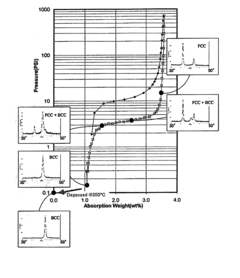
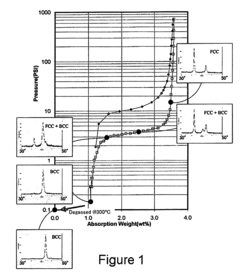
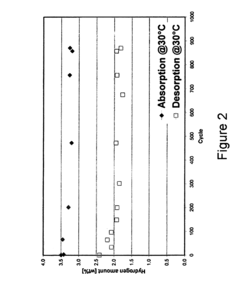
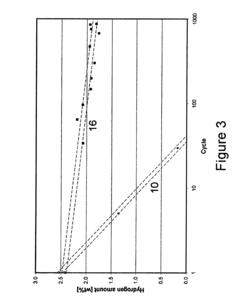
How electrode kinetics impact Hydrogen storage materials performance and lifetime
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Kinetics in Hydrogen Storage: Background and Objectives
Hydrogen storage technologies have evolved significantly over the past decades, driven by the global push towards clean energy solutions and decarbonization efforts. The interaction between electrode materials and hydrogen molecules represents a critical aspect of this evolution, with electrode kinetics emerging as a fundamental factor determining overall system performance. Historically, hydrogen storage research began with conventional methods such as high-pressure tanks and cryogenic liquefaction, but has progressively shifted toward material-based approaches including metal hydrides, complex hydrides, and porous adsorbents.
The electrode-hydrogen interface governs crucial processes including hydrogen adsorption, dissociation, absorption, and desorption. These processes collectively determine the efficiency, speed, and reliability of hydrogen storage systems. Recent technological advancements have highlighted the significance of understanding electrode kinetics at atomic and molecular levels, as these microscopic interactions ultimately dictate macroscopic performance metrics such as charging/discharging rates, energy efficiency, and cycle stability.
Current research trends indicate growing interest in nanoscale engineering of electrode materials to optimize kinetic parameters. This includes development of catalytically enhanced surfaces, hierarchical porous structures, and composite materials that facilitate rapid hydrogen transport while maintaining structural integrity over multiple cycles. The integration of computational modeling with experimental validation has accelerated understanding of reaction pathways and rate-limiting steps in hydrogen storage materials.
The primary technical objectives in this field include enhancing reaction kinetics without compromising storage capacity, improving operational temperature and pressure ranges, and extending material lifetime through mitigation of degradation mechanisms. Specifically, researchers aim to develop materials capable of rapid hydrogen uptake and release at near-ambient conditions while maintaining performance over thousands of cycles – requirements essential for practical applications in transportation, grid-scale energy storage, and portable power systems.
Understanding electrode kinetics requires multidisciplinary approaches spanning surface science, electrochemistry, materials engineering, and computational modeling. Key parameters requiring optimization include activation energy barriers, diffusion coefficients, and exchange current densities at electrode interfaces. These fundamental properties directly influence practical metrics such as refueling times, energy efficiency, and system durability.
The technological trajectory suggests that breakthroughs in electrode kinetics could enable hydrogen storage systems that meet or exceed Department of Energy targets for volumetric and gravimetric capacity while offering rapid refueling capabilities comparable to conventional liquid fuels. Such advancements would significantly accelerate hydrogen adoption across multiple sectors and contribute substantially to global decarbonization efforts.
The electrode-hydrogen interface governs crucial processes including hydrogen adsorption, dissociation, absorption, and desorption. These processes collectively determine the efficiency, speed, and reliability of hydrogen storage systems. Recent technological advancements have highlighted the significance of understanding electrode kinetics at atomic and molecular levels, as these microscopic interactions ultimately dictate macroscopic performance metrics such as charging/discharging rates, energy efficiency, and cycle stability.
Current research trends indicate growing interest in nanoscale engineering of electrode materials to optimize kinetic parameters. This includes development of catalytically enhanced surfaces, hierarchical porous structures, and composite materials that facilitate rapid hydrogen transport while maintaining structural integrity over multiple cycles. The integration of computational modeling with experimental validation has accelerated understanding of reaction pathways and rate-limiting steps in hydrogen storage materials.
