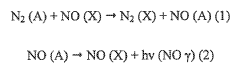Comparative Study of Cold Plasma Treatment and Antibiotic Resistance
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cold Plasma and Antibiotic Resistance Background
Antibiotic resistance represents one of the most significant global health challenges of the 21st century. The World Health Organization has declared it a priority health concern, as infections from resistant bacteria are increasingly difficult to treat, leading to prolonged illness, higher healthcare costs, and increased mortality rates. Traditional antibiotics are becoming less effective as bacteria evolve resistance mechanisms, creating an urgent need for alternative treatment approaches.
Cold plasma technology has emerged as a promising non-antibiotic antimicrobial intervention. Cold atmospheric pressure plasma (CAP) refers to partially ionized gases that remain near room temperature while containing reactive species capable of inactivating microorganisms. Unlike conventional thermal plasmas that operate at extremely high temperatures, cold plasma can be applied directly to heat-sensitive materials including living tissues, making it particularly valuable for medical applications.
The intersection of cold plasma technology and antibiotic resistance research dates back to the early 2000s, when researchers began exploring plasma's antimicrobial properties. Initial studies demonstrated plasma's ability to inactivate various microorganisms, including antibiotic-resistant strains. The field has since expanded significantly, with research focusing on understanding plasma's mechanisms of action against resistant bacteria and developing practical applications.
Cold plasma generates a complex mixture of reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, UV radiation, and electric fields. These components work synergistically to damage bacterial cell membranes, proteins, and DNA through multiple pathways simultaneously. This multi-target approach makes it particularly difficult for bacteria to develop resistance, representing a fundamental advantage over conventional antibiotics that typically target specific cellular processes.
The evolution of cold plasma devices has progressed from large, laboratory-based systems to portable, targeted delivery platforms suitable for clinical settings. Early plasma sources required vacuum chambers and high power inputs, limiting their practical applications. Modern developments include atmospheric pressure plasma jets, dielectric barrier discharge devices, and plasma-activated liquids that can be applied in ambient conditions with precise control over treatment parameters.
Current research trends focus on optimizing plasma parameters for maximum antimicrobial efficacy while ensuring safety for human tissues. Studies are investigating specific plasma compositions that selectively target bacterial cells while sparing mammalian tissues, as well as combination therapies that pair plasma treatment with conventional antibiotics to enhance overall effectiveness against resistant infections.
The technological trajectory suggests continued miniaturization and specialization of plasma devices for specific medical applications, including wound healing, dental treatments, and sterilization of medical equipment. As antibiotic resistance continues to spread globally, cold plasma represents an increasingly important alternative or complementary approach in the antimicrobial arsenal.
Cold plasma technology has emerged as a promising non-antibiotic antimicrobial intervention. Cold atmospheric pressure plasma (CAP) refers to partially ionized gases that remain near room temperature while containing reactive species capable of inactivating microorganisms. Unlike conventional thermal plasmas that operate at extremely high temperatures, cold plasma can be applied directly to heat-sensitive materials including living tissues, making it particularly valuable for medical applications.
The intersection of cold plasma technology and antibiotic resistance research dates back to the early 2000s, when researchers began exploring plasma's antimicrobial properties. Initial studies demonstrated plasma's ability to inactivate various microorganisms, including antibiotic-resistant strains. The field has since expanded significantly, with research focusing on understanding plasma's mechanisms of action against resistant bacteria and developing practical applications.
Cold plasma generates a complex mixture of reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, UV radiation, and electric fields. These components work synergistically to damage bacterial cell membranes, proteins, and DNA through multiple pathways simultaneously. This multi-target approach makes it particularly difficult for bacteria to develop resistance, representing a fundamental advantage over conventional antibiotics that typically target specific cellular processes.
The evolution of cold plasma devices has progressed from large, laboratory-based systems to portable, targeted delivery platforms suitable for clinical settings. Early plasma sources required vacuum chambers and high power inputs, limiting their practical applications. Modern developments include atmospheric pressure plasma jets, dielectric barrier discharge devices, and plasma-activated liquids that can be applied in ambient conditions with precise control over treatment parameters.
