Why Cold Plasma Treatment Enhances Antimicrobial Properties in Textiles
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cold Plasma Technology Evolution and Objectives
Cold plasma technology has evolved significantly over the past several decades, transitioning from a purely scientific curiosity to a practical industrial application. Initially discovered in the late 19th century, plasma was primarily studied as the fourth state of matter, with limited practical applications. The 1970s marked the beginning of cold plasma research for material modification, though applications remained largely confined to electronics and semiconductor industries.
The 1990s witnessed a pivotal shift when researchers began exploring cold plasma for surface modifications of polymers and textiles. This period established fundamental understanding of how low-temperature plasma could alter surface properties without affecting bulk material characteristics. By the early 2000s, researchers had documented the potential antimicrobial effects of plasma treatment on various substrates, opening new avenues for textile applications.
The evolution accelerated in the 2010s with the development of atmospheric pressure plasma systems, eliminating the need for vacuum chambers and making the technology more accessible for industrial textile processing. This advancement represented a critical turning point, as it addressed scalability challenges that had previously limited commercial adoption. Recent years have seen increasingly sophisticated plasma generation methods, including dielectric barrier discharge (DBD), corona discharge, and atmospheric pressure plasma jets, each offering specific advantages for textile treatment.
Current technological objectives focus on optimizing cold plasma parameters for enhanced antimicrobial efficacy while maintaining textile integrity. Researchers aim to identify ideal gas compositions, power settings, and exposure durations that maximize antimicrobial properties while minimizing energy consumption and treatment time. Another key objective involves understanding the fundamental mechanisms by which plasma treatment confers antimicrobial properties, including surface etching effects, functional group grafting, and nanostructure formation.
Looking forward, the field is moving toward developing sustainable plasma processes that reduce chemical usage in textile finishing. Integration with other emerging technologies, such as nanotechnology and smart textiles, represents another important objective. Researchers are also working to establish standardized testing protocols specifically designed for plasma-treated antimicrobial textiles, as current antimicrobial testing methods may not fully capture the unique properties of plasma-modified surfaces.
The ultimate goal remains creating commercially viable, environmentally friendly processes that can be seamlessly integrated into existing textile manufacturing lines, providing durable antimicrobial properties without compromising other textile characteristics such as comfort, breathability, and mechanical strength.
The 1990s witnessed a pivotal shift when researchers began exploring cold plasma for surface modifications of polymers and textiles. This period established fundamental understanding of how low-temperature plasma could alter surface properties without affecting bulk material characteristics. By the early 2000s, researchers had documented the potential antimicrobial effects of plasma treatment on various substrates, opening new avenues for textile applications.
The evolution accelerated in the 2010s with the development of atmospheric pressure plasma systems, eliminating the need for vacuum chambers and making the technology more accessible for industrial textile processing. This advancement represented a critical turning point, as it addressed scalability challenges that had previously limited commercial adoption. Recent years have seen increasingly sophisticated plasma generation methods, including dielectric barrier discharge (DBD), corona discharge, and atmospheric pressure plasma jets, each offering specific advantages for textile treatment.
Current technological objectives focus on optimizing cold plasma parameters for enhanced antimicrobial efficacy while maintaining textile integrity. Researchers aim to identify ideal gas compositions, power settings, and exposure durations that maximize antimicrobial properties while minimizing energy consumption and treatment time. Another key objective involves understanding the fundamental mechanisms by which plasma treatment confers antimicrobial properties, including surface etching effects, functional group grafting, and nanostructure formation.
Looking forward, the field is moving toward developing sustainable plasma processes that reduce chemical usage in textile finishing. Integration with other emerging technologies, such as nanotechnology and smart textiles, represents another important objective. Researchers are also working to establish standardized testing protocols specifically designed for plasma-treated antimicrobial textiles, as current antimicrobial testing methods may not fully capture the unique properties of plasma-modified surfaces.
The ultimate goal remains creating commercially viable, environmentally friendly processes that can be seamlessly integrated into existing textile manufacturing lines, providing durable antimicrobial properties without compromising other textile characteristics such as comfort, breathability, and mechanical strength.
