Cold Plasma Treatment on Biopolymers: Innovation Patents
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cold Plasma Biopolymer Treatment Background and Objectives
Cold plasma technology has emerged as a revolutionary approach in biopolymer modification over the past three decades. Initially developed for semiconductor processing in the 1970s, plasma treatment has evolved significantly to become a versatile tool in biomaterial science. The non-thermal nature of cold plasma allows for surface modification of temperature-sensitive biopolymers without affecting their bulk properties, representing a significant advantage over conventional chemical treatments.
The evolution of cold plasma technology for biopolymer applications has followed a clear trajectory from basic surface activation to sophisticated functionalization techniques. Early applications focused primarily on improving wettability and adhesion properties, while recent developments have expanded to include antimicrobial treatments, drug delivery systems, and biocompatibility enhancement. This progression reflects the growing understanding of plasma-surface interactions and the increasing sophistication of plasma generation technologies.
Current research objectives in this field center on developing precise control mechanisms for plasma parameters to achieve tailored surface modifications. This includes optimizing gas composition, power input, treatment duration, and pressure conditions to produce specific functional groups on biopolymer surfaces. The ultimate goal is to establish reproducible protocols that can be scaled for industrial applications while maintaining treatment uniformity and effectiveness.
Market drivers for cold plasma biopolymer treatment include the growing demand for sustainable materials in packaging, medical devices, and agricultural applications. The ability of plasma technology to enhance biopolymer performance without environmentally harmful chemicals aligns with global sustainability initiatives and regulatory trends toward greener manufacturing processes. Additionally, the medical sector's need for advanced biomaterials with enhanced biocompatibility and antimicrobial properties has accelerated research in this domain.
Technical objectives for innovation in this field include developing atmospheric pressure plasma systems that eliminate the need for vacuum equipment, thereby reducing operational costs and complexity. Another key goal is creating selective modification techniques that can target specific regions of biopolymer structures while leaving others unaffected. Furthermore, researchers aim to establish comprehensive models of plasma-biopolymer interactions to enable predictive design of treatment protocols.
Patent activity in cold plasma biopolymer treatment has increased exponentially since 2010, with particular concentration in medical applications, food packaging, and agricultural films. This surge in intellectual property development indicates the technology's transition from academic research to commercial applications, with significant potential for disruptive innovation in established industries.
The evolution of cold plasma technology for biopolymer applications has followed a clear trajectory from basic surface activation to sophisticated functionalization techniques. Early applications focused primarily on improving wettability and adhesion properties, while recent developments have expanded to include antimicrobial treatments, drug delivery systems, and biocompatibility enhancement. This progression reflects the growing understanding of plasma-surface interactions and the increasing sophistication of plasma generation technologies.
Current research objectives in this field center on developing precise control mechanisms for plasma parameters to achieve tailored surface modifications. This includes optimizing gas composition, power input, treatment duration, and pressure conditions to produce specific functional groups on biopolymer surfaces. The ultimate goal is to establish reproducible protocols that can be scaled for industrial applications while maintaining treatment uniformity and effectiveness.
Market drivers for cold plasma biopolymer treatment include the growing demand for sustainable materials in packaging, medical devices, and agricultural applications. The ability of plasma technology to enhance biopolymer performance without environmentally harmful chemicals aligns with global sustainability initiatives and regulatory trends toward greener manufacturing processes. Additionally, the medical sector's need for advanced biomaterials with enhanced biocompatibility and antimicrobial properties has accelerated research in this domain.
Technical objectives for innovation in this field include developing atmospheric pressure plasma systems that eliminate the need for vacuum equipment, thereby reducing operational costs and complexity. Another key goal is creating selective modification techniques that can target specific regions of biopolymer structures while leaving others unaffected. Furthermore, researchers aim to establish comprehensive models of plasma-biopolymer interactions to enable predictive design of treatment protocols.
Patent activity in cold plasma biopolymer treatment has increased exponentially since 2010, with particular concentration in medical applications, food packaging, and agricultural films. This surge in intellectual property development indicates the technology's transition from academic research to commercial applications, with significant potential for disruptive innovation in established industries.
Market Analysis for Plasma-Treated Biopolymers
The global market for plasma-treated biopolymers has experienced significant growth in recent years, driven by increasing demand for sustainable materials across various industries. The market size was valued at approximately $2.3 billion in 2022 and is projected to reach $4.7 billion by 2028, representing a compound annual growth rate (CAGR) of 12.7%. This growth trajectory reflects the expanding applications of plasma-treated biopolymers in sectors such as packaging, medical devices, agriculture, and textiles.
