care segment represents the largest application area for cold plasma devices, accounting for approximately 42% of the market share. This is attributed to the rising incidence of diabetic foot ulcers, pressure ulcers, and surgical site infections. With over 8.2 million patients requiring advanced wound care treatments annually, the demand for effective, non-pharmaceutical interventions continues to escalate.
Dermatological applications constitute another rapidly growing segment, with a 22% market share. The ability of cold plasma to treat various skin conditions, including acne, eczema, and psoriasis, has garnered significant attention from dermatologists and aesthetic medicine practitioners. Consumer surveys indicate that 65% of patients prefer non-pharmaceutical treatment options for chronic skin conditions, creating a substantial market opportunity for cold plasma technologies.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to exhibit the highest growth rate due to increasing healthcare expenditure, growing awareness about advanced medical technologies, and the rising burden of chronic diseases in countries like China, India, and Japan.
Market penetration challenges persist, primarily due to the high initial investment costs associated with cold plasma equipment and the need for specialized training. Healthcare facilities report that implementation costs and reimbursement uncertainties represent significant barriers to adoption, with 56% of potential buyers citing these factors as primary concerns. Nevertheless, the demonstrable clinical benefits and long-term cost savings potential continue to drive market expansion.
Global Cold Plasma Standards Landscape
The global cold plasma standards landscape for medical devices is characterized by a complex regulatory framework that varies significantly across regions. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for cold plasma medical devices under the 510(k) clearance pathway, with particular emphasis on safety parameters including ozone generation limits, electromagnetic compatibility, and biocompatibility testing requirements.
The European Union regulates cold plasma medical devices through the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021. This framework imposes stringent requirements for clinical evidence, post-market surveillance, and risk management specific to plasma-based technologies. The EU standards particularly focus on electrical safety parameters and treatment dosimetry validation.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specialized evaluation guidelines for plasma medicine applications, while China's National Medical Products Administration (NMPA) has recently introduced standards specifically addressing cold plasma sterilization efficacy requirements. South Korea has emerged as a leader in establishing specialized testing protocols for plasma wound care devices through the Korean Medical Device Information & Technology Assistance Center.
International standardization bodies play a crucial role in harmonizing these diverse regulatory approaches. The International Electrotechnical Commission (IEC) has developed IEC 60601-2-76, which specifically addresses safety requirements for plasma medicine equipment. Similarly, ISO 22441:2022 provides guidelines for the biological evaluation of medical devices treated with cold plasma, establishing standardized testing methodologies for biocompatibility assessment.
Industry consortia have also contributed significantly to standards development. The International Society for Plasma Medicine (ISPM) has published consensus guidelines for reporting plasma medicine research, while the Plasma Healthcare Alliance has established voluntary industry standards for device characterization and performance benchmarking.
Emerging trends in the standards landscape include the development of application-specific guidelines that address unique requirements for different medical applications such as wound healing, cancer treatment, and dental applications. Additionally, there is growing emphasis on establishing standardized protocols for plasma dose quantification and treatment parameter optimization to ensure reproducibility across different device platforms.
The fragmented nature of these standards presents challenges for manufacturers seeking global market access, often necessitating multiple certification processes. However, recent international harmonization efforts, particularly through the Medical Device Single Audit Program (MDSAP), are gradually facilitating more streamlined regulatory pathways for innovative plasma technologies in the medical device sector.
The European Union regulates cold plasma medical devices through the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021. This framework imposes stringent requirements for clinical evidence, post-market surveillance, and risk management specific to plasma-based technologies. The EU standards particularly focus on electrical safety parameters and treatment dosimetry validation.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specialized evaluation guidelines for plasma medicine applications, while China's National Medical Products Administration (NMPA) has recently introduced standards specifically addressing cold plasma sterilization efficacy requirements. South Korea has emerged as a leader in establishing specialized testing protocols for plasma wound care devices through the Korean Medical Device Information & Technology Assistance Center.
International standardization bodies play a crucial role in harmonizing these diverse regulatory approaches. The International Electrotechnical Commission (IEC) has developed IEC 60601-2-76, which specifically addresses safety requirements for plasma medicine equipment. Similarly, ISO 22441:2022 provides guidelines for the biological evaluation of medical devices treated with cold plasma, establishing standardized testing methodologies for biocompatibility assessment.