Antimicrobial Textile Market Analysis
The global antimicrobial textile market has been experiencing significant growth, driven by increasing awareness of hygiene and health concerns across various sectors. Currently valued at approximately 12.3 billion USD, the market is projected to reach 20.5 billion USD by 2026, representing a compound annual growth rate (CAGR) of 9.4%. This growth trajectory is particularly notable in healthcare, sportswear, and home textile segments where antimicrobial properties deliver substantial value.
Healthcare applications dominate the antimicrobial textile market, accounting for nearly 40% of the total market share. This dominance stems from the critical need to prevent healthcare-associated infections (HAIs) in medical facilities. The sportswear segment follows closely, driven by consumer demand for odor-resistant and hygiene-enhanced athletic apparel, particularly in premium product categories where performance features command price premiums.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 11.2% annually through 2026, primarily due to expanding healthcare infrastructure, rising disposable incomes, and increasing awareness about antimicrobial benefits in countries like China and India.
Consumer behavior studies indicate a growing willingness to pay premium prices for textiles with proven antimicrobial properties, with surveys showing that 67% of consumers consider antimicrobial features "important" or "very important" in their purchasing decisions for certain textile products. This trend has accelerated significantly since the COVID-19 pandemic, which heightened public awareness about pathogen transmission through surfaces.
The competitive landscape features established players like Milliken & Company, Trevira GmbH, and Microban International, alongside emerging specialists focusing exclusively on antimicrobial textile technologies. Cold plasma treatment represents a disruptive innovation in this space, potentially offering manufacturers a more sustainable and effective alternative to traditional chemical treatments.
Market challenges include regulatory hurdles, with varying standards across regions creating compliance complexities. Additionally, consumer skepticism regarding durability of antimicrobial properties after multiple wash cycles presents an adoption barrier that manufacturers must address through improved technology and transparent efficacy testing.
Healthcare applications dominate the antimicrobial textile market, accounting for nearly 40% of the total market share. This dominance stems from the critical need to prevent healthcare-associated infections (HAIs) in medical facilities. The sportswear segment follows closely, driven by consumer demand for odor-resistant and hygiene-enhanced athletic apparel, particularly in premium product categories where performance features command price premiums.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 11.2% annually through 2026, primarily due to expanding healthcare infrastructure, rising disposable incomes, and increasing awareness about antimicrobial benefits in countries like China and India.
Consumer behavior studies indicate a growing willingness to pay premium prices for textiles with proven antimicrobial properties, with surveys showing that 67% of consumers consider antimicrobial features "important" or "very important" in their purchasing decisions for certain textile products. This trend has accelerated significantly since the COVID-19 pandemic, which heightened public awareness about pathogen transmission through surfaces.
The competitive landscape features established players like Milliken & Company, Trevira GmbH, and Microban International, alongside emerging specialists focusing exclusively on antimicrobial textile technologies. Cold plasma treatment represents a disruptive innovation in this space, potentially offering manufacturers a more sustainable and effective alternative to traditional chemical treatments.
Market challenges include regulatory hurdles, with varying standards across regions creating compliance complexities. Additionally, consumer skepticism regarding durability of antimicrobial properties after multiple wash cycles presents an adoption barrier that manufacturers must address through improved technology and transparent efficacy testing.
Current Cold Plasma Treatment Challenges
Despite the promising antimicrobial potential of cold plasma treatment for textiles, several significant technical challenges currently impede its widespread industrial adoption. The primary obstacle remains scalability, as most research has been conducted at laboratory scale using small batch processes. Transitioning to continuous, high-throughput production lines presents considerable engineering difficulties in maintaining plasma uniformity across large textile surfaces and ensuring consistent treatment quality at industrial speeds.
Energy efficiency represents another major challenge, with current cold plasma systems requiring substantial power input relative to conventional textile treatments. This high energy consumption not only increases operational costs but also diminishes the environmental benefits that cold plasma technology potentially offers compared to chemical-intensive alternatives.
