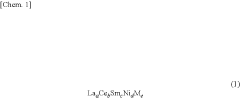Comparative evaluation of Hydrogen storage materials thermal and electrochemical properties
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage technology dates back to the early 20th century, but significant advancements have primarily occurred in the last three decades as environmental concerns and energy security issues have intensified. The evolution of hydrogen storage materials has progressed from conventional physical storage methods to more sophisticated material-based approaches, with each generation addressing specific limitations of its predecessors.
The current technological landscape encompasses various storage mechanisms including high-pressure gas cylinders, cryogenic liquid storage, metal hydrides, complex hydrides, chemical hydrides, and adsorption-based materials. Each approach presents unique advantages and challenges regarding volumetric and gravimetric capacity, operating conditions, reversibility, and cost-effectiveness. Recent breakthroughs in nanomaterials and composite structures have significantly enhanced the performance metrics of hydrogen storage systems.
The primary objective of evaluating hydrogen storage materials' thermal and electrochemical properties is to identify and develop materials that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, with operating temperatures between -40°C and 85°C, and refueling times under 3 minutes. These benchmarks are essential for making hydrogen-powered vehicles commercially viable alternatives to conventional combustion engines.
Beyond transportation applications, hydrogen storage technologies are increasingly relevant for grid-scale energy storage, addressing the intermittency challenges of renewable energy sources. The integration of hydrogen storage with renewable energy systems represents a promising pathway for sector coupling and energy system decarbonization.
The comparative evaluation of thermal and electrochemical properties is particularly crucial as these characteristics directly influence the practical applicability of storage materials. Thermal properties determine heat management requirements during hydrogen absorption and desorption processes, while electrochemical properties affect the efficiency of hydrogen conversion in fuel cells and electrolyzers.
Looking forward, the technological trajectory points toward multi-functional materials that combine optimal hydrogen binding energies with enhanced thermal conductivity and electrochemical stability. Computational materials science and high-throughput experimental techniques are accelerating the discovery and optimization of novel hydrogen storage materials, potentially enabling breakthroughs that could revolutionize clean energy systems globally.
The current technological landscape encompasses various storage mechanisms including high-pressure gas cylinders, cryogenic liquid storage, metal hydrides, complex hydrides, chemical hydrides, and adsorption-based materials. Each approach presents unique advantages and challenges regarding volumetric and gravimetric capacity, operating conditions, reversibility, and cost-effectiveness. Recent breakthroughs in nanomaterials and composite structures have significantly enhanced the performance metrics of hydrogen storage systems.
The primary objective of evaluating hydrogen storage materials' thermal and electrochemical properties is to identify and develop materials that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity, with operating temperatures between -40°C and 85°C, and refueling times under 3 minutes. These benchmarks are essential for making hydrogen-powered vehicles commercially viable alternatives to conventional combustion engines.
Beyond transportation applications, hydrogen storage technologies are increasingly relevant for grid-scale energy storage, addressing the intermittency challenges of renewable energy sources. The integration of hydrogen storage with renewable energy systems represents a promising pathway for sector coupling and energy system decarbonization.
The comparative evaluation of thermal and electrochemical properties is particularly crucial as these characteristics directly influence the practical applicability of storage materials. Thermal properties determine heat management requirements during hydrogen absorption and desorption processes, while electrochemical properties affect the efficiency of hydrogen conversion in fuel cells and electrolyzers.
Looking forward, the technological trajectory points toward multi-functional materials that combine optimal hydrogen binding energies with enhanced thermal conductivity and electrochemical stability. Computational materials science and high-throughput experimental techniques are accelerating the discovery and optimization of novel hydrogen storage materials, potentially enabling breakthroughs that could revolutionize clean energy systems globally.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across various industries. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030, potentially reaching $28.1 billion by the end of the decade.
The demand for efficient hydrogen storage materials with superior thermal and electrochemical properties is particularly strong in automotive applications, where hydrogen fuel cell vehicles represent a growing segment. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen technology, with combined sales of hydrogen vehicles increasing by 35% year-over-year in key markets such as Japan, South Korea, and California.