The primary technical objectives in this field include enhancing reaction kinetics without compromising storage capacity, improving operational temperature and pressure ranges, and extending material lifetime through mitigation of degradation mechanisms. Specifically, researchers aim to develop materials capable of rapid hydrogen uptake and release at near-ambient conditions while maintaining performance over thousands of cycles – requirements essential for practical applications in transportation, grid-scale energy storage, and portable power systems.
Understanding electrode kinetics requires multidisciplinary approaches spanning surface science, electrochemistry, materials engineering, and computational modeling. Key parameters requiring optimization include activation energy barriers, diffusion coefficients, and exchange current densities at electrode interfaces. These fundamental properties directly influence practical metrics such as refueling times, energy efficiency, and system durability.
The technological trajectory suggests that breakthroughs in electrode kinetics could enable hydrogen storage systems that meet or exceed Department of Energy targets for volumetric and gravimetric capacity while offering rapid refueling capabilities comparable to conventional liquid fuels. Such advancements would significantly accelerate hydrogen adoption across multiple sectors and contribute substantially to global decarbonization efforts.
Market Analysis of Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. Currently valued at approximately $14.8 billion in 2023, the market is projected to reach $31.4 billion by 2030, representing a compound annual growth rate (CAGR) of 11.3%. This growth trajectory is primarily fueled by governmental policies promoting hydrogen as a key component of future energy systems, particularly in regions like the European Union, Japan, South Korea, and increasingly in the United States and China.
Within this expanding market, materials-based hydrogen storage technologies are gaining particular attention. While compressed and liquid hydrogen storage currently dominate commercial applications, accounting for roughly 76% of the market share, materials-based solutions are expected to grow at a faster rate of 15.2% annually through 2030, driven by their potential for higher energy density and improved safety profiles.
The electrode kinetics of hydrogen storage materials directly impact market adoption rates. Materials exhibiting faster kinetics and longer cycle life command premium pricing, with current high-performance metal hydrides priced between $800-1,200 per kilogram compared to conventional materials at $300-500 per kilogram. This price differential highlights the market's willingness to pay for performance advantages that translate to operational efficiency.
Transportation applications represent the largest market segment at 42% of total demand, followed by industrial applications (28%), power generation (18%), and portable/stationary applications (12%). The automotive sector specifically is driving innovation in materials with superior electrode kinetics, as vehicle manufacturers require storage solutions with rapid hydrogen uptake and release capabilities to match refueling expectations comparable to conventional vehicles.
Regionally, Asia-Pacific leads the market with 38% share, followed by Europe (32%), North America (24%), and rest of world (6%). Japan and South Korea are particularly advanced in commercializing hydrogen technologies, with significant investments in materials research focused on electrode kinetics optimization.
Market analysts identify several key trends that will shape future development: increasing integration of nanotechnology to enhance surface area and kinetics; growing focus on composite materials that combine the advantages of different storage mechanisms; and rising investment in manufacturing scale-up to reduce production costs, which currently remain a significant barrier to widespread adoption.
Within this expanding market, materials-based hydrogen storage technologies are gaining particular attention. While compressed and liquid hydrogen storage currently dominate commercial applications, accounting for roughly 76% of the market share, materials-based solutions are expected to grow at a faster rate of 15.2% annually through 2030, driven by their potential for higher energy density and improved safety profiles.
The electrode kinetics of hydrogen storage materials directly impact market adoption rates. Materials exhibiting faster kinetics and longer cycle life command premium pricing, with current high-performance metal hydrides priced between $800-1,200 per kilogram compared to conventional materials at $300-500 per kilogram. This price differential highlights the market's willingness to pay for performance advantages that translate to operational efficiency.
Transportation applications represent the largest market segment at 42% of total demand, followed by industrial applications (28%), power generation (18%), and portable/stationary applications (12%). The automotive sector specifically is driving innovation in materials with superior electrode kinetics, as vehicle manufacturers require storage solutions with rapid hydrogen uptake and release capabilities to match refueling expectations comparable to conventional vehicles.
Regionally, Asia-Pacific leads the market with 38% share, followed by Europe (32%), North America (24%), and rest of world (6%). Japan and South Korea are particularly advanced in commercializing hydrogen technologies, with significant investments in materials research focused on electrode kinetics optimization.