Current research trends focus on optimizing plasma parameters for maximum antimicrobial efficacy while ensuring safety for human tissues. Studies are investigating specific plasma compositions that selectively target bacterial cells while sparing mammalian tissues, as well as combination therapies that pair plasma treatment with conventional antibiotics to enhance overall effectiveness against resistant infections.
The technological trajectory suggests continued miniaturization and specialization of plasma devices for specific medical applications, including wound healing, dental treatments, and sterilization of medical equipment. As antibiotic resistance continues to spread globally, cold plasma represents an increasingly important alternative or complementary approach in the antimicrobial arsenal.
Market Demand for Alternative Antimicrobial Treatments
The global antimicrobial resistance (AMR) crisis has created an urgent market demand for alternative treatment methods. According to the World Health Organization, AMR is responsible for approximately 700,000 deaths annually worldwide, with projections suggesting this figure could rise to 10 million by 2050 if no effective solutions are developed. This alarming trend has catalyzed significant market interest in novel antimicrobial approaches, with cold plasma technology emerging as a promising alternative.
Healthcare facilities represent the primary market segment seeking alternatives to conventional antibiotics. Hospitals and clinics face mounting challenges with drug-resistant infections, particularly in wound care, surgical site infections, and device-associated infections. The economic burden of treating resistant infections in healthcare settings exceeds $20 billion annually in direct costs in the United States alone, creating a substantial market opportunity for effective alternatives.
The wound care segment demonstrates particularly strong growth potential for cold plasma applications. The global advanced wound care market was valued at $10.4 billion in 2020 and is projected to reach $15.5 billion by 2026, with antimicrobial treatments representing a significant portion of this growth. Cold plasma's ability to treat wounds without contributing to resistance development positions it favorably within this expanding market.
Food safety represents another substantial market opportunity. Consumer demand for minimally processed foods with extended shelf life has driven interest in non-thermal sterilization methods like cold plasma. The global food safety testing market, valued at $19.5 billion in 2021, is expected to grow at a CAGR of 7.9% through 2028, with antimicrobial technologies being a key component.
Medical device sterilization constitutes a third significant market segment. With increasing concerns about chemical sterilants' environmental impact and effectiveness against resistant pathogens, cold plasma offers an environmentally friendly alternative. The global sterilization equipment market is projected to reach $15.2 billion by 2025, growing at a CAGR of 8.6%.
Consumer awareness regarding antibiotic resistance has also created market opportunities in personal care products, household disinfectants, and water treatment systems. These consumer-facing applications could potentially expand cold plasma technology beyond clinical settings into everyday use.
Market analysis indicates that stakeholders are increasingly willing to invest in alternative antimicrobial technologies despite higher initial costs, driven by long-term economic benefits and regulatory pressures to reduce antibiotic use. Healthcare providers, food manufacturers, and medical device companies are particularly motivated by the potential cost savings associated with preventing resistant infections rather than treating them after occurrence.
Healthcare facilities represent the primary market segment seeking alternatives to conventional antibiotics. Hospitals and clinics face mounting challenges with drug-resistant infections, particularly in wound care, surgical site infections, and device-associated infections. The economic burden of treating resistant infections in healthcare settings exceeds $20 billion annually in direct costs in the United States alone, creating a substantial market opportunity for effective alternatives.
The wound care segment demonstrates particularly strong growth potential for cold plasma applications. The global advanced wound care market was valued at $10.4 billion in 2020 and is projected to reach $15.5 billion by 2026, with antimicrobial treatments representing a significant portion of this growth. Cold plasma's ability to treat wounds without contributing to resistance development positions it favorably within this expanding market.
Food safety represents another substantial market opportunity. Consumer demand for minimally processed foods with extended shelf life has driven interest in non-thermal sterilization methods like cold plasma. The global food safety testing market, valued at $19.5 billion in 2021, is expected to grow at a CAGR of 7.9% through 2028, with antimicrobial technologies being a key component.