Industry consortia have also contributed significantly to standards development. The International Society for Plasma Medicine (ISPM) has published consensus guidelines for reporting plasma medicine research, while the Plasma Healthcare Alliance has established voluntary industry standards for device characterization and performance benchmarking.
Emerging trends in the standards landscape include the development of application-specific guidelines that address unique requirements for different medical applications such as wound healing, cancer treatment, and dental applications. Additionally, there is growing emphasis on establishing standardized protocols for plasma dose quantification and treatment parameter optimization to ensure reproducibility across different device platforms.
The fragmented nature of these standards presents challenges for manufacturers seeking global market access, often necessitating multiple certification processes. However, recent international harmonization efforts, particularly through the Medical Device Single Audit Program (MDSAP), are gradually facilitating more streamlined regulatory pathways for innovative plasma technologies in the medical device sector.
Current Cold Plasma Sterilization Methods
01 Medical applications of cold plasma treatment
Cold plasma treatment has significant applications in the medical field, particularly for wound healing, sterilization, and tissue regeneration. The non-thermal plasma can effectively kill bacteria and promote healing without damaging surrounding tissues. This technology is being developed for treating chronic wounds, surgical site infections, and various skin conditions. The controlled application of cold plasma allows for targeted treatment with minimal side effects compared to traditional methods.- Medical applications of cold plasma treatment: Cold plasma technology is being utilized in various medical applications for treatment of tissues and wounds. The non-thermal plasma can effectively kill bacteria and promote healing without damaging surrounding healthy tissue. These treatments can be applied to chronic wounds, dermatological conditions, and even in dental procedures. The controlled application of cold plasma allows for targeted therapy with minimal side effects compared to traditional treatments.
- Cold plasma devices and delivery systems: Various devices and systems have been developed for the generation and delivery of cold plasma for treatment purposes. These include handheld devices, plasma jets, dielectric barrier discharge systems, and specialized applicators designed for specific treatment areas. The engineering of these devices focuses on controlling plasma parameters such as temperature, gas composition, and energy levels to ensure safe and effective treatment while maintaining the 'cold' nature of the plasma.
- Cold plasma for surface modification and sterilization: Cold plasma treatment is effective for modifying surface properties of materials and sterilizing various objects. The technology can alter surface chemistry, improve wettability, and enhance adhesion properties of materials. In sterilization applications, cold plasma can inactivate microorganisms on heat-sensitive materials without causing thermal damage. This makes it particularly valuable in medical device manufacturing, food processing, and pharmaceutical industries where traditional heat sterilization methods may be unsuitable.
- Biological effects and mechanisms of cold plasma: Research into the biological mechanisms of cold plasma treatment reveals complex interactions with living tissues and cells. The reactive species generated by cold plasma, including reactive oxygen and nitrogen species, can trigger cellular responses that promote healing, reduce inflammation, and induce selective apoptosis in damaged cells. Understanding these mechanisms is crucial for optimizing treatment parameters and developing new therapeutic applications while ensuring safety and efficacy.
- Industrial and agricultural applications of cold plasma: Cold plasma technology extends beyond medical applications to various industrial and agricultural uses. In agriculture, it can be used for seed treatment to enhance germination rates and plant growth, as well as for pest control. Industrial applications include surface cleaning, coating preparation, polymer modification, and waste treatment. The environmentally friendly nature of cold plasma, which typically requires only electricity and ambient air or other gases, makes it an attractive alternative to chemical-based processes in these sectors.