Process standardization remains elusive due to the complex interplay of plasma parameters. Treatment outcomes are highly sensitive to variations in gas composition, power input, exposure time, pressure conditions, and electrode configurations. This sensitivity makes it difficult to establish universal protocols that can be reliably reproduced across different manufacturing environments and equipment setups.
The durability of antimicrobial effects after repeated washing cycles continues to be problematic. While initial antimicrobial efficacy may be impressive, many plasma-treated textiles show significant reduction in performance after standard laundering procedures. This limitation severely restricts application in products requiring long-term antimicrobial properties, such as healthcare textiles and athletic wear.
Material compatibility issues further complicate implementation, as plasma treatments can adversely affect certain synthetic fibers, causing unwanted changes in mechanical properties, color, or texture. Particularly challenging are temperature-sensitive materials that may degrade under plasma exposure, even at relatively low temperatures compared to thermal processes.
Equipment cost presents a substantial barrier to entry, especially for small and medium-sized textile manufacturers. The specialized nature of plasma generation equipment, vacuum systems (for low-pressure treatments), and associated control systems requires significant capital investment that may be difficult to justify without clear return-on-investment metrics.
Regulatory uncertainty compounds these technical challenges, as standardized testing protocols specifically designed for plasma-treated antimicrobial textiles are still evolving. This creates difficulties in validating claims about antimicrobial efficacy and comparing performance across different treatment technologies, ultimately slowing market acceptance and commercial deployment.
Energy efficiency represents another major challenge, with current cold plasma systems requiring substantial power input relative to conventional textile treatments. This high energy consumption not only increases operational costs but also diminishes the environmental benefits that cold plasma technology potentially offers compared to chemical-intensive alternatives.
Process standardization remains elusive due to the complex interplay of plasma parameters. Treatment outcomes are highly sensitive to variations in gas composition, power input, exposure time, pressure conditions, and electrode configurations. This sensitivity makes it difficult to establish universal protocols that can be reliably reproduced across different manufacturing environments and equipment setups.
The durability of antimicrobial effects after repeated washing cycles continues to be problematic. While initial antimicrobial efficacy may be impressive, many plasma-treated textiles show significant reduction in performance after standard laundering procedures. This limitation severely restricts application in products requiring long-term antimicrobial properties, such as healthcare textiles and athletic wear.
Material compatibility issues further complicate implementation, as plasma treatments can adversely affect certain synthetic fibers, causing unwanted changes in mechanical properties, color, or texture. Particularly challenging are temperature-sensitive materials that may degrade under plasma exposure, even at relatively low temperatures compared to thermal processes.
Equipment cost presents a substantial barrier to entry, especially for small and medium-sized textile manufacturers. The specialized nature of plasma generation equipment, vacuum systems (for low-pressure treatments), and associated control systems requires significant capital investment that may be difficult to justify without clear return-on-investment metrics.
Regulatory uncertainty compounds these technical challenges, as standardized testing protocols specifically designed for plasma-treated antimicrobial textiles are still evolving. This creates difficulties in validating claims about antimicrobial efficacy and comparing performance across different treatment technologies, ultimately slowing market acceptance and commercial deployment.
Existing Cold Plasma Antimicrobial Solutions
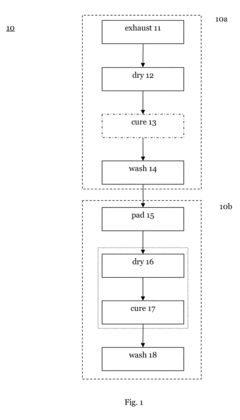
01 Cold plasma sterilization mechanisms
Cold plasma treatment generates reactive species such as ions, electrons, and free radicals that can effectively inactivate microorganisms. These reactive species damage microbial cell membranes, proteins, and DNA, leading to cell death. The non-thermal nature of cold plasma allows for antimicrobial treatment without causing thermal damage to heat-sensitive materials, making it suitable for various applications including medical device sterilization and food preservation.- Cold plasma sterilization mechanisms: Cold plasma treatment generates reactive species such as ions, electrons, and free radicals that can effectively inactivate microorganisms. These reactive species damage microbial cell membranes, proteins, and DNA, leading to cell death. The non-thermal nature of cold plasma allows for antimicrobial treatment without causing thermal damage to heat-sensitive materials, making it suitable for various applications including medical device sterilization and food preservation.