Medical device sterilization constitutes a third significant market segment. With increasing concerns about chemical sterilants' environmental impact and effectiveness against resistant pathogens, cold plasma offers an environmentally friendly alternative. The global sterilization equipment market is projected to reach $15.2 billion by 2025, growing at a CAGR of 8.6%.
Consumer awareness regarding antibiotic resistance has also created market opportunities in personal care products, household disinfectants, and water treatment systems. These consumer-facing applications could potentially expand cold plasma technology beyond clinical settings into everyday use.
Market analysis indicates that stakeholders are increasingly willing to invest in alternative antimicrobial technologies despite higher initial costs, driven by long-term economic benefits and regulatory pressures to reduce antibiotic use. Healthcare providers, food manufacturers, and medical device companies are particularly motivated by the potential cost savings associated with preventing resistant infections rather than treating them after occurrence.
Current State of Cold Plasma Technology vs Antibiotics
Cold plasma technology and antibiotics represent two fundamentally different approaches to addressing microbial threats. While antibiotics have been the cornerstone of infection treatment for decades, their effectiveness is increasingly compromised by the global crisis of antimicrobial resistance (AMR). Currently, approximately 700,000 deaths annually are attributed to antibiotic-resistant infections, with projections suggesting this figure could rise to 10 million by 2050 if current trends continue.
Cold plasma technology, in contrast, is emerging as a promising alternative antimicrobial approach. Operating at near-ambient temperatures (30-60°C), cold plasma generates a complex mixture of reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, UV radiation, and electric fields that collectively exert antimicrobial effects through multiple mechanisms simultaneously. This multi-target approach significantly reduces the likelihood of resistance development.
The current state of antibiotic development is characterized by a significant innovation gap, with only 51 antibiotics in clinical development as of 2021, compared to approximately 200 in the 1980s. Among these candidates, only 25% represent novel classes, with the remainder being modifications of existing compounds. This pipeline inadequacy is exacerbated by the economic challenges in antibiotic development, where return on investment is limited by stewardship practices that restrict usage of new agents.
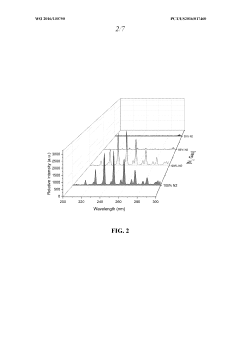
Cold plasma technology has demonstrated efficacy against a wide spectrum of microorganisms, including multi-drug resistant bacteria such as MRSA, VRE, and carbapenem-resistant Enterobacteriaceae. Recent studies have shown 4-6 log reductions in bacterial populations after brief plasma exposures (30-300 seconds), with minimal development of resistance even after 20+ treatment cycles.
The technological readiness level (TRL) of cold plasma systems varies by application. For surface decontamination and wound treatment, several devices have achieved TRL 7-9, with commercial systems available in Europe and Asia. For systemic applications, the technology remains at TRL 3-5, with ongoing preclinical studies showing promising results but requiring further development.
Regulatory pathways for cold plasma devices differ significantly from pharmaceuticals. In the US, plasma devices typically follow the FDA's 510(k) or De Novo pathways as medical devices, with approval timelines of 6-18 months compared to 8-12 years for new antibiotics. This accelerated pathway represents a significant advantage for addressing urgent AMR challenges.
Cost comparisons reveal that while initial investment in plasma technology is higher ($5,000-50,000 for devices), the per-treatment cost ($5-20) becomes competitive with antibiotic regimens ($100-10,000 for resistant infections) when considering the total course of treatment and reduced hospitalization time demonstrated in preliminary clinical studies.