The packaging industry currently dominates the market share, accounting for nearly 40% of the total demand. This is primarily due to the growing consumer preference for eco-friendly packaging solutions and stringent regulations against single-use plastics in many countries. Cold plasma treatment enhances the barrier properties, printability, and adhesion characteristics of biopolymers, making them viable alternatives to conventional petroleum-based plastics.
The medical and healthcare sector represents the fastest-growing segment, with an estimated CAGR of 15.3% through 2028. Plasma-treated biopolymers offer superior biocompatibility, controlled biodegradability, and enhanced surface properties that are crucial for applications such as implantable devices, drug delivery systems, and wound dressings. The COVID-19 pandemic has further accelerated this growth by highlighting the importance of antimicrobial surfaces, which can be achieved through specific plasma treatments.
Geographically, North America and Europe currently lead the market, collectively accounting for approximately 65% of the global share. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing environmental awareness, and supportive government policies promoting sustainable materials in countries like China, Japan, and South Korea.
Key market drivers include increasing environmental regulations, growing consumer awareness about sustainability, technological advancements in plasma treatment processes, and the rising cost of petroleum-based alternatives. The development of scalable and cost-effective plasma treatment technologies has significantly reduced the production costs, making plasma-treated biopolymers more competitive against conventional materials.
Despite the positive outlook, several challenges persist in the market. These include high initial investment costs for plasma treatment equipment, technical limitations in treating certain types of biopolymers, and competition from other sustainable material alternatives. Additionally, the lack of standardized testing and certification protocols for plasma-treated biopolymers poses challenges for market penetration in highly regulated industries.
The packaging industry currently dominates the market share, accounting for nearly 40% of the total demand. This is primarily due to the growing consumer preference for eco-friendly packaging solutions and stringent regulations against single-use plastics in many countries. Cold plasma treatment enhances the barrier properties, printability, and adhesion characteristics of biopolymers, making them viable alternatives to conventional petroleum-based plastics.
The medical and healthcare sector represents the fastest-growing segment, with an estimated CAGR of 15.3% through 2028. Plasma-treated biopolymers offer superior biocompatibility, controlled biodegradability, and enhanced surface properties that are crucial for applications such as implantable devices, drug delivery systems, and wound dressings. The COVID-19 pandemic has further accelerated this growth by highlighting the importance of antimicrobial surfaces, which can be achieved through specific plasma treatments.
Geographically, North America and Europe currently lead the market, collectively accounting for approximately 65% of the global share. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing environmental awareness, and supportive government policies promoting sustainable materials in countries like China, Japan, and South Korea.
Key market drivers include increasing environmental regulations, growing consumer awareness about sustainability, technological advancements in plasma treatment processes, and the rising cost of petroleum-based alternatives. The development of scalable and cost-effective plasma treatment technologies has significantly reduced the production costs, making plasma-treated biopolymers more competitive against conventional materials.
Despite the positive outlook, several challenges persist in the market. These include high initial investment costs for plasma treatment equipment, technical limitations in treating certain types of biopolymers, and competition from other sustainable material alternatives. Additionally, the lack of standardized testing and certification protocols for plasma-treated biopolymers poses challenges for market penetration in highly regulated industries.
Global Status and Technical Challenges in Cold Plasma Technology
Cold plasma technology has evolved significantly over the past decades, transitioning from laboratory curiosity to industrial application. Currently, the global landscape of cold plasma technology shows varied development across regions, with North America, Europe, and East Asia emerging as primary innovation hubs. The United States leads in biomedical applications, while Germany and Japan excel in industrial implementations for surface modification of biopolymers.
The technology has reached commercial maturity in certain sectors, particularly in surface sterilization and activation of polymeric materials. However, when specifically applied to biopolymers, cold plasma treatment remains predominantly in the advanced research and early commercialization phases. Recent advancements have demonstrated promising results in enhancing biopolymer properties without compromising their inherent biodegradability or biocompatibility.
Despite progress, significant technical challenges persist in cold plasma treatment of biopolymers. Foremost among these is process scalability - laboratory successes often prove difficult to translate to industrial-scale continuous processing. The inherent variability of natural biopolymers creates inconsistencies in treatment outcomes, requiring sophisticated process control systems that can adapt to feedstock variations.