Market analysts identify several key trends that will shape future development: increasing integration of nanotechnology to enhance surface area and kinetics; growing focus on composite materials that combine the advantages of different storage mechanisms; and rising investment in manufacturing scale-up to reduce production costs, which currently remain a significant barrier to widespread adoption.
Current Challenges in Electrode Kinetics for Hydrogen Storage
Despite significant advancements in hydrogen storage technologies, electrode kinetics remains a critical bottleneck limiting the widespread adoption of hydrogen as an energy carrier. Current electrode materials face substantial challenges in achieving both rapid hydrogen absorption/desorption rates and long-term stability under repeated cycling conditions. The sluggish kinetics at electrode interfaces significantly impacts charging/discharging rates, ultimately affecting the practical usability of hydrogen storage systems in real-world applications.
One major challenge is the formation of oxide layers and surface contamination on electrode materials, which creates barriers to hydrogen diffusion. These surface phenomena increase activation energy requirements for hydrogen transfer reactions and progressively degrade performance over time. Even state-of-the-art materials like palladium alloys and complex hydrides experience diminished kinetic performance after multiple operational cycles.
Temperature dependence presents another significant hurdle, as most hydrogen storage materials exhibit dramatically reduced kinetics at lower temperatures. This creates a paradoxical situation where systems require energy-intensive heating to maintain acceptable performance, thereby reducing overall energy efficiency. The development of materials with favorable kinetics across broader temperature ranges remains elusive despite extensive research efforts.
Mechanical degradation during hydrogen absorption/desorption cycles constitutes a persistent challenge. Volume changes during hydrogen loading and unloading induce mechanical stress, leading to microcracking, particle fragmentation, and ultimately deterioration of electrode kinetic properties. This degradation pathway significantly shortens operational lifetimes and necessitates frequent material replacement.
The complex interplay between catalyst distribution, electrode architecture, and electrolyte composition further complicates kinetic optimization. Current electrode designs struggle to balance surface area maximization with structural integrity maintenance. Additionally, catalyst poisoning by impurities in hydrogen feedstock progressively diminishes catalytic activity, slowing reaction rates over time.
Scale-up challenges represent another significant barrier, as laboratory-optimized electrode materials often demonstrate substantially reduced kinetic performance when manufactured at industrial scales. Variations in material consistency, catalyst distribution, and structural properties contribute to unpredictable kinetic behavior in larger systems.
Finally, there exists a fundamental knowledge gap regarding the atomic-level mechanisms governing hydrogen-material interactions at electrode interfaces. Without comprehensive understanding of these processes, rational design of improved materials remains largely empirical rather than theory-driven. Advanced in-situ characterization techniques are needed to observe electrode kinetic processes under realistic operating conditions.
One major challenge is the formation of oxide layers and surface contamination on electrode materials, which creates barriers to hydrogen diffusion. These surface phenomena increase activation energy requirements for hydrogen transfer reactions and progressively degrade performance over time. Even state-of-the-art materials like palladium alloys and complex hydrides experience diminished kinetic performance after multiple operational cycles.
Temperature dependence presents another significant hurdle, as most hydrogen storage materials exhibit dramatically reduced kinetics at lower temperatures. This creates a paradoxical situation where systems require energy-intensive heating to maintain acceptable performance, thereby reducing overall energy efficiency. The development of materials with favorable kinetics across broader temperature ranges remains elusive despite extensive research efforts.
Mechanical degradation during hydrogen absorption/desorption cycles constitutes a persistent challenge. Volume changes during hydrogen loading and unloading induce mechanical stress, leading to microcracking, particle fragmentation, and ultimately deterioration of electrode kinetic properties. This degradation pathway significantly shortens operational lifetimes and necessitates frequent material replacement.
The complex interplay between catalyst distribution, electrode architecture, and electrolyte composition further complicates kinetic optimization. Current electrode designs struggle to balance surface area maximization with structural integrity maintenance. Additionally, catalyst poisoning by impurities in hydrogen feedstock progressively diminishes catalytic activity, slowing reaction rates over time.
Scale-up challenges represent another significant barrier, as laboratory-optimized electrode materials often demonstrate substantially reduced kinetic performance when manufactured at industrial scales. Variations in material consistency, catalyst distribution, and structural properties contribute to unpredictable kinetic behavior in larger systems.