Cold plasma technology, in contrast, is emerging as a promising alternative antimicrobial approach. Operating at near-ambient temperatures (30-60°C), cold plasma generates a complex mixture of reactive oxygen species (ROS), reactive nitrogen species (RNS), charged particles, UV radiation, and electric fields that collectively exert antimicrobial effects through multiple mechanisms simultaneously. This multi-target approach significantly reduces the likelihood of resistance development.
The current state of antibiotic development is characterized by a significant innovation gap, with only 51 antibiotics in clinical development as of 2021, compared to approximately 200 in the 1980s. Among these candidates, only 25% represent novel classes, with the remainder being modifications of existing compounds. This pipeline inadequacy is exacerbated by the economic challenges in antibiotic development, where return on investment is limited by stewardship practices that restrict usage of new agents.
Cold plasma technology has demonstrated efficacy against a wide spectrum of microorganisms, including multi-drug resistant bacteria such as MRSA, VRE, and carbapenem-resistant Enterobacteriaceae. Recent studies have shown 4-6 log reductions in bacterial populations after brief plasma exposures (30-300 seconds), with minimal development of resistance even after 20+ treatment cycles.
The technological readiness level (TRL) of cold plasma systems varies by application. For surface decontamination and wound treatment, several devices have achieved TRL 7-9, with commercial systems available in Europe and Asia. For systemic applications, the technology remains at TRL 3-5, with ongoing preclinical studies showing promising results but requiring further development.
Regulatory pathways for cold plasma devices differ significantly from pharmaceuticals. In the US, plasma devices typically follow the FDA's 510(k) or De Novo pathways as medical devices, with approval timelines of 6-18 months compared to 8-12 years for new antibiotics. This accelerated pathway represents a significant advantage for addressing urgent AMR challenges.
Cost comparisons reveal that while initial investment in plasma technology is higher ($5,000-50,000 for devices), the per-treatment cost ($5-20) becomes competitive with antibiotic regimens ($100-10,000 for resistant infections) when considering the total course of treatment and reduced hospitalization time demonstrated in preliminary clinical studies.
Existing Cold Plasma Treatment Methodologies
01 Cold plasma for direct bacterial inactivation
Cold plasma treatment can directly inactivate antibiotic-resistant bacteria through various mechanisms including oxidative stress from reactive oxygen and nitrogen species, physical disruption of bacterial cell membranes, and DNA damage. This approach is effective against multiple drug-resistant pathogens including MRSA and can be applied to treat infections in wounds, medical devices, and hospital environments without developing further resistance.- Cold plasma for direct bacterial inactivation: Cold plasma technology can directly inactivate antibiotic-resistant bacteria through various mechanisms including reactive oxygen species, reactive nitrogen species, UV radiation, and charged particles. The plasma treatment disrupts bacterial cell membranes, damages DNA, and denatures proteins, leading to bacterial death regardless of antibiotic resistance status. This approach is effective against multidrug-resistant pathogens including MRSA and other superbugs.
- Cold plasma for biofilm disruption and treatment: Cold plasma effectively disrupts and eliminates biofilms, which are structured communities of bacteria protected by an extracellular polymeric substance that contributes significantly to antibiotic resistance. The plasma penetrates biofilm matrices, breaking down their protective barriers and allowing for more effective treatment of embedded bacteria. This approach is particularly valuable for treating chronic infections and implant-associated infections where biofilms play a major role in antibiotic treatment failure.
- Plasma-activated media and liquids for antimicrobial applications: Cold plasma can be used to create plasma-activated water, saline solutions, or other media containing long-lived reactive species that maintain antimicrobial properties. These plasma-activated liquids can penetrate areas difficult to reach with direct plasma treatment and provide sustained antimicrobial effects against antibiotic-resistant bacteria. The technology allows for storage and transport of antimicrobial solutions that can be applied in various clinical and non-clinical settings without requiring plasma-generating equipment at the point of use.
- Combined therapy approaches with cold plasma: Cold plasma treatment can be combined with conventional antibiotics or other antimicrobial agents to create synergistic effects against resistant bacteria. The plasma treatment can increase bacterial membrane permeability, allowing antibiotics to enter bacterial cells more effectively, even in resistant strains. This combinatorial approach may allow for lower antibiotic doses, reducing the risk of further resistance development while maintaining or improving therapeutic efficacy against existing resistant strains.