02 Cold plasma devices and delivery systems
Various devices and systems have been developed for generating and delivering cold plasma for therapeutic and industrial applications. These include handheld devices, plasma jets, dielectric barrier discharge systems, and atmospheric pressure plasma sources. The designs focus on controlling plasma parameters such as temperature, gas composition, and energy levels to achieve specific treatment outcomes. Innovations in electrode configurations and power supply systems have improved the efficiency and safety of cold plasma delivery.Expand Specific Solutions03 Surface modification and material treatment
Cold plasma treatment is widely used for surface modification of materials, including polymers, textiles, metals, and biomaterials. The treatment can alter surface properties such as wettability, adhesion, and biocompatibility without affecting bulk material properties. This technology enables improved coating adhesion, enhanced material performance, and creation of functional surfaces. Applications include preparing surfaces for bonding, sterilizing medical devices, and creating bioactive interfaces for cell growth.Expand Specific Solutions04 Agricultural and food processing applications
Cold plasma technology has emerging applications in agriculture and food processing, including seed treatment, pest control, and food preservation. The treatment can enhance seed germination rates, decontaminate food products without heat damage, and extend shelf life by inactivating microorganisms. This environmentally friendly approach reduces the need for chemical pesticides and preservatives while maintaining food quality and safety. The non-thermal nature of cold plasma makes it particularly suitable for heat-sensitive agricultural products.Expand Specific Solutions05 Environmental and industrial applications
Cold plasma treatment has significant environmental and industrial applications, including water purification, air cleaning, and waste treatment. The technology can break down pollutants, remove contaminants, and neutralize harmful substances through oxidation processes. Industrial applications include surface cleaning, etching, and deposition of thin films. The versatility of cold plasma allows for customization to specific environmental challenges and manufacturing requirements, offering energy-efficient alternatives to conventional chemical processes.Expand Specific Solutions
Key Medical Device Manufacturers and Regulators
The cold plasma treatment in medical devices market is currently in a growth phase, characterized by increasing adoption across various medical applications. The market size is expanding rapidly, driven by the rising demand for minimally invasive treatments and advanced sterilization technologies. From a technological maturity perspective, the field is evolving with established players like Tokyo Electron Ltd. and Lam Research Corp. bringing semiconductor plasma expertise to medical applications, while specialized companies such as Plasmology4, US Medical Innovations, and CAPS Medical are developing targeted cold plasma solutions for specific medical treatments. Larger healthcare corporations including L'Oréal, Koninklijke Philips, and Olympus are investing in this technology, indicating its growing commercial viability. Academic institutions like Technion Research Foundation and Wuhan University are advancing fundamental research, bridging the gap between theoretical plasma physics and practical medical applications.
Plasmology4, Inc.
Technical Solution: Plasmology4 has developed the PK4™ cold plasma system specifically designed for medical device surface treatment and sterilization. Their technology utilizes a proprietary electrode configuration that generates non-thermal plasma at atmospheric pressure with temperatures maintained below 40°C. The system complies with IEC 60601 standards for medical electrical equipment safety and has undergone extensive validation according to AAMI TIR75:2018 guidelines for plasma sterilization processes. Plasmology4's technology incorporates precise control of plasma parameters including power input, gas composition, and treatment duration to ensure consistent antimicrobial efficacy while preserving material integrity of treated devices. Their cold plasma generators produce controlled concentrations of reactive oxygen and nitrogen species that have been validated through standardized testing methods to effectively inactivate a broad spectrum of pathogens including bacteria, viruses, and fungi. The company has developed specific protocols for different medical device materials and geometries, with validated processes that meet ISO 13485 quality management requirements. Their technology includes automated documentation systems that record treatment parameters for regulatory compliance and traceability.
Strengths: Specialized focus on medical applications with technology specifically designed for healthcare environments; comprehensive validation data supporting antimicrobial efficacy; user-friendly systems designed for integration into existing medical device manufacturing processes. Weaknesses: Smaller company with potentially limited resources compared to larger competitors; relatively focused application range compared to broader plasma technology providers.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced cold plasma technology for medical device applications that complies with multiple international standards. Their systems utilize low-temperature atmospheric pressure plasma jets (APPJ) that operate below 40°C while delivering controlled doses of reactive species for antimicrobial and surface modification purposes. Philips' technology adheres to IEC 60601 series standards for medical electrical equipment safety and has undergone extensive biocompatibility testing according to ISO 10993 requirements. Their plasma generation systems incorporate real-time monitoring of critical parameters including gas flow rates, electrical input, and plasma chemistry to ensure consistent performance within defined safety parameters. The company has developed standardized protocols for plasma treatment of various medical device materials including polymers, metals, and ceramics, with validated processes that meet ISO 13485 quality management requirements. Philips actively participates in international standards development through organizations like IEC and ISO, contributing to the evolution of standards governing cold plasma applications in healthcare. Their technology includes comprehensive documentation systems that record treatment parameters for regulatory compliance and traceability.