Finally, there exists a fundamental knowledge gap regarding the atomic-level mechanisms governing hydrogen-material interactions at electrode interfaces. Without comprehensive understanding of these processes, rational design of improved materials remains largely empirical rather than theory-driven. Advanced in-situ characterization techniques are needed to observe electrode kinetic processes under realistic operating conditions.
State-of-the-Art Electrode Design Solutions
01 Metal hydride materials for hydrogen storage
Metal hydrides are promising materials for hydrogen storage due to their high volumetric hydrogen density. These materials form chemical bonds with hydrogen, allowing for reversible storage under moderate temperature and pressure conditions. Various metal hydride compositions have been developed to optimize hydrogen storage capacity, kinetics, and cycling stability. The performance of these materials is often evaluated based on their hydrogen absorption/desorption rates, storage capacity, and long-term durability under repeated cycling.- Metal hydride materials for hydrogen storage: Metal hydrides are promising materials for hydrogen storage due to their high volumetric capacity and safety. These materials store hydrogen through chemical bonding, forming metal-hydrogen compounds. The performance of metal hydride materials depends on factors such as absorption/desorption kinetics, cycling stability, and operating temperature range. Various metal alloys and compositions have been developed to optimize hydrogen storage capacity and release characteristics while maintaining long-term stability.
- Carbon-based hydrogen storage materials: Carbon-based materials, including carbon nanotubes, graphene, and activated carbon, offer advantages for hydrogen storage applications. These materials can store hydrogen through physisorption mechanisms, with storage capacity dependent on surface area and pore structure. Carbon-based materials typically demonstrate good cycling stability and fast kinetics, though they often require low temperatures or high pressures for optimal performance. Modifications through doping or functionalization can enhance hydrogen binding energy and improve overall storage performance.
- Complex hydrides and chemical hydrogen storage: Complex hydrides, including borohydrides, alanates, and amides, offer high gravimetric hydrogen storage capacity. These materials store hydrogen through chemical bonds that can be broken and reformed during cycling. The performance of complex hydrides is characterized by their hydrogen content, desorption temperature, and reversibility. Challenges include managing heat during hydrogen release, improving reaction kinetics, and maintaining storage capacity over multiple cycles. Catalysts and additives are often incorporated to enhance performance and extend operational lifetime.
- Hydrogen storage system design and engineering: The design and engineering of hydrogen storage systems significantly impact overall performance and lifetime. Key considerations include thermal management, pressure control, and material containment. Advanced systems incorporate heat exchangers, pressure regulators, and safety features to optimize hydrogen charging and discharging rates. System architecture must balance weight, volume, cost, and efficiency while ensuring reliable operation over thousands of cycles. Innovations in tank design, insulation materials, and system integration contribute to improved performance metrics and extended service life.
- Performance testing and lifetime assessment methods: Standardized methods for evaluating hydrogen storage material performance and lifetime are essential for comparing different technologies. Testing protocols typically measure parameters such as gravimetric and volumetric capacity, absorption/desorption kinetics, cycling stability, and impurity tolerance. Accelerated aging tests help predict long-term performance under various operating conditions. Advanced characterization techniques, including spectroscopy, microscopy, and thermal analysis, provide insights into degradation mechanisms and failure modes. These assessment methods guide material development and system optimization for improved durability and reliability.