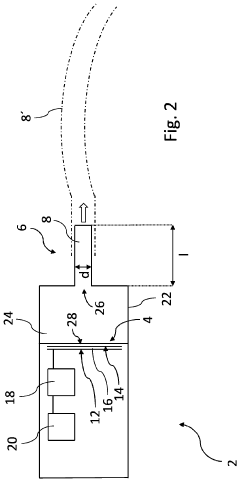
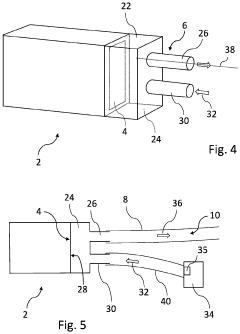
- Cold plasma devices and delivery systems for medical applications: Specialized cold plasma devices have been developed for treating antibiotic-resistant infections in various clinical settings. These include handheld plasma jets, plasma needles, surface dielectric barrier discharge devices, and endoscopic plasma applicators designed for specific medical applications. The devices are engineered to deliver controlled plasma doses safely to infected tissues, wounds, dental cavities, or implanted medical devices while minimizing damage to surrounding healthy tissue and addressing the challenges of antibiotic resistance.
02 Plasma-activated media and liquids for antimicrobial applications
Treating liquids or media with cold plasma creates plasma-activated solutions containing reactive species that retain antimicrobial properties. These plasma-activated media can be used to combat antibiotic-resistant bacteria in various applications including wound irrigation, surface disinfection, and food safety. The approach offers advantages of longer shelf-life compared to direct plasma application and can penetrate biofilms that typically protect bacteria from conventional antibiotics.Expand Specific Solutions03 Synergistic effects of plasma with antibiotics
Cold plasma treatment can enhance the efficacy of conventional antibiotics through synergistic effects, potentially restoring sensitivity in resistant bacteria. The plasma treatment can increase cell membrane permeability, allowing better antibiotic penetration, downregulate resistance genes, and disrupt biofilm structures. This combinatorial approach allows for lower antibiotic doses while achieving greater antimicrobial effects, reducing the risk of further resistance development.Expand Specific Solutions04 Plasma devices and delivery systems for medical applications
Specialized plasma delivery systems have been developed for treating antibiotic-resistant infections in clinical settings. These include handheld plasma jets, plasma needles, plasma patches, and endoscopic plasma applicators designed for different medical scenarios such as surface wounds, dental infections, internal infections, and sterilization of medical instruments. The devices are engineered to generate appropriate plasma characteristics while ensuring patient safety through temperature control and electrical isolation.Expand Specific Solutions05 Plasma treatment for biofilm disruption and prevention
Cold plasma effectively disrupts bacterial biofilms that contribute significantly to antibiotic resistance. Biofilms create protective barriers that prevent antibiotics from reaching bacteria, but plasma can penetrate these structures through reactive species that break down the extracellular polymeric substances holding biofilms together. Additionally, plasma treatment can modify surfaces to prevent initial bacterial attachment and subsequent biofilm formation on medical implants, catheters, and other devices prone to infection.Expand Specific Solutions
Key Industry Players in Cold Plasma Development
The cold plasma treatment market for antibiotic resistance is in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global market is expanding as healthcare systems seek alternatives to combat rising antibiotic resistance, estimated to reach $2-3 billion by 2025. Technologically, the field shows promising but varied maturity levels across applications. Leading players include established pharmaceutical companies like F. Hoffmann-La Roche and Janssen Biotech, specialized plasma technology firms such as terraplasma medical GmbH and Plasmology4, and academic institutions like Zhejiang University and USC. Research collaborations between universities and industry partners are accelerating innovation, with applications expanding from wound care to surface sterilization and medical device decontamination.