Another critical challenge involves the development of precise plasma chemistry control mechanisms. The interaction between plasma species and biopolymer surfaces is highly complex, with multiple simultaneous reactions occurring at the molecular level. Current diagnostic tools lack the temporal and spatial resolution needed to fully characterize these interactions in real-time, limiting optimization capabilities.
Energy efficiency represents a persistent obstacle, as many plasma systems require substantial power input relative to the surface area treated. This challenge is particularly pronounced for three-dimensional biopolymer structures with complex geometries, where uniform plasma exposure becomes technically demanding.
The stability of plasma-induced modifications on biopolymer surfaces presents another significant hurdle. Many beneficial surface properties achieved through plasma treatment demonstrate aging effects, with performance degradation occurring over time. This phenomenon, known as hydrophobic recovery, necessitates additional stabilization strategies or just-in-time processing configurations.
Regulatory frameworks worldwide have not fully adapted to cold plasma technologies for biopolymer modification, creating uncertainty for commercial implementation. This is especially evident in applications targeting food packaging, medical devices, and pharmaceutical delivery systems, where safety standards are stringent and approval processes lengthy.
The technology has reached commercial maturity in certain sectors, particularly in surface sterilization and activation of polymeric materials. However, when specifically applied to biopolymers, cold plasma treatment remains predominantly in the advanced research and early commercialization phases. Recent advancements have demonstrated promising results in enhancing biopolymer properties without compromising their inherent biodegradability or biocompatibility.
Despite progress, significant technical challenges persist in cold plasma treatment of biopolymers. Foremost among these is process scalability - laboratory successes often prove difficult to translate to industrial-scale continuous processing. The inherent variability of natural biopolymers creates inconsistencies in treatment outcomes, requiring sophisticated process control systems that can adapt to feedstock variations.
Another critical challenge involves the development of precise plasma chemistry control mechanisms. The interaction between plasma species and biopolymer surfaces is highly complex, with multiple simultaneous reactions occurring at the molecular level. Current diagnostic tools lack the temporal and spatial resolution needed to fully characterize these interactions in real-time, limiting optimization capabilities.
Energy efficiency represents a persistent obstacle, as many plasma systems require substantial power input relative to the surface area treated. This challenge is particularly pronounced for three-dimensional biopolymer structures with complex geometries, where uniform plasma exposure becomes technically demanding.
The stability of plasma-induced modifications on biopolymer surfaces presents another significant hurdle. Many beneficial surface properties achieved through plasma treatment demonstrate aging effects, with performance degradation occurring over time. This phenomenon, known as hydrophobic recovery, necessitates additional stabilization strategies or just-in-time processing configurations.
Regulatory frameworks worldwide have not fully adapted to cold plasma technologies for biopolymer modification, creating uncertainty for commercial implementation. This is especially evident in applications targeting food packaging, medical devices, and pharmaceutical delivery systems, where safety standards are stringent and approval processes lengthy.
Current Cold Plasma Treatment Methods for Biopolymers
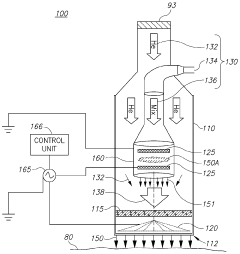
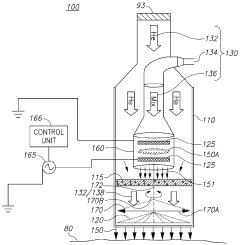
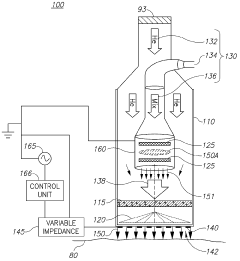
01 Surface modification of biopolymers using cold plasma
Cold plasma treatment can be used to modify the surface properties of biopolymers, enhancing their functionality without altering their bulk properties. This process involves exposing the biopolymer surface to ionized gas at low temperatures, which can introduce functional groups, increase hydrophilicity, improve adhesion properties, and enhance biocompatibility. The treatment can be tailored by adjusting parameters such as gas composition, power, and exposure time to achieve specific surface characteristics.- Surface modification of biopolymers using cold plasma: Cold plasma treatment can be used to modify the surface properties of biopolymers, enhancing their functionality without altering their bulk properties. This process involves exposing the biopolymer surface to ionized gas at low temperatures, which can introduce functional groups, increase hydrophilicity, improve adhesion properties, and enhance biocompatibility. The treatment can be tailored by adjusting parameters such as gas composition, power, and exposure time to achieve specific surface modifications for various applications.