- Medical applications of cold plasma antimicrobial treatment: Cold plasma technology is utilized in medical settings for its antimicrobial properties to treat wounds, sterilize medical devices, and disinfect surfaces. The treatment can effectively eliminate bacteria, viruses, and fungi without damaging human tissues when properly controlled. Cold plasma devices have been developed specifically for wound healing, dental applications, and surgical site preparation, offering alternatives to traditional antimicrobial approaches while reducing the risk of antimicrobial resistance.
- Cold plasma treatment systems and devices: Various systems and devices have been developed to generate and apply cold plasma for antimicrobial purposes. These include atmospheric pressure plasma jets, dielectric barrier discharge systems, and portable plasma-generating devices. The designs focus on controlling plasma parameters such as gas composition, power input, and treatment duration to optimize antimicrobial efficacy while ensuring safety. Advanced systems incorporate feedback mechanisms to monitor and adjust plasma characteristics during treatment.
- Surface decontamination and material treatment: Cold plasma technology is effective for decontaminating surfaces and treating materials to impart antimicrobial properties. The treatment can be applied to various materials including polymers, textiles, metals, and food packaging to create antimicrobial surfaces. Cold plasma modification can alter surface properties to prevent microbial adhesion and biofilm formation. This approach is particularly valuable in healthcare settings, food processing facilities, and for creating antimicrobial consumer products.
- Food safety and preservation applications: Cold plasma treatment offers a non-thermal approach to food decontamination and preservation. The technology can inactivate foodborne pathogens and spoilage microorganisms on food surfaces without affecting nutritional quality or sensory attributes. Applications include treatment of fresh produce, meat products, and food contact surfaces. Cold plasma can extend shelf life while maintaining food quality and safety, providing an alternative to chemical preservatives and thermal processing methods.
02 Medical applications of cold plasma antimicrobial treatment
Cold plasma technology is utilized in medical settings for its antimicrobial properties to treat wounds, sterilize medical devices, and disinfect surfaces. The treatment can effectively eliminate bacteria, viruses, and fungi without damaging human tissues when properly controlled. Cold plasma devices have been developed specifically for wound healing applications, combining antimicrobial action with stimulation of tissue regeneration, providing a dual therapeutic effect in clinical settings.Expand Specific Solutions03 Cold plasma treatment systems and devices
Various systems and devices have been developed to generate and apply cold plasma for antimicrobial purposes. These include atmospheric pressure plasma jets, dielectric barrier discharge systems, and portable handheld devices. The design of these systems focuses on controlling plasma parameters such as gas composition, power input, and treatment duration to optimize antimicrobial efficacy while ensuring safety for the treated materials or tissues. Advanced systems incorporate feedback mechanisms to maintain consistent plasma characteristics during operation.Expand Specific Solutions04 Surface decontamination and material treatment
Cold plasma technology is effective for surface decontamination of various materials including polymers, metals, and textiles. The treatment can inactivate microorganisms on surfaces without altering the bulk properties of the materials. This makes it particularly valuable for decontaminating heat-sensitive materials and complex geometries that are difficult to treat with conventional methods. The process can be tailored to specific surface types by adjusting plasma parameters to achieve optimal antimicrobial effects while preserving material integrity.Expand Specific Solutions05 Food safety and preservation applications
Cold plasma treatment is emerging as a novel non-thermal food preservation technology with significant antimicrobial properties. It can effectively reduce microbial contamination on food surfaces, packaging materials, and processing equipment. The treatment extends shelf life while maintaining nutritional quality and sensory characteristics of food products. Cold plasma can inactivate foodborne pathogens and spoilage microorganisms without leaving chemical residues, making it an environmentally friendly alternative to conventional chemical treatments in the food industry.Expand Specific Solutions
Leading Cold Plasma Technology Providers
Cold plasma treatment for textiles is emerging as a significant innovation in the antimicrobial textiles market, which is currently in a growth phase with increasing market size driven by healthcare and consumer demands. The global antimicrobial textile market is expanding rapidly, with technological maturity varying across applications. Leading research institutions like Zhejiang University and Jiangnan University are advancing fundamental research, while commercial players demonstrate varying levels of technological implementation. Companies like US Medical Innovations and Plasmology4 are pioneering medical applications, while textile-focused firms such as APJeT and MTIX Ltd. are developing industrial-scale solutions. Consumer product companies including L'Oréal and Henkel are exploring applications in specialized textiles, indicating the technology's cross-industry potential despite being in relatively early commercial stages.