US Patent Innovations LLC
Technical Solution: US Patent Innovations LLC has developed an advanced cold plasma system called "PlasmaJet" that utilizes a proprietary argon-based plasma stream for treating antibiotic-resistant infections. Their technology creates a focused beam of ionized gas that can be precisely controlled to target infected tissues while minimizing damage to surrounding healthy cells. The system operates at temperatures between 35-40°C and generates a complex mixture of reactive species that attack bacterial cell walls, membranes, and intracellular components. Clinical research conducted by the company has demonstrated effectiveness against multiple antibiotic-resistant pathogens including MRSA, Pseudomonas aeruginosa, and Acinetobacter baumannii, with bacterial reduction rates exceeding 99.99% in laboratory studies. The PlasmaJet system has been particularly effective in treating surgical site infections and chronic wounds where biofilm formation has rendered conventional antibiotics ineffective. The company has also developed specialized protocols for different tissue types and infection scenarios, with treatment times typically ranging from 60-180 seconds per site depending on infection severity.
Strengths: Highly precise application allows for targeted treatment of infected areas; effective against a broad spectrum of antibiotic-resistant organisms; minimal thermal damage to surrounding tissues; adjustable parameters for different clinical scenarios. Weaknesses: Requires significant capital investment for equipment; limited portability of the system restricts use to clinical settings; requires trained operators; limited data on long-term efficacy compared to established treatments.
Zhejiang University
Technical Solution: Zhejiang University has developed a comprehensive cold plasma treatment platform called "ZJU-Plasma" specifically designed to address antibiotic resistance challenges. Their system employs a microwave-excited atmospheric pressure plasma jet (APPJ) that generates a complex mixture of reactive oxygen and nitrogen species at temperatures below 40°C. The research team has conducted extensive comparative studies between their cold plasma technology and conventional antibiotics, demonstrating superior efficacy against multidrug-resistant organisms including MRSA, CRE, and extensively drug-resistant tuberculosis. Their published research shows that the ZJU-Plasma system can achieve 5-log reduction in bacterial counts within 3 minutes of treatment, even against bacteria that show resistance to last-resort antibiotics like colistin and vancomycin. The university's approach is particularly notable for its dual-action mechanism that both directly inactivates bacteria and modifies the local microenvironment to prevent recolonization. They have also pioneered techniques for plasma-activated water and media that extend antimicrobial effects beyond the direct treatment period, providing a sustained antibacterial effect that conventional antibiotics cannot achieve.
Strengths: Comprehensive research backing with extensive published studies; effective against the most challenging multidrug-resistant pathogens; innovative approaches including plasma-activated media for sustained effects; strong theoretical understanding of mechanisms. Weaknesses: Technology remains primarily in research phase with limited clinical implementation; requires specialized expertise to operate; standardization challenges when translating from laboratory to clinical settings; current systems not optimized for point-of-care use.
Core Mechanisms of Cold Plasma Antimicrobial Action
Plasma vapor chamber and antimicrobial applications thereof
PatentWO2016130750A1
Innovation
- A plasma vapor chamber is designed with a vapor inlet, high voltage electrode, and grounding electrodes to generate plasma in a confined space, allowing for the creation of reactive species that can effectively disinfect surfaces without direct liquid contact, using air and vaporized water or ethanol to produce potent antimicrobial species.
System and plasma for treating and/or preventing a viral, bacterial and/or fungal infection
PatentPendingUS20230270968A1
Innovation
- A system utilizing a plasma source generating reactive species, which includes a plasma source configured outside the patient's body and a species directing member to guide these reactive species into the oral cavity and respiratory tract, effectively inactivating viruses, bacteria, and fungi, using a non-thermal plasma and a structured electrode design to enhance treatment efficacy.