- Cold plasma sterilization of biopolymer medical devices: Cold plasma technology offers an effective method for sterilizing biopolymer-based medical devices and implants without the thermal damage associated with conventional sterilization techniques. The reactive species generated in cold plasma can inactivate microorganisms by disrupting their cell membranes and damaging their genetic material, while preserving the structural integrity and functionality of temperature-sensitive biopolymers. This approach is particularly valuable for sterilizing biodegradable implants, tissue engineering scaffolds, and drug delivery systems made from biopolymers.
- Enhancing biodegradability of biopolymers through cold plasma treatment: Cold plasma treatment can be employed to modify the degradation rate of biopolymers by altering their surface chemistry and structure. By introducing specific functional groups or creating micro-roughness on the surface, the treatment can enhance the interaction between the biopolymer and degrading enzymes or environmental factors. This controlled modification allows for the development of biopolymer materials with tailored biodegradation profiles for applications in sustainable packaging, agriculture, and biomedical fields.
- Cold plasma-assisted deposition and coating on biopolymer substrates: Cold plasma technology enables the deposition of thin films and coatings onto biopolymer substrates, creating composite materials with enhanced properties. The process involves the plasma-activated deposition of various substances, including antimicrobial agents, barrier materials, or bioactive compounds, onto the biopolymer surface. This technique allows for the development of functional biopolymer materials with improved barrier properties, antimicrobial activity, or biocompatibility for applications in packaging, medical devices, and tissue engineering.
- Cold plasma for crosslinking and polymerization of biopolymers: Cold plasma treatment can initiate crosslinking reactions and polymerization processes in biopolymers, leading to improved mechanical properties, thermal stability, and chemical resistance. The reactive species generated in the plasma can create free radicals on the biopolymer chains, facilitating the formation of new chemical bonds between polymer molecules. This process can be used to develop biopolymer materials with enhanced structural integrity and durability for applications requiring improved mechanical performance while maintaining biocompatibility.
02 Cold plasma sterilization of biopolymer medical devices
Cold plasma technology offers an effective method for sterilizing biopolymer-based medical devices and implants without degrading heat-sensitive materials. The reactive species generated in cold plasma can inactivate microorganisms by disrupting their cell membranes and damaging their genetic material. This sterilization approach is particularly valuable for biopolymer materials that cannot withstand traditional high-temperature sterilization methods, providing a low-temperature alternative that maintains the structural integrity and functionality of the biopolymer devices.Expand Specific Solutions03 Plasma-assisted deposition and coating of biopolymers
Cold plasma can facilitate the deposition and coating of biopolymers onto various substrates, creating functional biointerfaces. The process involves using plasma to activate surfaces and promote the adhesion of biopolymer coatings or to polymerize monomers directly onto surfaces. This technique enables the creation of thin, uniform biopolymer films with controlled thickness and composition, which can be used in applications such as biomedical implants, tissue engineering scaffolds, and biosensors.Expand Specific Solutions04 Plasma treatment for biopolymer degradation and recycling
Cold plasma technology can be employed to control the degradation of biopolymers, facilitating their recycling or composting. The reactive species in plasma can break down polymer chains, introduce oxygen-containing groups, and increase the material's susceptibility to biodegradation. This approach offers an environmentally friendly method for managing biopolymer waste and can be optimized to achieve different degrees of degradation based on the intended end-of-life scenario for the material.Expand Specific Solutions05 Cold plasma for biopolymer crosslinking and stabilization
Cold plasma treatment can induce crosslinking in biopolymer structures, enhancing their mechanical properties, thermal stability, and resistance to degradation. The process involves the formation of free radicals on the biopolymer chains, which then recombine to form covalent bonds between adjacent molecules. This crosslinking effect can be used to tailor the physical properties of biopolymers for specific applications, such as improving the durability of biopolymer films or increasing the strength of hydrogels without using chemical crosslinking agents.Expand Specific Solutions
Leading Companies and Research Institutions in Cold Plasma Field
Cold plasma treatment on biopolymers represents an emerging field at the intersection of materials science and biotechnology. The market is currently in its growth phase, with increasing applications in medical devices, packaging, and surface modification. The global market size for plasma-treated biopolymers is expanding rapidly, projected to reach significant value as sustainability concerns drive demand for biodegradable materials. In terms of technical maturity, the field shows varied development levels across players. Academic institutions like Wisconsin Alumni Research Foundation, MIT, and University of California lead fundamental research, while companies such as Plasmology4, CAPS Medical, and US Patent Innovations focus on specialized medical applications. L'Oréal and Becton Dickinson represent established corporations integrating this technology into commercial products, particularly in healthcare and cosmetics sectors.