Strengths: Extensive resources for R&D and regulatory compliance; global distribution network for technology implementation; comprehensive quality management systems aligned with international standards. Weaknesses: Broad corporate focus may limit specialization in plasma technology compared to dedicated plasma companies; technologies may be optimized for integration with other Philips products rather than standalone applications.
Critical Patents in Medical Cold Plasma Applications
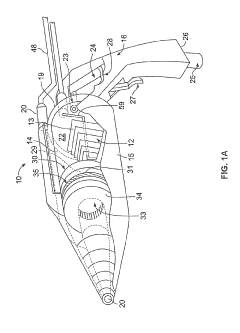
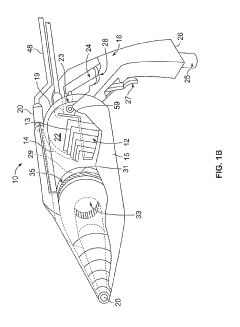
Cold Plasma Treatment Devices and Associated Methods
PatentInactiveUS20190254154A1
Innovation
- A hand-held cold plasma device with a magnet-free gas compartment and a modular electrode configuration, coupled to a high voltage electrical inlet, generates cold plasma in the range of 65 to 120 degrees Fahrenheit, using a capacitor charging power supply and a double tuned RF transformer to produce a rich harmonic output voltage, allowing for adjustable settings and easy component insertion/removal.
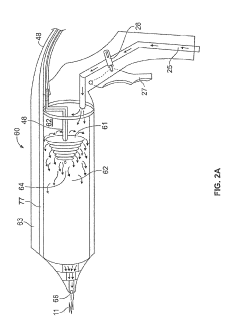
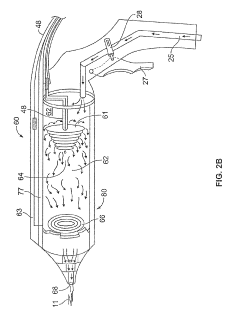
Cold plasma medical device
PatentPendingUS20210145499A1
Innovation
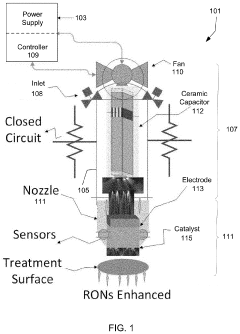
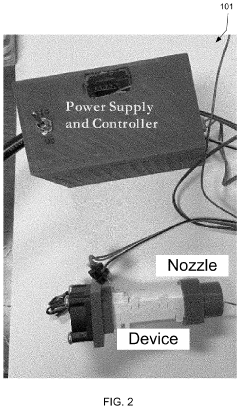
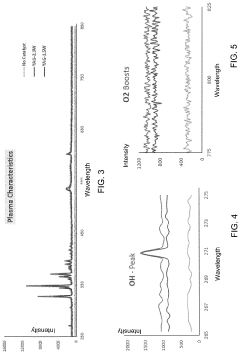
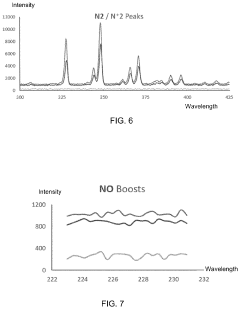
- A portable, non-contact, chemical-free, and low power-consuming cold plasma medical device that generates enhanced reactive oxygen and nitrogen species (RONs) for selective wound application, using a plasma generator with a nozzle and catalyst to produce RONs at safe levels, less than 4 watts, and incorporates a photocatalyst like titanium dioxide or graphene oxide to enhance radical production.
Safety and Efficacy Validation Protocols
The validation of cold plasma treatment safety and efficacy for medical devices requires rigorous protocols that meet international standards. These protocols typically follow a multi-phase approach beginning with in vitro testing to establish fundamental safety parameters. Cell viability assays, mutagenicity tests, and cytotoxicity evaluations form the cornerstone of initial validation, providing critical data on cellular responses to plasma exposure. These tests must be conducted across multiple cell lines relevant to the intended application area to ensure comprehensive safety profiling.