Koninklijke Philips NV
Technical Solution: Koninklijke Philips NV has developed a sophisticated cold plasma treatment system for antimicrobial textiles using their proprietary Dielectric Barrier Discharge (DBD) plasma technology. Their approach utilizes a controlled atmospheric pressure plasma that generates reactive oxygen and nitrogen species to modify textile surfaces without thermal damage. The Philips system employs a unique electrode configuration that ensures uniform plasma distribution across textile surfaces, creating consistent antimicrobial properties. Their technology incorporates precise power control systems that optimize the plasma chemistry for different textile substrates, allowing customization based on fiber type and desired antimicrobial properties. The plasma treatment creates functional groups on fiber surfaces that enhance the binding of antimicrobial agents while simultaneously increasing surface energy and wettability. Philips has also developed specialized plasma chemistries that incorporate precursors during the treatment process, allowing for one-step functionalization with antimicrobial properties. Their research shows that plasma-activated textiles exhibit enhanced uptake of antimicrobial agents with improved durability through multiple washing cycles.
Strengths: Highly scalable technology suitable for industrial production; precise control of plasma parameters for consistent quality; versatile application across various textile types including synthetics and natural fibers. Weaknesses: Higher initial capital investment compared to conventional treatments; requires technical expertise for operation and maintenance; energy consumption considerations for large-scale implementation.
US Medical Innovations LLC
Technical Solution: US Medical Innovations LLC has developed advanced cold plasma technology for antimicrobial textile treatment through their patented Canady Helios Cold Plasma (CHCP) system. This technology generates non-thermal plasma at atmospheric pressure using noble gases like argon or helium, creating reactive oxygen and nitrogen species that effectively target microbial cell membranes. Their approach involves a controlled plasma discharge that modifies textile surface properties without thermal damage, creating functional groups that bind antimicrobial agents more effectively. The company's plasma treatment increases the surface energy of textiles, improving wettability and allowing for deeper penetration of antimicrobial compounds. Their process is particularly effective because it creates micro-roughness on fiber surfaces that enhances antimicrobial agent adhesion while simultaneously generating reactive species that directly inactivate pathogens through oxidative stress mechanisms.
Strengths: Highly effective pathogen reduction (>99.9% against common bacteria and fungi); environmentally friendly process requiring minimal water and chemicals; treatment durability lasting through multiple wash cycles. Weaknesses: Requires specialized equipment with precise control parameters; higher initial implementation costs compared to conventional treatments; potential for uneven treatment on complex textile structures.
Key Patents in Plasma-Treated Antimicrobial Textiles
Textiles having antimicrobial properties
PatentInactiveUS20180368401A1
Innovation
- A textile material is treated with antimicrobial agents using a process involving liquor application, heat treatment, and drying to ensure strong bonding of these agents, allowing the textile to act as a disinfectant and filter, while being wash-durable and non-leaching, and used in water filtration and medical garments.
Textile fiber treatment, devices therefore as well as products obtained with the process
PatentWO1992003591A1
Innovation
- A process involving the use of cold plasma to induce polymerization of monomers or prepolymers on textile fibers, creating a homogeneous sheath, utilizing a device with a microwave resonant cavity and nitrogen plasma, which enhances adhesion and deposition efficiency.
Environmental Impact Assessment
The environmental impact assessment of cold plasma treatment for enhancing antimicrobial properties in textiles reveals several significant considerations that must be evaluated when implementing this technology at scale.