Industrial applications constitute another significant market segment, with petroleum refining and ammonia production industries being traditional consumers of hydrogen. These sectors are increasingly seeking advanced storage solutions that offer improved safety profiles and energy efficiency. The chemical industry alone accounts for approximately 38% of current hydrogen consumption globally.
Regionally, Asia-Pacific dominates the hydrogen storage materials market, representing approximately 42% of global demand, followed by Europe at 31% and North America at 22%. Japan and South Korea lead in technological adoption, while China is rapidly scaling up investments in hydrogen infrastructure, committing over $17 billion to hydrogen projects through 2025.
The market for materials with enhanced thermal properties is growing at a faster rate (11.3% CAGR) compared to those focused solely on electrochemical properties (8.5% CAGR), indicating industry preference for solutions that address heat management challenges in addition to storage capacity.
Consumer awareness and acceptance of hydrogen technologies have improved substantially, with recent surveys indicating that 67% of consumers in developed markets now view hydrogen as a viable alternative to conventional fuels, up from 41% in 2018. This shift in public perception has created a more favorable environment for market expansion.
Government policies and incentives are playing a crucial role in market development, with over 30 countries having established national hydrogen strategies as of 2023. The European Union's Green Deal includes €470 billion in hydrogen investments by 2050, while the U.S. Infrastructure Investment and Jobs Act allocates $9.5 billion specifically for clean hydrogen initiatives.
The demand for efficient hydrogen storage materials with superior thermal and electrochemical properties is particularly strong in automotive applications, where hydrogen fuel cell vehicles represent a growing segment. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen technology, with combined sales of hydrogen vehicles increasing by 35% year-over-year in key markets such as Japan, South Korea, and California.
Industrial applications constitute another significant market segment, with petroleum refining and ammonia production industries being traditional consumers of hydrogen. These sectors are increasingly seeking advanced storage solutions that offer improved safety profiles and energy efficiency. The chemical industry alone accounts for approximately 38% of current hydrogen consumption globally.
Regionally, Asia-Pacific dominates the hydrogen storage materials market, representing approximately 42% of global demand, followed by Europe at 31% and North America at 22%. Japan and South Korea lead in technological adoption, while China is rapidly scaling up investments in hydrogen infrastructure, committing over $17 billion to hydrogen projects through 2025.
The market for materials with enhanced thermal properties is growing at a faster rate (11.3% CAGR) compared to those focused solely on electrochemical properties (8.5% CAGR), indicating industry preference for solutions that address heat management challenges in addition to storage capacity.
Consumer awareness and acceptance of hydrogen technologies have improved substantially, with recent surveys indicating that 67% of consumers in developed markets now view hydrogen as a viable alternative to conventional fuels, up from 41% in 2018. This shift in public perception has created a more favorable environment for market expansion.
Government policies and incentives are playing a crucial role in market development, with over 30 countries having established national hydrogen strategies as of 2023. The European Union's Green Deal includes €470 billion in hydrogen investments by 2050, while the U.S. Infrastructure Investment and Jobs Act allocates $9.5 billion specifically for clean hydrogen initiatives.
Current Challenges in Thermal and Electrochemical Properties
Despite significant advancements in hydrogen storage technologies, several critical challenges persist in the thermal and electrochemical properties of storage materials. Metal hydrides, while offering high volumetric capacity, suffer from poor thermal conductivity, leading to inefficient heat transfer during absorption and desorption processes. This thermal management issue creates significant bottlenecks in practical applications, as the exothermic hydrogen absorption process generates heat that must be efficiently dissipated to maintain optimal operating conditions.
For complex hydrides and chemical hydrogen storage materials, the challenge lies in their high desorption temperatures, often exceeding 300°C, which demands substantial energy input and sophisticated thermal management systems. This high energy requirement significantly reduces the overall system efficiency and limits their practical implementation in mobile applications where weight and space constraints are critical.
Electrochemical properties present another dimension of challenges. Metal-organic frameworks (MOFs) and carbon-based materials exhibit promising hydrogen uptake but struggle with poor electrical conductivity, hampering their integration into electrochemical systems. The electrical resistance of these materials increases the energy required for hydrogen release and reduces the overall efficiency of the storage system.