Following successful in vitro validation, protocols advance to ex vivo testing using tissue models that more closely approximate the target treatment environment. This intermediate step bridges the gap between cellular studies and animal models, allowing for assessment of tissue-specific responses without the full complexity of in vivo systems. Tissue penetration depth measurements and histological analyses are essential components of this phase.
Animal model testing represents a critical validation milestone, with protocols designed to evaluate both acute and chronic exposure effects. These studies must monitor not only the target therapeutic outcomes but also potential systemic effects, inflammatory responses, and tissue healing dynamics. Long-term follow-up periods are necessary to detect delayed adverse reactions that may not manifest immediately after treatment.
Human clinical trials for cold plasma medical devices follow a progressive structure from safety-focused Phase I studies through efficacy-centered Phase II and III investigations. Validation protocols must include standardized outcome measures specific to the intended therapeutic application, whether wound healing, pathogen reduction, or cancer treatment. Patient-reported outcomes should complement objective clinical measurements to provide a comprehensive efficacy profile.
Biocompatibility testing according to ISO 10993 standards forms an essential component of validation protocols, with particular emphasis on parts 1 (evaluation and testing), 5 (cytotoxicity), and 10 (irritation and skin sensitization). For devices with prolonged tissue contact, additional testing for genotoxicity and carcinogenicity may be required under parts 3 and 4 of the standard.
Technical performance validation must establish the consistency and reproducibility of plasma generation parameters across multiple device units and operating conditions. This includes measurements of reactive species production, temperature profiles, and electrical safety parameters. Shelf-life testing and stability studies are necessary to determine how storage conditions and time affect device performance and safety characteristics.
Documentation requirements for validation protocols are extensive, necessitating detailed records of test methods, acceptance criteria, statistical analyses, and deviation management. These records form the foundation for regulatory submissions and must demonstrate traceability between validation activities and the device's intended use claims.
Following successful in vitro validation, protocols advance to ex vivo testing using tissue models that more closely approximate the target treatment environment. This intermediate step bridges the gap between cellular studies and animal models, allowing for assessment of tissue-specific responses without the full complexity of in vivo systems. Tissue penetration depth measurements and histological analyses are essential components of this phase.
Animal model testing represents a critical validation milestone, with protocols designed to evaluate both acute and chronic exposure effects. These studies must monitor not only the target therapeutic outcomes but also potential systemic effects, inflammatory responses, and tissue healing dynamics. Long-term follow-up periods are necessary to detect delayed adverse reactions that may not manifest immediately after treatment.
Human clinical trials for cold plasma medical devices follow a progressive structure from safety-focused Phase I studies through efficacy-centered Phase II and III investigations. Validation protocols must include standardized outcome measures specific to the intended therapeutic application, whether wound healing, pathogen reduction, or cancer treatment. Patient-reported outcomes should complement objective clinical measurements to provide a comprehensive efficacy profile.
Biocompatibility testing according to ISO 10993 standards forms an essential component of validation protocols, with particular emphasis on parts 1 (evaluation and testing), 5 (cytotoxicity), and 10 (irritation and skin sensitization). For devices with prolonged tissue contact, additional testing for genotoxicity and carcinogenicity may be required under parts 3 and 4 of the standard.
Technical performance validation must establish the consistency and reproducibility of plasma generation parameters across multiple device units and operating conditions. This includes measurements of reactive species production, temperature profiles, and electrical safety parameters. Shelf-life testing and stability studies are necessary to determine how storage conditions and time affect device performance and safety characteristics.
Documentation requirements for validation protocols are extensive, necessitating detailed records of test methods, acceptance criteria, statistical analyses, and deviation management. These records form the foundation for regulatory submissions and must demonstrate traceability between validation activities and the device's intended use claims.
Biocompatibility and Material Interaction Considerations
Cold plasma treatment of medical devices introduces complex interactions between the plasma-activated surfaces and biological systems. The biocompatibility of plasma-treated medical devices is paramount, as these devices directly contact human tissues and fluids. Research indicates that plasma treatment can significantly alter surface properties without compromising bulk material characteristics, potentially enhancing biocompatibility through improved wettability, reduced bacterial adhesion, and controlled protein adsorption.