Cold plasma treatment offers substantial environmental advantages compared to conventional chemical antimicrobial treatments. Traditional methods often rely on heavy metals like silver or copper, quaternary ammonium compounds, or triclosan, which can persist in the environment and potentially harm aquatic ecosystems. In contrast, cold plasma primarily utilizes electricity and benign gases such as air, oxygen, nitrogen, or argon, substantially reducing chemical waste streams.
Energy consumption represents a critical environmental factor for cold plasma systems. While modern plasma generators have become increasingly efficient, the process still requires significant electrical input. Life cycle assessments indicate that the environmental footprint largely depends on the energy source powering the plasma equipment. Renewable energy integration can dramatically reduce the carbon footprint of plasma treatment facilities.
Water conservation presents another notable environmental benefit. Unlike wet chemical processes that consume substantial volumes of water and generate contaminated effluent, cold plasma operates as a dry process. Studies demonstrate water savings of up to 90% when replacing certain conventional antimicrobial finishing methods with plasma technology, contributing significantly to sustainable textile manufacturing.
Atmospheric emissions from plasma systems must also be considered. While generally minimal, certain plasma configurations may produce trace amounts of ozone or nitrogen oxides. Modern plasma systems incorporate filtration and catalytic conversion technologies that effectively mitigate these emissions, ensuring compliance with air quality regulations.
The durability of antimicrobial properties achieved through plasma treatment directly impacts environmental sustainability. Research indicates that plasma-treated textiles maintain their antimicrobial efficacy through multiple washing cycles, reducing the need for frequent reapplication of antimicrobial agents and extending product lifespan. This durability translates to fewer replacement products and reduced material consumption over time.
End-of-life considerations for plasma-treated textiles appear favorable. Unlike fabrics treated with persistent chemical antimicrobials, plasma-modified textiles generally do not introduce additional barriers to recycling or biodegradation processes. This compatibility with circular economy principles represents an important advantage as the textile industry faces increasing pressure to reduce waste and environmental impact.
Cold plasma treatment offers substantial environmental advantages compared to conventional chemical antimicrobial treatments. Traditional methods often rely on heavy metals like silver or copper, quaternary ammonium compounds, or triclosan, which can persist in the environment and potentially harm aquatic ecosystems. In contrast, cold plasma primarily utilizes electricity and benign gases such as air, oxygen, nitrogen, or argon, substantially reducing chemical waste streams.
Energy consumption represents a critical environmental factor for cold plasma systems. While modern plasma generators have become increasingly efficient, the process still requires significant electrical input. Life cycle assessments indicate that the environmental footprint largely depends on the energy source powering the plasma equipment. Renewable energy integration can dramatically reduce the carbon footprint of plasma treatment facilities.
Water conservation presents another notable environmental benefit. Unlike wet chemical processes that consume substantial volumes of water and generate contaminated effluent, cold plasma operates as a dry process. Studies demonstrate water savings of up to 90% when replacing certain conventional antimicrobial finishing methods with plasma technology, contributing significantly to sustainable textile manufacturing.
Atmospheric emissions from plasma systems must also be considered. While generally minimal, certain plasma configurations may produce trace amounts of ozone or nitrogen oxides. Modern plasma systems incorporate filtration and catalytic conversion technologies that effectively mitigate these emissions, ensuring compliance with air quality regulations.
The durability of antimicrobial properties achieved through plasma treatment directly impacts environmental sustainability. Research indicates that plasma-treated textiles maintain their antimicrobial efficacy through multiple washing cycles, reducing the need for frequent reapplication of antimicrobial agents and extending product lifespan. This durability translates to fewer replacement products and reduced material consumption over time.
End-of-life considerations for plasma-treated textiles appear favorable. Unlike fabrics treated with persistent chemical antimicrobials, plasma-modified textiles generally do not introduce additional barriers to recycling or biodegradation processes. This compatibility with circular economy principles represents an important advantage as the textile industry faces increasing pressure to reduce waste and environmental impact.