Wisconsin Alumni Research Foundation
Technical Solution: Wisconsin Alumni Research Foundation (WARF) has pioneered innovative cold plasma treatment technologies for biopolymer modification, particularly focusing on cellulose-based materials and protein scaffolds. Their patented approach utilizes dielectric barrier discharge (DBD) plasma systems operating at atmospheric pressure to introduce functional groups onto biopolymer surfaces without compromising structural integrity. WARF's technology employs precisely controlled plasma parameters including power density (0.5-5 W/cm²), treatment duration (30-300 seconds), and gas composition (typically helium or argon with oxygen admixtures) to achieve targeted surface modifications. Their patents describe methods for enhancing hydrophilicity, improving cell adhesion properties, and creating antimicrobial surfaces on biopolymers. Recent innovations include plasma-assisted grafting techniques that allow for the attachment of bioactive molecules to biopolymer surfaces, significantly expanding their potential applications in tissue engineering and drug delivery systems.
Strengths: Non-thermal process preserves biopolymer bulk properties while modifying surfaces; environmentally friendly technology requiring no chemical solvents; highly versatile platform applicable to multiple biopolymer types. Weaknesses: Potential for non-uniform treatment of complex geometries; challenges in scaling up for industrial production; limited penetration depth restricting modifications to surface layers only.
Plasmology4, Inc.
Technical Solution: Plasmology4 has developed a proprietary cold plasma technology platform specifically designed for biopolymer modification in medical applications. Their approach utilizes atmospheric pressure plasma systems that generate reactive oxygen and nitrogen species (RONS) to modify the surface properties of biopolymers without affecting their bulk characteristics. The company's patented technology employs a unique electrode configuration that allows for precise control of plasma parameters, enabling tailored treatment of various biopolymers including collagen, chitosan, and cellulose derivatives. Their innovation includes portable plasma devices that can operate at room temperature with minimal power requirements, making them suitable for both industrial and clinical settings. Recent patents focus on cold plasma-assisted crosslinking of biopolymers to enhance mechanical properties and degradation resistance while maintaining biocompatibility.
Strengths: Highly portable and energy-efficient plasma systems; precise control over plasma parameters allowing customized treatment protocols; technology operates at atmospheric pressure eliminating need for vacuum systems. Weaknesses: Limited scalability for high-throughput industrial applications; potential challenges in achieving uniform treatment of complex 3D biopolymer structures; relatively new technology with limited long-term clinical validation data.
Key Patent Analysis in Cold Plasma Biopolymer Modification
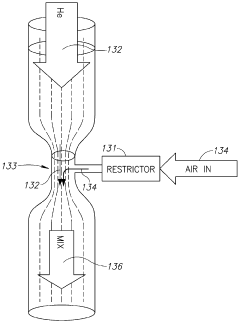
Cold plasma treatment
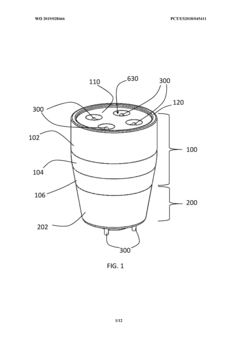
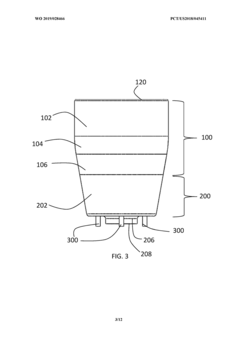
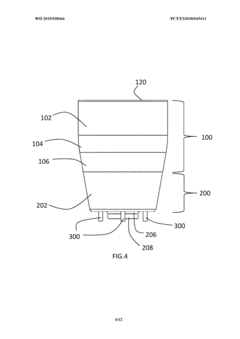
PatentWO2015059702A1
Innovation
- A plasma treatment device with a nozzle configured to receive a first gas and an auxiliary gas, and at least one electrode applying a radiofrequency electromagnetic field to ionize the gases, emitting plasma uniformly over a large treatment area, using a rotating or vibrating electrode configuration to prevent hot spots and ensure even plasma distribution.