Material interactions during cold plasma treatment vary significantly based on the substrate composition. Polymeric materials commonly used in medical devices, such as polyethylene, polypropylene, and polyurethane, exhibit different responses to plasma exposure. Surface oxidation, cross-linking, and chain scission are common reactions that modify surface chemistry and topography. These modifications must be carefully controlled to ensure they enhance rather than compromise biocompatibility.
The plasma treatment parameters—including gas composition, power density, exposure time, and pressure—directly influence the resulting material-biological interactions. For instance, oxygen-based plasmas typically increase surface hydrophilicity and introduce oxygen-containing functional groups, while nitrogen-based plasmas can introduce amine groups that promote cell adhesion. Standards must address the correlation between these treatment parameters and the resulting biological responses.
Cytotoxicity testing represents a critical component of biocompatibility assessment for plasma-treated devices. ISO 10993-5 provides standardized methods for evaluating potential toxic effects of materials on cellular systems. Evidence suggests that properly optimized plasma treatments generally do not introduce cytotoxic effects, but validation is essential for each specific application and material combination.
Hemocompatibility considerations are particularly important for blood-contacting devices. Plasma treatment can modify surface properties to reduce platelet adhesion and activation, potentially decreasing thrombogenicity. However, these effects are highly dependent on the specific plasma chemistry and treatment conditions, necessitating comprehensive hemocompatibility testing according to ISO 10993-4.
Long-term stability of plasma-induced surface modifications presents another critical consideration. Surface aging effects, including hydrophobic recovery and reorganization of surface chemistry, can diminish the beneficial properties initially conferred by plasma treatment. Standards must address stability testing protocols and establish acceptable limits for changes in surface properties over the device's intended lifetime.
Sterilization compatibility represents an additional challenge, as common sterilization methods (ethylene oxide, gamma irradiation, steam) may interact with plasma-modified surfaces, potentially altering their properties. Standards must ensure that the biocompatibility benefits of plasma treatment persist through sterilization processes and throughout the device's shelf life.
Material interactions during cold plasma treatment vary significantly based on the substrate composition. Polymeric materials commonly used in medical devices, such as polyethylene, polypropylene, and polyurethane, exhibit different responses to plasma exposure. Surface oxidation, cross-linking, and chain scission are common reactions that modify surface chemistry and topography. These modifications must be carefully controlled to ensure they enhance rather than compromise biocompatibility.
The plasma treatment parameters—including gas composition, power density, exposure time, and pressure—directly influence the resulting material-biological interactions. For instance, oxygen-based plasmas typically increase surface hydrophilicity and introduce oxygen-containing functional groups, while nitrogen-based plasmas can introduce amine groups that promote cell adhesion. Standards must address the correlation between these treatment parameters and the resulting biological responses.
Cytotoxicity testing represents a critical component of biocompatibility assessment for plasma-treated devices. ISO 10993-5 provides standardized methods for evaluating potential toxic effects of materials on cellular systems. Evidence suggests that properly optimized plasma treatments generally do not introduce cytotoxic effects, but validation is essential for each specific application and material combination.
Hemocompatibility considerations are particularly important for blood-contacting devices. Plasma treatment can modify surface properties to reduce platelet adhesion and activation, potentially decreasing thrombogenicity. However, these effects are highly dependent on the specific plasma chemistry and treatment conditions, necessitating comprehensive hemocompatibility testing according to ISO 10993-4.
Long-term stability of plasma-induced surface modifications presents another critical consideration. Surface aging effects, including hydrophobic recovery and reorganization of surface chemistry, can diminish the beneficial properties initially conferred by plasma treatment. Standards must address stability testing protocols and establish acceptable limits for changes in surface properties over the device's intended lifetime.
Sterilization compatibility represents an additional challenge, as common sterilization methods (ethylene oxide, gamma irradiation, steam) may interact with plasma-modified surfaces, potentially altering their properties. Standards must ensure that the biocompatibility benefits of plasma treatment persist through sterilization processes and throughout the device's shelf life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!