Cycling stability remains a persistent issue across most hydrogen storage materials. Repeated hydrogen absorption-desorption cycles lead to structural degradation, particle agglomeration, and consequent reduction in storage capacity. This degradation is particularly pronounced in complex hydrides and intermetallic compounds, where phase segregation during cycling compromises long-term performance.
The interface between the storage material and the surrounding system components presents additional challenges. Contact resistance at these interfaces impedes both thermal and electrical transport, creating performance bottlenecks that are difficult to address through material design alone. This necessitates a systems-level approach to material integration.
Environmental sensitivity poses another significant challenge. Many promising hydrogen storage materials, particularly complex hydrides and reactive metal systems, are highly sensitive to oxygen and moisture contamination, which can rapidly degrade their performance and even pose safety risks through uncontrolled reactions.
The trade-off between gravimetric capacity, operating temperature, and reaction kinetics remains unresolved. Materials with high hydrogen content typically require elevated temperatures for hydrogen release, while those operating at lower temperatures often demonstrate insufficient storage capacity for practical applications. Balancing these competing properties represents perhaps the most fundamental challenge in the field.
For complex hydrides and chemical hydrogen storage materials, the challenge lies in their high desorption temperatures, often exceeding 300°C, which demands substantial energy input and sophisticated thermal management systems. This high energy requirement significantly reduces the overall system efficiency and limits their practical implementation in mobile applications where weight and space constraints are critical.
Electrochemical properties present another dimension of challenges. Metal-organic frameworks (MOFs) and carbon-based materials exhibit promising hydrogen uptake but struggle with poor electrical conductivity, hampering their integration into electrochemical systems. The electrical resistance of these materials increases the energy required for hydrogen release and reduces the overall efficiency of the storage system.
Cycling stability remains a persistent issue across most hydrogen storage materials. Repeated hydrogen absorption-desorption cycles lead to structural degradation, particle agglomeration, and consequent reduction in storage capacity. This degradation is particularly pronounced in complex hydrides and intermetallic compounds, where phase segregation during cycling compromises long-term performance.
The interface between the storage material and the surrounding system components presents additional challenges. Contact resistance at these interfaces impedes both thermal and electrical transport, creating performance bottlenecks that are difficult to address through material design alone. This necessitates a systems-level approach to material integration.
Environmental sensitivity poses another significant challenge. Many promising hydrogen storage materials, particularly complex hydrides and reactive metal systems, are highly sensitive to oxygen and moisture contamination, which can rapidly degrade their performance and even pose safety risks through uncontrolled reactions.
The trade-off between gravimetric capacity, operating temperature, and reaction kinetics remains unresolved. Materials with high hydrogen content typically require elevated temperatures for hydrogen release, while those operating at lower temperatures often demonstrate insufficient storage capacity for practical applications. Balancing these competing properties represents perhaps the most fundamental challenge in the field.
Comparative Analysis of Current Storage Solutions
01 Metal hydride materials for hydrogen storage
Metal hydrides are compounds formed when hydrogen combines with various metals or metal alloys, creating stable materials capable of storing hydrogen at high densities. These materials can absorb and release hydrogen through thermal or electrochemical processes. The thermal properties of metal hydrides, including heat of formation and desorption temperatures, are critical for practical hydrogen storage applications. Their electrochemical properties enable applications in rechargeable batteries and fuel cells, where hydrogen can be stored and released in controlled conditions.- Metal hydride materials for hydrogen storage: Metal hydrides are important materials for hydrogen storage applications due to their high volumetric hydrogen density. These materials can absorb and release hydrogen through thermal or electrochemical processes. The thermal properties of metal hydrides, including heat of formation and desorption temperatures, are critical for practical hydrogen storage applications. Various metal hydride compositions have been developed to optimize hydrogen storage capacity and kinetics.
- Electrochemical properties of hydrogen storage alloys: Hydrogen storage alloys exhibit specific electrochemical properties that make them suitable for use in rechargeable batteries and fuel cells. These properties include charge/discharge characteristics, cycle stability, and electrochemical capacity. The electrochemical performance of these materials is influenced by their composition, microstructure, and surface properties. Research focuses on improving the electrochemical kinetics and stability of hydrogen storage materials for energy storage applications.