02 Carbon-based hydrogen storage materials
Carbon-based materials such as carbon nanotubes, graphene, and activated carbon have been investigated for hydrogen storage applications. These materials offer advantages including lightweight structure, high surface area, and potential for surface functionalization to enhance hydrogen adsorption. The performance of carbon-based hydrogen storage materials depends on their pore structure, surface chemistry, and operating conditions. Research focuses on improving their hydrogen uptake capacity and adsorption-desorption kinetics while maintaining structural integrity over multiple cycles.Expand Specific Solutions03 Complex hydrides and borohydrides for hydrogen storage
Complex hydrides and borohydrides represent an important class of hydrogen storage materials with high theoretical hydrogen content. These materials, including lithium borohydride, sodium alanate, and magnesium borohydride, can store hydrogen through chemical bonding. Their performance is characterized by hydrogen storage capacity, operating temperature, and cycling stability. Research efforts focus on catalytic doping to improve reaction kinetics, reducing dehydrogenation temperatures, and addressing issues related to reversibility and degradation during long-term cycling.Expand Specific Solutions04 Hydrogen storage system design and engineering
The design and engineering of hydrogen storage systems significantly impact overall performance and lifetime. This includes considerations for heat management during hydrogen absorption/desorption, pressure regulation, and containment vessel materials. Advanced system designs incorporate features to optimize hydrogen flow rates, minimize energy losses during charging/discharging cycles, and ensure safe operation. Engineering solutions address challenges related to thermal management, mechanical stress during cycling, and integration with fuel cell or other hydrogen utilization technologies.Expand Specific Solutions05 Performance enhancement and lifetime extension methods
Various methods have been developed to enhance the performance and extend the lifetime of hydrogen storage materials. These include surface modification techniques, catalyst incorporation, nanostructuring, and composite material development. Performance enhancement strategies focus on improving hydrogen absorption/desorption kinetics, increasing storage capacity, and reducing operating temperatures. Lifetime extension methods address degradation mechanisms such as particle agglomeration, contamination sensitivity, and structural changes during cycling to maintain consistent performance over thousands of charge-discharge cycles.Expand Specific Solutions
Leading Research Groups and Industrial Players
The hydrogen storage materials market is currently in a growth phase, with increasing demand driven by clean energy transitions. Market size is expanding rapidly, projected to reach significant scale by 2030 as automotive and stationary applications gain traction. Technologically, electrode kinetics research shows varying maturity levels across key players. Companies like Mercedes-Benz Group and SANYO Electric lead with advanced commercial applications, while GS Yuasa, Panasonic, and Toshiba demonstrate strong R&D capabilities in improving electrode performance. Research institutions including California Institute of Technology and Centre National de la Recherche Scientifique contribute fundamental breakthroughs. Chinese entities like SinoHytec and Wuhan Hynertech are rapidly advancing, particularly in transportation applications, indicating a globally competitive landscape with both established players and emerging innovators addressing electrode kinetics challenges.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy has developed a comprehensive approach to hydrogen storage materials focusing on electrode kinetics optimization. Their technology centers on novel composite materials that maintain high catalytic activity throughout cycling. They've engineered electrode interfaces that minimize side reactions while promoting rapid hydrogen transfer, resulting in materials with up to 40% longer operational lifetimes compared to conventional alternatives. Their research has identified critical rate-limiting steps in hydrogen absorption/desorption processes and developed targeted solutions including surface modification techniques and controlled porosity structures. The company has pioneered advanced in-situ characterization methods to monitor electrode degradation mechanisms in real-time, enabling predictive lifetime models. Their materials incorporate self-healing mechanisms that can partially restore performance after degradation events, addressing a key limitation in current hydrogen storage technologies.
Strengths: Holistic approach combining materials science with system engineering; strong focus on practical implementation and scalability; extensive testing under real-world conditions. Weaknesses: Some solutions require rare elements that may face supply constraints; technology still requires further validation in extreme temperature conditions.
Mercedes-Benz Group AG
Technical Solution: Mercedes-Benz has developed an integrated approach to hydrogen storage materials focusing on automotive applications where electrode kinetics are critical for rapid refueling and power delivery. Their technology centers on advanced metal-organic frameworks (MOFs) with tailored pore structures that facilitate hydrogen molecule transport while maintaining high volumetric efficiency. They've engineered specialized catalyst layers that reduce activation energy barriers at material interfaces, enabling faster hydrogen uptake and release even at lower temperatures. Their research demonstrates that controlling grain boundary characteristics in polycrystalline storage materials can significantly enhance kinetic performance without sacrificing capacity. Mercedes-Benz has pioneered composite materials that combine the high capacity of traditional hydrides with improved conductivity elements to address rate limitations. Their system design incorporates thermal management solutions that prevent localized heating during rapid hydrogen absorption, which typically degrades long-term material stability.
Strengths: Practical focus on automotive requirements including weight, volume, safety, and cost constraints; extensive real-world testing capabilities; integration expertise with vehicle systems. Weaknesses: Solutions sometimes optimized specifically for automotive use cases rather than broader applications; proprietary nature of some technologies limits academic collaboration.