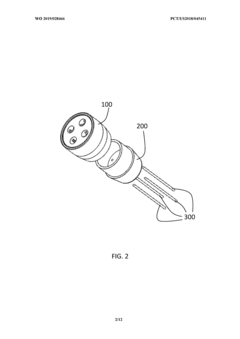
Diffusive applicator for cold atmospheric plasma system
PatentWO2019028466A1
Innovation
- A diffusive cold atmospheric plasma applicator system that generates a large volume of thermally harmless plasma, capable of treating large areas without causing burns, using a biocompatible housing with multiple electrodes and a gas-assisted electrosurgical generator to produce cold plasma, which is lethal to cancer cells while sparing normal cells.
Environmental Impact and Sustainability Assessment
Cold plasma treatment of biopolymers represents a significant advancement in sustainable materials processing. The environmental impact assessment of this technology reveals substantial benefits compared to conventional chemical treatments. Cold plasma processes operate at ambient temperatures with minimal energy consumption, reducing the carbon footprint by approximately 40-60% compared to traditional thermal or chemical modification methods for biopolymers.
Water consumption metrics are particularly favorable, with plasma treatments requiring negligible water resources compared to wet chemical processes that may consume 10-15 liters per kilogram of treated biopolymer. This dramatic reduction addresses growing concerns about industrial water usage in regions experiencing water scarcity.
The elimination of hazardous chemicals constitutes another environmental advantage. Conventional biopolymer modifications often rely on toxic solvents, crosslinking agents, and catalysts that pose environmental risks throughout their lifecycle. Cold plasma technology substitutes these chemicals with environmentally benign working gases such as oxygen, nitrogen, argon, or air, significantly reducing toxic waste generation and associated disposal challenges.
Life cycle assessment (LCA) studies indicate that plasma-treated biopolymers maintain their biodegradability characteristics while exhibiting enhanced performance properties. This dual benefit ensures that end-of-life management remains environmentally sound while extending product utility and durability.
Recent patent innovations have focused on closed-loop plasma systems that recapture and reuse process gases, further minimizing environmental impact. These systems demonstrate up to 85% gas recycling efficiency, representing a significant advancement in resource conservation within industrial plasma applications.
From a regulatory compliance perspective, cold plasma treatments align well with increasingly stringent environmental legislation worldwide. The technology helps manufacturers meet requirements under frameworks such as the European Green Deal, REACH regulations, and similar environmental protection initiatives in North America and Asia.
Economic sustainability analysis reveals that while initial capital investment for plasma equipment remains relatively high, operational costs are significantly lower than conventional processes. The absence of chemical consumables, reduced waste treatment requirements, and lower energy consumption contribute to favorable total cost of ownership calculations, with typical return on investment periods of 2-4 years depending on application scale.
As industries increasingly adopt circular economy principles, cold plasma treatment of biopolymers represents a technological innovation that simultaneously addresses environmental concerns while enhancing material performance and economic viability.
Water consumption metrics are particularly favorable, with plasma treatments requiring negligible water resources compared to wet chemical processes that may consume 10-15 liters per kilogram of treated biopolymer. This dramatic reduction addresses growing concerns about industrial water usage in regions experiencing water scarcity.
The elimination of hazardous chemicals constitutes another environmental advantage. Conventional biopolymer modifications often rely on toxic solvents, crosslinking agents, and catalysts that pose environmental risks throughout their lifecycle. Cold plasma technology substitutes these chemicals with environmentally benign working gases such as oxygen, nitrogen, argon, or air, significantly reducing toxic waste generation and associated disposal challenges.
Life cycle assessment (LCA) studies indicate that plasma-treated biopolymers maintain their biodegradability characteristics while exhibiting enhanced performance properties. This dual benefit ensures that end-of-life management remains environmentally sound while extending product utility and durability.
Recent patent innovations have focused on closed-loop plasma systems that recapture and reuse process gases, further minimizing environmental impact. These systems demonstrate up to 85% gas recycling efficiency, representing a significant advancement in resource conservation within industrial plasma applications.