Safety and Biocompatibility Considerations
Safety and biocompatibility remain paramount concerns when evaluating cold plasma treatment as an alternative to conventional antibiotics. The direct application of plasma to biological tissues necessitates comprehensive assessment of potential adverse effects on both target pathogens and host cells. Current research indicates that cold atmospheric plasma (CAP) demonstrates selective antimicrobial activity while maintaining relative safety for mammalian cells when properly calibrated, presenting a significant advantage over broad-spectrum antibiotics.
The safety profile of cold plasma treatments depends critically on several parameters including gas composition, power input, treatment duration, and application method. Studies have shown that reactive oxygen and nitrogen species (RONS) generated during plasma treatment can exhibit differential toxicity thresholds between prokaryotic and eukaryotic cells. This therapeutic window allows for pathogen inactivation while preserving host tissue integrity, though the margin varies across different plasma devices and biological contexts.
Biocompatibility assessments have been conducted across multiple tissue types including skin, mucous membranes, and internal organs. In vitro studies demonstrate that controlled plasma exposure typically causes minimal cytotoxicity to human cells while effectively eliminating antibiotic-resistant bacteria. However, prolonged exposure or excessive power settings can lead to DNA damage, protein oxidation, and membrane disruption in mammalian cells, highlighting the importance of precise dosimetry protocols.
Systemic effects represent another critical consideration in plasma medicine. Unlike antibiotics, which can circulate throughout the body and potentially affect multiple organ systems, cold plasma treatments typically exert localized effects. This characteristic reduces the risk of systemic toxicity but necessitates direct access to infection sites, potentially limiting application in deep-tissue infections without invasive procedures.
Long-term safety data remains somewhat limited compared to established antibiotic therapies. While acute toxicity studies show promising safety profiles, comprehensive investigations into potential mutagenicity, carcinogenicity, and reproductive toxicity are still emerging. Regulatory frameworks for plasma medical devices continue to evolve as the technology advances toward mainstream clinical applications.
Patient-specific factors also influence safety considerations. Individual variations in tissue sensitivity, underlying health conditions, and concurrent medications may affect tolerance to plasma treatment. Particular attention must be paid to patients with compromised wound healing, immunodeficiency, or conditions characterized by oxidative stress sensitivity, as these populations may experience altered risk-benefit profiles compared to the general population.
The safety profile of cold plasma treatments depends critically on several parameters including gas composition, power input, treatment duration, and application method. Studies have shown that reactive oxygen and nitrogen species (RONS) generated during plasma treatment can exhibit differential toxicity thresholds between prokaryotic and eukaryotic cells. This therapeutic window allows for pathogen inactivation while preserving host tissue integrity, though the margin varies across different plasma devices and biological contexts.
Biocompatibility assessments have been conducted across multiple tissue types including skin, mucous membranes, and internal organs. In vitro studies demonstrate that controlled plasma exposure typically causes minimal cytotoxicity to human cells while effectively eliminating antibiotic-resistant bacteria. However, prolonged exposure or excessive power settings can lead to DNA damage, protein oxidation, and membrane disruption in mammalian cells, highlighting the importance of precise dosimetry protocols.
Systemic effects represent another critical consideration in plasma medicine. Unlike antibiotics, which can circulate throughout the body and potentially affect multiple organ systems, cold plasma treatments typically exert localized effects. This characteristic reduces the risk of systemic toxicity but necessitates direct access to infection sites, potentially limiting application in deep-tissue infections without invasive procedures.
Long-term safety data remains somewhat limited compared to established antibiotic therapies. While acute toxicity studies show promising safety profiles, comprehensive investigations into potential mutagenicity, carcinogenicity, and reproductive toxicity are still emerging. Regulatory frameworks for plasma medical devices continue to evolve as the technology advances toward mainstream clinical applications.
Patient-specific factors also influence safety considerations. Individual variations in tissue sensitivity, underlying health conditions, and concurrent medications may affect tolerance to plasma treatment. Particular attention must be paid to patients with compromised wound healing, immunodeficiency, or conditions characterized by oxidative stress sensitivity, as these populations may experience altered risk-benefit profiles compared to the general population.