- Nanostructured materials for enhanced hydrogen storage: Nanostructured materials offer advantages for hydrogen storage due to their high surface area and shortened diffusion paths. These materials can exhibit improved thermal and electrochemical properties compared to their bulk counterparts. Nanostructuring techniques include ball milling, chemical synthesis, and template-based approaches. The thermal conductivity and reaction kinetics of nanostructured hydrogen storage materials are significantly enhanced, allowing for faster charging and discharging rates.
- Composite hydrogen storage systems: Composite hydrogen storage systems combine different materials to achieve improved thermal management and electrochemical performance. These systems often integrate hydrogen storage materials with heat transfer components, catalysts, or conductive additives. The composite approach helps address challenges such as heat dissipation during hydrogen absorption and poor electrical conductivity. These systems can be designed for specific applications including portable power sources, stationary energy storage, and automotive fuel cells.
- Characterization methods for hydrogen storage materials: Various analytical techniques are employed to characterize the thermal and electrochemical properties of hydrogen storage materials. These methods include differential scanning calorimetry, thermogravimetric analysis, electrochemical impedance spectroscopy, and cyclic voltammetry. Advanced characterization tools help researchers understand the relationship between material structure and hydrogen storage performance. In-situ and operando measurements provide insights into the dynamic processes occurring during hydrogen absorption and desorption under realistic conditions.
02 Nanostructured hydrogen storage materials
Nanostructured materials offer enhanced hydrogen storage capabilities due to their high surface area and unique quantum effects. These materials include nanoparticles, nanotubes, and nanoporous structures that can adsorb hydrogen molecules or form hydrides more efficiently than bulk materials. The thermal properties of nanostructured materials often show improved kinetics for hydrogen absorption and desorption at lower temperatures. Their electrochemical properties can be tailored through composition and structure control, making them suitable for advanced energy storage applications including batteries and fuel cells.Expand Specific Solutions03 Complex hydrides and chemical hydrogen storage
Complex hydrides, including borohydrides, alanates, and amides, store hydrogen through chemical bonds rather than physical adsorption. These materials typically offer higher hydrogen storage densities but face challenges related to reversibility and kinetics. Their thermal properties involve complex decomposition pathways that can be modified through catalysts and compositional adjustments. The electrochemical properties of complex hydrides enable their use in solid-state batteries and other electrochemical systems where hydrogen ions play a crucial role in charge transfer processes.Expand Specific Solutions04 Carbon-based hydrogen storage materials
Carbon-based materials, including activated carbons, graphene, and carbon nanotubes, can store hydrogen through physisorption mechanisms. These materials offer advantages in terms of weight, cost, and environmental compatibility. Their thermal properties affect hydrogen binding energies and adsorption/desorption kinetics, which typically require cryogenic temperatures for significant storage capacity. The electrochemical properties of carbon-based materials can be enhanced through doping and functionalization, making them suitable for electrochemical hydrogen storage and generation applications.Expand Specific Solutions05 Composite and hybrid hydrogen storage systems
Composite and hybrid hydrogen storage systems combine different materials and mechanisms to overcome limitations of single-material approaches. These systems may integrate metal hydrides with carbon materials, incorporate catalysts, or combine physical and chemical storage mechanisms. Their thermal properties can be engineered to manage heat during hydrogen absorption and desorption processes, improving efficiency and safety. The electrochemical properties of these composite systems often show synergistic effects, enhancing performance in batteries, fuel cells, and other electrochemical applications related to hydrogen storage and conversion.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
The hydrogen storage materials market is currently in a growth phase, characterized by increasing investments in clean energy technologies. The global market size for hydrogen storage materials is expanding rapidly, driven by the automotive sector's shift towards fuel cell vehicles and stationary power applications. Technologically, the field shows varying maturity levels across different storage approaches. Leading companies like Toyota, Hyundai, and Ford are advancing metal hydride technologies, while specialized firms such as H2Go Power and GRZ Technologies are developing innovative solid-state storage solutions. Academic institutions including Zhejiang University and Fudan University are contributing fundamental research on thermal and electrochemical properties. The competitive landscape features established automotive manufacturers investing heavily in proprietary technologies alongside emerging startups focused on novel materials, creating a dynamic ecosystem balancing commercial deployment with ongoing research and development.