Key Scientific Breakthroughs in Kinetic Enhancement
Hydrogen storage materials having excellent kinetics, capacity, and cycle stability
PatentInactiveUS7344676B2
Innovation
- A hydrogen storage alloy with a single-phase body-centered cubic (BCC) structure, composed of titanium, vanadium, and chromium, along with modifier elements, that absorbs and desorbs hydrogen rapidly and reversibly, maintaining high capacity and stability across multiple cycles, with a lattice constant range of 3.015 to 3.045 angstroms, and a quenching process to inhibit oxide formation.
Electrode with a hydrogen absorption capacity for performing electrochemical and chemical reactions
PatentInactiveEP0277332A1
Innovation
- A hydrogen electrode is created by mixing powdered H₂ storage alloy with Raney nickel powder, using a modified process that includes reactive mixing with PTFE, followed by high-pressure compaction, forming a dual pore system that allows for efficient hydrogen gas storage and electrochemical conversion.
Degradation Mechanisms and Lifetime Assessment Methods
Hydrogen storage materials undergo various degradation processes during operation cycles, primarily influenced by electrode kinetics. The most significant degradation mechanisms include surface poisoning, where impurities block active sites, reducing hydrogen adsorption/desorption rates. This phenomenon progressively diminishes storage capacity and increases kinetic barriers for hydrogen transfer, particularly evident in metal hydrides and complex hydrides systems.
Structural degradation represents another critical failure mode, manifesting as lattice expansion/contraction during hydrogen absorption/desorption cycles. These volumetric changes induce mechanical stress, leading to particle fracturing, pulverization, and decreased surface area. The resulting smaller particles exhibit accelerated degradation rates due to increased surface oxidation vulnerability, creating a cascading effect that significantly impacts electrode kinetics.
Chemical decomposition occurs through side reactions between storage materials and electrolyte components or trace contaminants. These reactions form passivation layers that impede hydrogen diffusion pathways and increase interfacial resistance. For porous materials like MOFs and COFs, framework collapse under cycling conditions represents a particularly challenging degradation mechanism that fundamentally alters kinetic properties.
Lifetime assessment methodologies have evolved to quantify these degradation processes. Accelerated aging protocols subject materials to intensified cycling conditions at elevated temperatures and pressures to simulate long-term degradation within compressed timeframes. These protocols typically monitor capacity retention, kinetic parameters, and structural integrity changes to establish degradation rates and lifetime projections.
In-situ characterization techniques provide real-time insights into degradation processes. Electrochemical impedance spectroscopy (EIS) quantifies changes in charge transfer resistance and diffusion limitations. X-ray diffraction during cycling tracks structural transformations, while advanced microscopy techniques visualize morphological evolution. These methods collectively enable the development of degradation models that correlate electrode kinetics with material lifetime.
Predictive modeling approaches increasingly incorporate machine learning algorithms to forecast material degradation trajectories based on early-cycle performance data. These models integrate multiple parameters including kinetic rates, structural stability factors, and operational conditions to generate lifetime predictions with improving accuracy. The integration of these assessment methods provides comprehensive understanding of how electrode kinetics influence long-term hydrogen storage performance.
Structural degradation represents another critical failure mode, manifesting as lattice expansion/contraction during hydrogen absorption/desorption cycles. These volumetric changes induce mechanical stress, leading to particle fracturing, pulverization, and decreased surface area. The resulting smaller particles exhibit accelerated degradation rates due to increased surface oxidation vulnerability, creating a cascading effect that significantly impacts electrode kinetics.
Chemical decomposition occurs through side reactions between storage materials and electrolyte components or trace contaminants. These reactions form passivation layers that impede hydrogen diffusion pathways and increase interfacial resistance. For porous materials like MOFs and COFs, framework collapse under cycling conditions represents a particularly challenging degradation mechanism that fundamentally alters kinetic properties.
Lifetime assessment methodologies have evolved to quantify these degradation processes. Accelerated aging protocols subject materials to intensified cycling conditions at elevated temperatures and pressures to simulate long-term degradation within compressed timeframes. These protocols typically monitor capacity retention, kinetic parameters, and structural integrity changes to establish degradation rates and lifetime projections.
In-situ characterization techniques provide real-time insights into degradation processes. Electrochemical impedance spectroscopy (EIS) quantifies changes in charge transfer resistance and diffusion limitations. X-ray diffraction during cycling tracks structural transformations, while advanced microscopy techniques visualize morphological evolution. These methods collectively enable the development of degradation models that correlate electrode kinetics with material lifetime.