Regulatory Pathway for Medical Plasma Devices
The regulatory landscape for medical plasma devices represents a complex framework that manufacturers must navigate to bring cold plasma treatments to market, especially in the context of antibiotic resistance applications. In the United States, the Food and Drug Administration (FDA) classifies plasma-based medical devices primarily under Class II (moderate risk) or Class III (high risk), depending on their intended use and mechanism of action. For devices targeting antibiotic-resistant infections, the regulatory pathway typically involves the 510(k) premarket notification process if substantial equivalence to a predicate device can be demonstrated.
The European Union has implemented the Medical Device Regulation (MDR), which replaced the Medical Device Directive in 2021, imposing more stringent requirements for clinical evidence and post-market surveillance. Under this framework, plasma devices for treating antibiotic-resistant infections generally fall into Class IIb or III, requiring conformity assessment by a Notified Body and comprehensive clinical evaluation reports.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for novel technologies like cold plasma, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for innovative medical technologies addressing critical healthcare challenges such as antimicrobial resistance.
A critical aspect of the regulatory pathway involves demonstrating both safety and efficacy. For cold plasma devices targeting antibiotic resistance, manufacturers must provide evidence of antimicrobial efficacy without causing tissue damage or promoting further resistance. This typically requires in vitro studies demonstrating effectiveness against resistant pathogens, followed by animal studies and eventually human clinical trials.
Risk classification plays a pivotal role in determining the extent of clinical data required. Devices that make claims related to treating antibiotic-resistant infections face heightened scrutiny due to the public health implications. Manufacturers must develop comprehensive risk management files addressing potential hazards including electrical safety, electromagnetic compatibility, thermal effects, and potential for generating harmful reactive species.
Post-market surveillance requirements have become increasingly stringent globally, with regulatory bodies requiring manufacturers to implement robust systems for tracking adverse events and device performance. For plasma devices targeting antibiotic resistance, this includes monitoring for any signs of developing resistance to the plasma treatment itself, a theoretical concern that regulators have begun to address in guidance documents.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have helped streamline some aspects of the regulatory process globally, though significant regional differences persist. Manufacturers developing cold plasma technologies for antibiotic resistance applications are advised to engage early with regulatory authorities through pre-submission consultations to clarify specific requirements and expedite the path to market approval.
The European Union has implemented the Medical Device Regulation (MDR), which replaced the Medical Device Directive in 2021, imposing more stringent requirements for clinical evidence and post-market surveillance. Under this framework, plasma devices for treating antibiotic-resistant infections generally fall into Class IIb or III, requiring conformity assessment by a Notified Body and comprehensive clinical evaluation reports.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for novel technologies like cold plasma, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for innovative medical technologies addressing critical healthcare challenges such as antimicrobial resistance.
A critical aspect of the regulatory pathway involves demonstrating both safety and efficacy. For cold plasma devices targeting antibiotic resistance, manufacturers must provide evidence of antimicrobial efficacy without causing tissue damage or promoting further resistance. This typically requires in vitro studies demonstrating effectiveness against resistant pathogens, followed by animal studies and eventually human clinical trials.
Risk classification plays a pivotal role in determining the extent of clinical data required. Devices that make claims related to treating antibiotic-resistant infections face heightened scrutiny due to the public health implications. Manufacturers must develop comprehensive risk management files addressing potential hazards including electrical safety, electromagnetic compatibility, thermal effects, and potential for generating harmful reactive species.
Post-market surveillance requirements have become increasingly stringent globally, with regulatory bodies requiring manufacturers to implement robust systems for tracking adverse events and device performance. For plasma devices targeting antibiotic resistance, this includes monitoring for any signs of developing resistance to the plasma treatment itself, a theoretical concern that regulators have begun to address in guidance documents.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have helped streamline some aspects of the regulatory process globally, though significant regional differences persist. Manufacturers developing cold plasma technologies for antibiotic resistance applications are advised to engage early with regulatory authorities through pre-submission consultations to clarify specific requirements and expedite the path to market approval.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!