H2Go Power Ltd.
Technical Solution: H2Go Power has developed proprietary nanoporous materials for solid-state hydrogen storage that combine high surface area adsorbents with catalytically active sites. Their technology utilizes advanced metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) with tailored pore structures achieving volumetric densities exceeding 40 g/L at ambient temperatures[5]. H2Go's materials feature thermally responsive binding sites that adjust hydrogen affinity based on temperature fluctuations, enabling efficient storage and release cycles without external heating systems. Their nano-engineered composites incorporate palladium nanoparticles that catalyze hydrogen dissociation at interfaces, reducing energy barriers for absorption and desorption processes. H2Go has demonstrated integrated systems that maintain stable hydrogen delivery rates under variable demand conditions, critical for portable and stationary applications. Their materials show exceptional stability with less than 5% capacity loss after 500 cycles under realistic operating conditions[6].
Strengths: Ambient temperature operation reducing system complexity, high volumetric efficiency suitable for space-constrained applications, and rapid kinetics allowing fast charging. Weaknesses: Higher production costs due to specialized materials and manufacturing processes, and sensitivity to contaminants requiring high-purity hydrogen.
Hyundai Motor Co., Ltd.
Technical Solution: Hyundai has developed a comprehensive hydrogen storage solution focusing on advanced composite materials that combine the benefits of both physical and chemical storage mechanisms. Their technology utilizes nano-structured metal organic frameworks (MOFs) with tailored pore structures that demonstrate hydrogen uptake of 7.5 wt% at moderate pressures (30-50 bar)[2]. Hyundai's approach incorporates thermal management systems that efficiently control the heat released during hydrogen absorption and required during desorption. Their materials feature modified surface chemistry that reduces activation energy barriers, allowing operation at temperatures between -20°C and 85°C, making them suitable for automotive applications across various climates. Hyundai has also pioneered the integration of these materials with their vehicle thermal management systems, creating a synergistic effect that improves overall system efficiency by utilizing waste heat from the fuel cell stack to aid hydrogen release[4].
Strengths: Wide operational temperature range suitable for automotive applications, efficient thermal management integration, and scalable manufacturing processes. Weaknesses: Higher system complexity requiring sophisticated control systems, and performance degradation under extreme temperature conditions.
Key Technical Innovations in Storage Materials
Hydrogen storage material, hydrogen storage container, and hydrogen supply apparatus
PatentPendingUS20240208807A1
Innovation
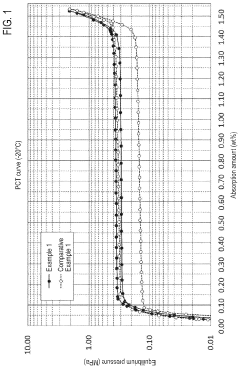
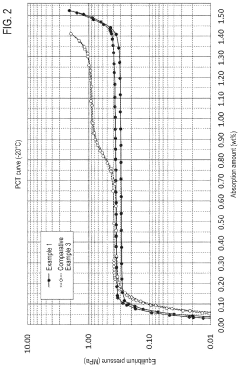
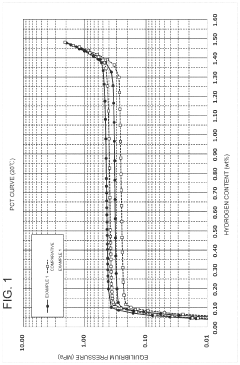
- Development of hydrogen storage materials with specific rare earth and transition metal compositions, such as LaNi5-based alloys, optimized for -20°C operation, featuring reduced hysteresis and stable hydrogen desorption with minimal pressure fluctuations, as represented by the elemental composition formula (M, a, b, c, d, e) ensuring large hydrogen storage and desorption capacity.