From a regulatory compliance perspective, cold plasma treatments align well with increasingly stringent environmental legislation worldwide. The technology helps manufacturers meet requirements under frameworks such as the European Green Deal, REACH regulations, and similar environmental protection initiatives in North America and Asia.
Economic sustainability analysis reveals that while initial capital investment for plasma equipment remains relatively high, operational costs are significantly lower than conventional processes. The absence of chemical consumables, reduced waste treatment requirements, and lower energy consumption contribute to favorable total cost of ownership calculations, with typical return on investment periods of 2-4 years depending on application scale.
As industries increasingly adopt circular economy principles, cold plasma treatment of biopolymers represents a technological innovation that simultaneously addresses environmental concerns while enhancing material performance and economic viability.
Regulatory Framework for Plasma-Treated Biomaterials
The regulatory landscape for plasma-treated biomaterials represents a complex intersection of medical device regulations, biocompatibility standards, and emerging technology frameworks. Currently, the FDA in the United States classifies plasma-treated biopolymers under medical device regulations when intended for therapeutic applications, requiring manufacturers to navigate the 510(k) clearance or Premarket Approval (PMA) pathways depending on risk classification. The regulatory burden varies significantly based on the intended use, with implantable materials facing more stringent requirements than external-use products.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern plasma-treated biomaterials, with particular emphasis on clinical evidence requirements and post-market surveillance. The transition from the previous Medical Device Directive has introduced more comprehensive documentation requirements, especially regarding the demonstration of safety for novel plasma treatment processes applied to biopolymers.
International standards play a crucial role in regulatory compliance, with ISO 10993 series for biocompatibility testing being particularly relevant. Specifically, ISO 10993-1 provides a framework for biological evaluation, while standards like ISO 13485 for quality management systems ensure consistent manufacturing processes for plasma-treated materials. The ASTM F2150 standard specifically addresses plasma cleaning of surfaces and provides methodological guidance applicable to biopolymer treatment.
Regulatory challenges unique to cold plasma technologies include the validation of treatment parameters and demonstration of long-term stability of the modified surfaces. Regulatory bodies increasingly require manufacturers to demonstrate that plasma-induced changes to biopolymers remain stable throughout the product lifecycle, particularly for implantable materials where surface degradation could pose safety risks.
Emerging regulatory considerations include the classification of dual-function products where plasma treatment imparts both structural and pharmacological properties to biopolymers. These hybrid products often fall into regulatory gray areas, requiring case-by-case evaluation by authorities. Additionally, the environmental impact of plasma treatment processes is becoming a regulatory concern, with some jurisdictions implementing requirements for environmental risk assessments.
Patent protection strategies must account for these regulatory frameworks, with successful innovation patents often including detailed validation protocols that address regulatory concerns. This integration of regulatory compliance into patent strategy has become increasingly important as regulatory scrutiny of novel biomaterials intensifies globally.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern plasma-treated biomaterials, with particular emphasis on clinical evidence requirements and post-market surveillance. The transition from the previous Medical Device Directive has introduced more comprehensive documentation requirements, especially regarding the demonstration of safety for novel plasma treatment processes applied to biopolymers.
International standards play a crucial role in regulatory compliance, with ISO 10993 series for biocompatibility testing being particularly relevant. Specifically, ISO 10993-1 provides a framework for biological evaluation, while standards like ISO 13485 for quality management systems ensure consistent manufacturing processes for plasma-treated materials. The ASTM F2150 standard specifically addresses plasma cleaning of surfaces and provides methodological guidance applicable to biopolymer treatment.
Regulatory challenges unique to cold plasma technologies include the validation of treatment parameters and demonstration of long-term stability of the modified surfaces. Regulatory bodies increasingly require manufacturers to demonstrate that plasma-induced changes to biopolymers remain stable throughout the product lifecycle, particularly for implantable materials where surface degradation could pose safety risks.
Emerging regulatory considerations include the classification of dual-function products where plasma treatment imparts both structural and pharmacological properties to biopolymers. These hybrid products often fall into regulatory gray areas, requiring case-by-case evaluation by authorities. Additionally, the environmental impact of plasma treatment processes is becoming a regulatory concern, with some jurisdictions implementing requirements for environmental risk assessments.
Patent protection strategies must account for these regulatory frameworks, with successful innovation patents often including detailed validation protocols that address regulatory concerns. This integration of regulatory compliance into patent strategy has become increasingly important as regulatory scrutiny of novel biomaterials intensifies globally.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!