Regulatory Framework for Antimicrobial Textiles
The regulatory landscape governing antimicrobial textiles has evolved significantly in response to growing concerns about public health and environmental impact. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial textiles under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) when manufacturers make public health claims. Products claiming to kill or control microorganisms must be registered with the EPA and undergo rigorous testing to verify efficacy and safety.
The European Union implements more stringent regulations through the Biocidal Products Regulation (BPR), which specifically addresses textiles treated with antimicrobial substances. Under this framework, cold plasma-treated textiles must demonstrate both effectiveness and environmental safety. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation further controls the chemical substances used in textile treatments, including those enhanced by cold plasma technology.
In Asia, Japan's Ministry of Health, Labor and Welfare has established the SEK (Seiketsu, meaning hygienic) standard specifically for antimicrobial textiles. China has implemented GB/T 20944 standards that evaluate antimicrobial efficacy in textiles, with special provisions for novel technologies like cold plasma treatment. These regulations focus on ensuring consistent antimicrobial performance while minimizing potential health risks.
International standards organizations play a crucial role in harmonizing global requirements. ISO 20743 provides standardized methods for determining antimicrobial activity in textiles, while AATCC Test Method 100 is widely recognized for evaluating antibacterial finishes on textile materials. These standards are particularly relevant for cold plasma treatments, as they establish quantifiable metrics for antimicrobial efficacy.
Healthcare-specific regulations impose additional requirements on antimicrobial textiles used in medical settings. The FDA in the United States classifies antimicrobial medical textiles as medical devices when intended to prevent infection, subjecting them to premarket approval processes. Similar classifications exist in the EU under the Medical Device Regulation (MDR).
Labeling requirements represent another critical regulatory aspect. The Federal Trade Commission (FTC) in the US and equivalent bodies in other regions monitor claims made about antimicrobial properties to prevent misleading marketing. Manufacturers must provide scientific evidence supporting specific claims about cold plasma-enhanced antimicrobial properties.
Emerging regulations increasingly focus on sustainability aspects of antimicrobial treatments. Cold plasma technology has gained favorable regulatory consideration due to its reduced chemical usage and lower environmental impact compared to conventional antimicrobial treatments, aligning with global shifts toward greener textile processing methods.
The European Union implements more stringent regulations through the Biocidal Products Regulation (BPR), which specifically addresses textiles treated with antimicrobial substances. Under this framework, cold plasma-treated textiles must demonstrate both effectiveness and environmental safety. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation further controls the chemical substances used in textile treatments, including those enhanced by cold plasma technology.
In Asia, Japan's Ministry of Health, Labor and Welfare has established the SEK (Seiketsu, meaning hygienic) standard specifically for antimicrobial textiles. China has implemented GB/T 20944 standards that evaluate antimicrobial efficacy in textiles, with special provisions for novel technologies like cold plasma treatment. These regulations focus on ensuring consistent antimicrobial performance while minimizing potential health risks.
International standards organizations play a crucial role in harmonizing global requirements. ISO 20743 provides standardized methods for determining antimicrobial activity in textiles, while AATCC Test Method 100 is widely recognized for evaluating antibacterial finishes on textile materials. These standards are particularly relevant for cold plasma treatments, as they establish quantifiable metrics for antimicrobial efficacy.
Healthcare-specific regulations impose additional requirements on antimicrobial textiles used in medical settings. The FDA in the United States classifies antimicrobial medical textiles as medical devices when intended to prevent infection, subjecting them to premarket approval processes. Similar classifications exist in the EU under the Medical Device Regulation (MDR).
Labeling requirements represent another critical regulatory aspect. The Federal Trade Commission (FTC) in the US and equivalent bodies in other regions monitor claims made about antimicrobial properties to prevent misleading marketing. Manufacturers must provide scientific evidence supporting specific claims about cold plasma-enhanced antimicrobial properties.
Emerging regulations increasingly focus on sustainability aspects of antimicrobial treatments. Cold plasma technology has gained favorable regulatory consideration due to its reduced chemical usage and lower environmental impact compared to conventional antimicrobial treatments, aligning with global shifts toward greener textile processing methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!