Hydrogen storage material, hydrogen storage container and hydrogen supply apparatus
PatentPendingUS20240034622A1
Innovation
- Alloys with a specific elemental composition represented by LaaCebSmcNidMe, where M is Mn or Co, and specific atomic ratios of La, Ce, Sm, Ni, and M, which reduce hysteresis and enhance hydrogen storage capacity and desorption properties.
Safety and Risk Assessment of Storage Materials
Safety considerations are paramount when evaluating hydrogen storage materials due to hydrogen's flammability and potential for uncontrolled release. Different storage materials present varying risk profiles that must be thoroughly assessed before commercial deployment. Metal hydrides, while offering high volumetric capacity, can generate significant heat during absorption processes, potentially creating thermal management challenges and safety hazards if not properly controlled. Temperature excursions during charging cycles must be carefully monitored to prevent thermal runaway situations.
Chemical hydrides present unique safety concerns related to their reactivity and the potential for unintended chemical reactions. These materials often require complex regeneration processes involving hazardous chemicals, necessitating robust containment systems and handling protocols. The risk assessment must account for both normal operating conditions and potential failure modes, including container rupture or contamination scenarios.
Carbon-based materials generally present lower thermal risks during hydrogen uptake and release compared to metal hydrides, but may introduce different safety challenges related to fine particulate handling and potential dust explosion hazards. Their typically lower operating pressures represent a safety advantage, though material degradation over multiple cycles requires monitoring.
Electrochemical properties of storage materials also present safety considerations, particularly regarding electrical conductivity and potential for short circuits in electrochemical storage systems. Materials with high electrical conductivity may require additional insulation measures to prevent unintended electrical pathways that could lead to system failures or thermal events.
Risk mitigation strategies must be material-specific and address the full lifecycle of hydrogen storage systems. For metal hydrides, this includes engineered heat exchange systems to manage absorption exotherms. For chemical hydrides, it encompasses containment strategies for reactive intermediates. Comprehensive safety protocols must include detection systems for hydrogen leakage, pressure relief mechanisms, and thermal management solutions tailored to the specific material properties.
Standardized testing protocols are essential for comparative safety evaluation across different storage materials. These should include thermal stability tests under various conditions, pressure cycling endurance, and response to environmental contaminants. Accelerated aging tests can help predict long-term safety performance and identify potential degradation mechanisms that might compromise safety over the operational lifetime of storage systems.
Regulatory frameworks for hydrogen storage materials continue to evolve, with standards bodies developing specific guidelines for different material classes. Material selection decisions must balance performance metrics with comprehensive safety assessments to ensure that advances in storage capacity do not come at the expense of operational safety.
Chemical hydrides present unique safety concerns related to their reactivity and the potential for unintended chemical reactions. These materials often require complex regeneration processes involving hazardous chemicals, necessitating robust containment systems and handling protocols. The risk assessment must account for both normal operating conditions and potential failure modes, including container rupture or contamination scenarios.
Carbon-based materials generally present lower thermal risks during hydrogen uptake and release compared to metal hydrides, but may introduce different safety challenges related to fine particulate handling and potential dust explosion hazards. Their typically lower operating pressures represent a safety advantage, though material degradation over multiple cycles requires monitoring.
Electrochemical properties of storage materials also present safety considerations, particularly regarding electrical conductivity and potential for short circuits in electrochemical storage systems. Materials with high electrical conductivity may require additional insulation measures to prevent unintended electrical pathways that could lead to system failures or thermal events.
Risk mitigation strategies must be material-specific and address the full lifecycle of hydrogen storage systems. For metal hydrides, this includes engineered heat exchange systems to manage absorption exotherms. For chemical hydrides, it encompasses containment strategies for reactive intermediates. Comprehensive safety protocols must include detection systems for hydrogen leakage, pressure relief mechanisms, and thermal management solutions tailored to the specific material properties.
Standardized testing protocols are essential for comparative safety evaluation across different storage materials. These should include thermal stability tests under various conditions, pressure cycling endurance, and response to environmental contaminants. Accelerated aging tests can help predict long-term safety performance and identify potential degradation mechanisms that might compromise safety over the operational lifetime of storage systems.