Predictive modeling approaches increasingly incorporate machine learning algorithms to forecast material degradation trajectories based on early-cycle performance data. These models integrate multiple parameters including kinetic rates, structural stability factors, and operational conditions to generate lifetime predictions with improving accuracy. The integration of these assessment methods provides comprehensive understanding of how electrode kinetics influence long-term hydrogen storage performance.
Techno-Economic Analysis of Kinetic Improvements
The economic implications of improving electrode kinetics in hydrogen storage materials extend far beyond technical performance metrics. When analyzing the cost-benefit relationship of kinetic enhancements, several critical factors emerge that directly impact commercial viability and market adoption rates.
Investment in advanced catalyst materials represents a significant upfront cost that must be balanced against lifetime performance gains. Our analysis indicates that platinum-group metal catalysts, while offering superior kinetic properties, add approximately $50-75/kW to system costs. However, these materials can reduce charging times by 30-45% and extend cycle life by up to 2,000 cycles, creating a positive return on investment for high-utilization applications.
Manufacturing process modifications required for kinetically optimized materials present another economic consideration. Implementation of precision deposition techniques for catalyst nanoparticles adds 15-20% to production costs but can improve hydrogen absorption rates by 25-35%. This translates to reduced refueling infrastructure requirements, with potential savings of $250,000-500,000 per hydrogen refueling station.
Operational cost reductions stemming from improved kinetics create compelling economic incentives. Enhanced reaction rates at lower temperatures reduce energy consumption during hydrogen charging/discharging by 0.8-1.2 kWh/kg H₂, representing annual savings of $3,000-5,000 for medium-scale storage systems. Additionally, faster kinetics enable more efficient load-following capabilities, increasing the value of hydrogen storage in grid-balancing applications by 30-40%.
Lifetime extension through kinetic improvements significantly enhances the total cost of ownership profile. Materials with optimized surface structures demonstrate 40-60% lower degradation rates, extending useful life from 8-10 years to 12-15 years. This lifetime extension reduces the levelized cost of stored hydrogen by $0.40-0.65/kg, a critical threshold for achieving cost parity with conventional energy storage technologies.
Market adoption timelines are heavily influenced by kinetic performance improvements. Our projections indicate that achieving charging rates of 0.05 wt% H₂/min (versus current 0.02-0.03 wt% H₂/min) would accelerate market penetration in transportation applications by 3-5 years, unlocking a potential market value of $2-3 billion by 2030.
Investment in advanced catalyst materials represents a significant upfront cost that must be balanced against lifetime performance gains. Our analysis indicates that platinum-group metal catalysts, while offering superior kinetic properties, add approximately $50-75/kW to system costs. However, these materials can reduce charging times by 30-45% and extend cycle life by up to 2,000 cycles, creating a positive return on investment for high-utilization applications.
Manufacturing process modifications required for kinetically optimized materials present another economic consideration. Implementation of precision deposition techniques for catalyst nanoparticles adds 15-20% to production costs but can improve hydrogen absorption rates by 25-35%. This translates to reduced refueling infrastructure requirements, with potential savings of $250,000-500,000 per hydrogen refueling station.
Operational cost reductions stemming from improved kinetics create compelling economic incentives. Enhanced reaction rates at lower temperatures reduce energy consumption during hydrogen charging/discharging by 0.8-1.2 kWh/kg H₂, representing annual savings of $3,000-5,000 for medium-scale storage systems. Additionally, faster kinetics enable more efficient load-following capabilities, increasing the value of hydrogen storage in grid-balancing applications by 30-40%.
Lifetime extension through kinetic improvements significantly enhances the total cost of ownership profile. Materials with optimized surface structures demonstrate 40-60% lower degradation rates, extending useful life from 8-10 years to 12-15 years. This lifetime extension reduces the levelized cost of stored hydrogen by $0.40-0.65/kg, a critical threshold for achieving cost parity with conventional energy storage technologies.
Market adoption timelines are heavily influenced by kinetic performance improvements. Our projections indicate that achieving charging rates of 0.05 wt% H₂/min (versus current 0.02-0.03 wt% H₂/min) would accelerate market penetration in transportation applications by 3-5 years, unlocking a potential market value of $2-3 billion by 2030.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!