Regulatory frameworks for hydrogen storage materials continue to evolve, with standards bodies developing specific guidelines for different material classes. Material selection decisions must balance performance metrics with comprehensive safety assessments to ensure that advances in storage capacity do not come at the expense of operational safety.
Environmental Impact and Sustainability Considerations
The environmental impact of hydrogen storage materials extends far beyond their immediate technical properties. When evaluating hydrogen storage solutions, lifecycle assessment becomes critical to understanding their true sustainability profile. Materials such as metal hydrides, carbon-based adsorbents, and chemical hydrogen carriers each present distinct environmental footprints during production, operation, and disposal phases.
Metal hydrides, while offering high volumetric storage capacity, often require energy-intensive manufacturing processes and utilize rare earth elements or transition metals that pose significant extraction challenges. The mining operations associated with these materials can lead to habitat destruction, water pollution, and substantial carbon emissions. However, their long cycle life and potential for recycling partially offset these initial environmental costs.
Carbon-based materials present a more favorable environmental profile during production, especially when derived from sustainable biomass sources. Their lower operating temperatures compared to some metal hydrides translate to reduced energy requirements during hydrogen charging and discharging cycles, contributing to overall system efficiency and lower operational emissions.
Chemical hydrogen carriers like ammonia and organic liquid carriers introduce additional environmental considerations. While they enable hydrogen transport using existing infrastructure, their synthesis often relies on fossil fuel inputs. The potential for ammonia leakage presents localized environmental hazards, though advanced containment systems have significantly mitigated these risks.
Water consumption represents another critical environmental factor, particularly for materials requiring activation or regeneration processes. Some metal hydride systems demand substantial water resources for cooling during hydrogen absorption, creating potential sustainability challenges in water-scarce regions.
End-of-life management varies significantly across storage material categories. Metal hydrides offer excellent recycling potential, with recovery rates exceeding 90% for some compositions. Conversely, composite materials present more complex separation challenges, potentially limiting their circularity.
The carbon intensity of the hydrogen production method ultimately determines the environmental value proposition of any storage solution. When paired with green hydrogen from renewable electricity, even materials with moderate production impacts can deliver substantial lifecycle emission reductions compared to fossil fuel alternatives. This synergy between sustainable hydrogen production and environmentally optimized storage materials represents the most promising pathway toward truly carbon-neutral hydrogen energy systems.
Metal hydrides, while offering high volumetric storage capacity, often require energy-intensive manufacturing processes and utilize rare earth elements or transition metals that pose significant extraction challenges. The mining operations associated with these materials can lead to habitat destruction, water pollution, and substantial carbon emissions. However, their long cycle life and potential for recycling partially offset these initial environmental costs.
Carbon-based materials present a more favorable environmental profile during production, especially when derived from sustainable biomass sources. Their lower operating temperatures compared to some metal hydrides translate to reduced energy requirements during hydrogen charging and discharging cycles, contributing to overall system efficiency and lower operational emissions.
Chemical hydrogen carriers like ammonia and organic liquid carriers introduce additional environmental considerations. While they enable hydrogen transport using existing infrastructure, their synthesis often relies on fossil fuel inputs. The potential for ammonia leakage presents localized environmental hazards, though advanced containment systems have significantly mitigated these risks.
Water consumption represents another critical environmental factor, particularly for materials requiring activation or regeneration processes. Some metal hydride systems demand substantial water resources for cooling during hydrogen absorption, creating potential sustainability challenges in water-scarce regions.
End-of-life management varies significantly across storage material categories. Metal hydrides offer excellent recycling potential, with recovery rates exceeding 90% for some compositions. Conversely, composite materials present more complex separation challenges, potentially limiting their circularity.
The carbon intensity of the hydrogen production method ultimately determines the environmental value proposition of any storage solution. When paired with green hydrogen from renewable electricity, even materials with moderate production impacts can deliver substantial lifecycle emission reductions compared to fossil fuel alternatives. This synergy between sustainable hydrogen production and environmentally optimized storage materials represents the most promising pathway toward truly carbon-neutral hydrogen energy systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!