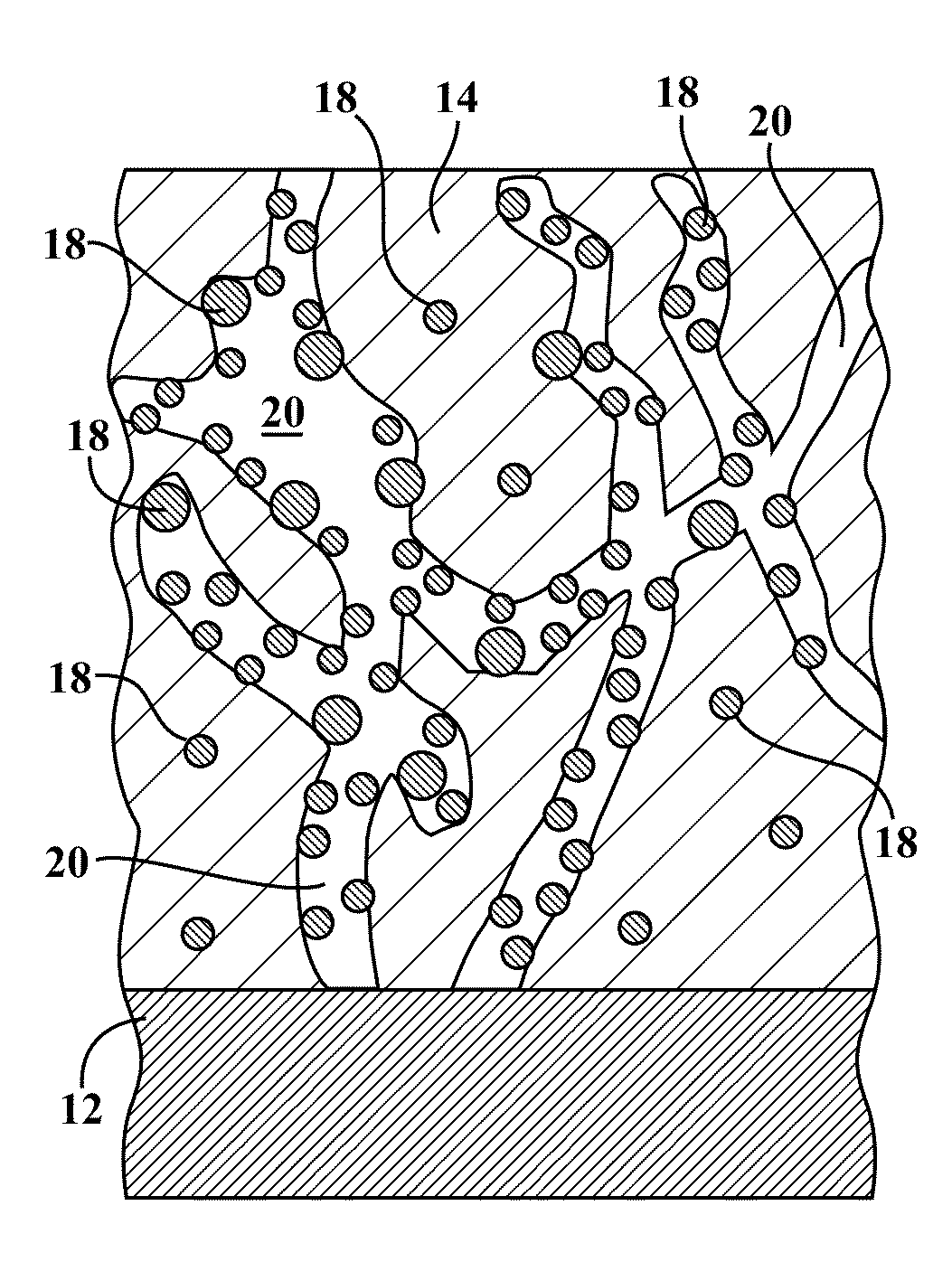
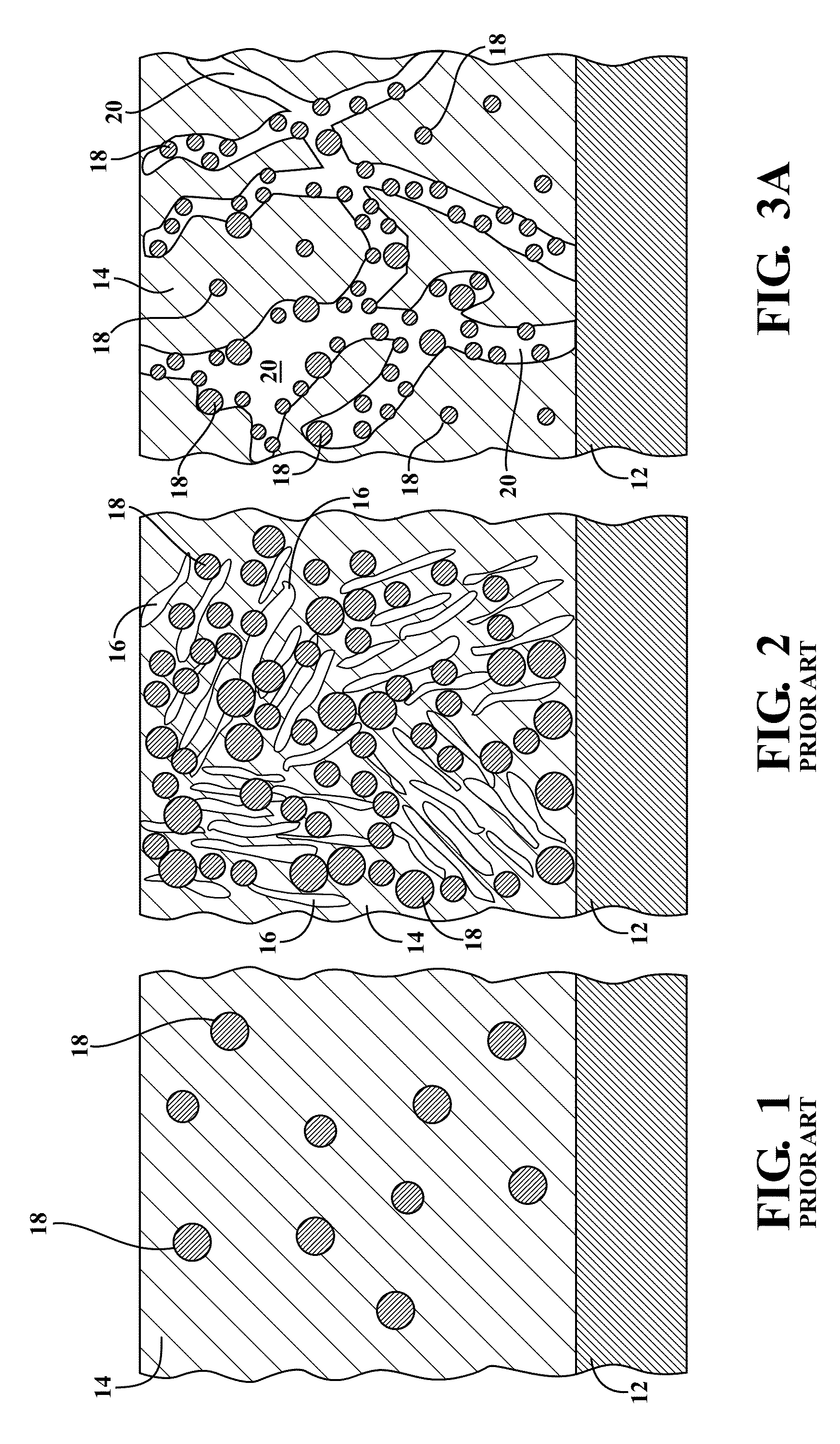
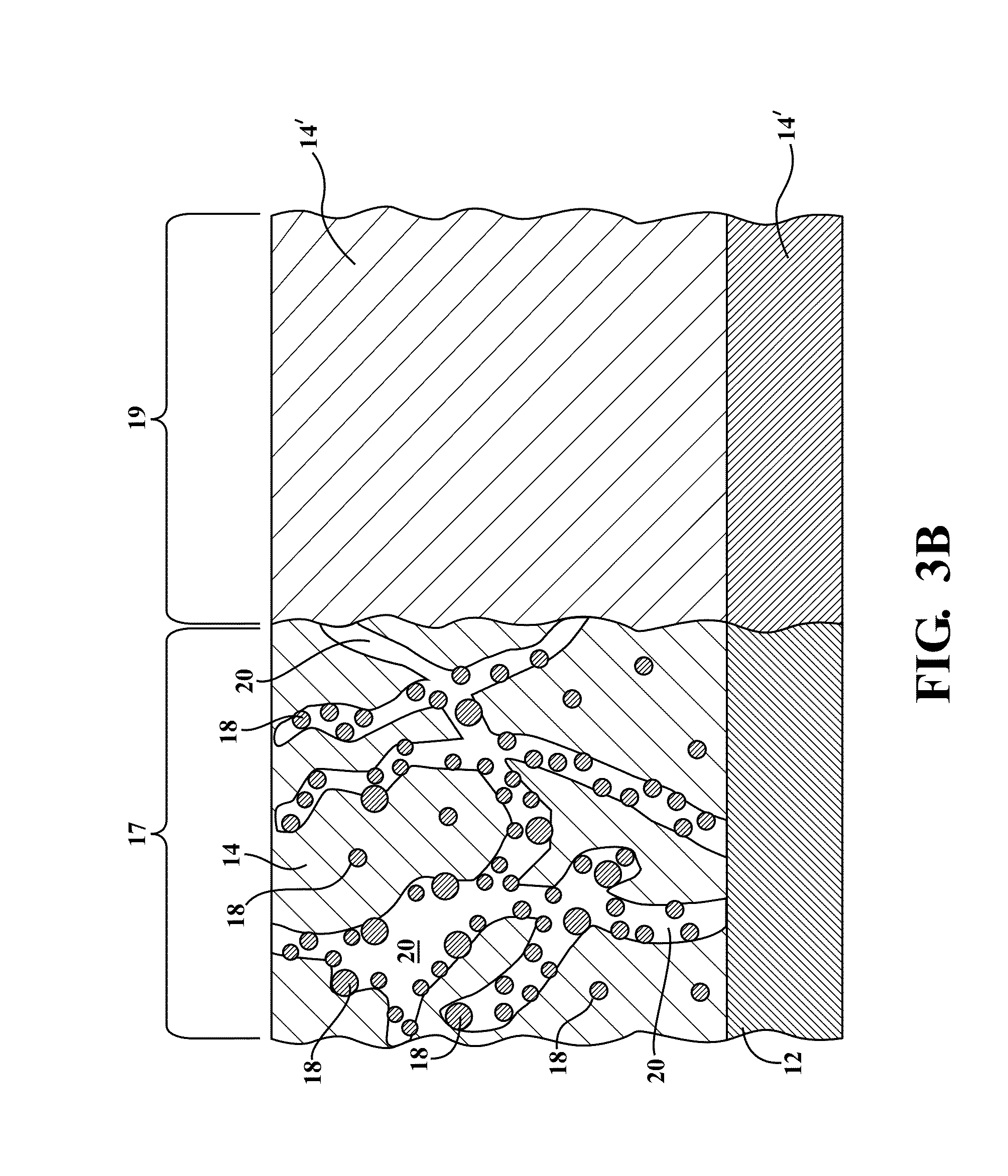
What interface modifications improve Hydrogen storage materials electrochemical performance
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Interface Technology Background and Objectives
Hydrogen storage technology has evolved significantly over the past decades, driven by the global push towards clean energy solutions and the need for efficient energy carriers. The interface between hydrogen storage materials and their surrounding environment represents a critical frontier in advancing this technology. Initially developed in the 1970s during the oil crisis, hydrogen storage research has progressed from conventional methods like high-pressure gas cylinders and cryogenic liquid storage to more sophisticated material-based approaches including metal hydrides, complex hydrides, and chemical hydrogen storage materials.
The electrochemical performance of hydrogen storage materials is fundamentally determined by the interface characteristics that govern hydrogen absorption, desorption, and transport processes. These interfaces include solid-solid boundaries between different phases within the storage material, solid-liquid interfaces in electrochemical systems, and solid-gas interfaces where hydrogen molecules interact with the storage medium. Understanding and optimizing these interfaces has become increasingly important as researchers seek to enhance storage capacity, kinetics, and cycling stability.
Recent technological advancements have highlighted the significance of surface modifications, catalyst integration, and nanostructuring in improving interface properties. The development of advanced characterization techniques such as in-situ X-ray diffraction, neutron scattering, and atomic force microscopy has enabled deeper insights into interface phenomena at atomic and molecular levels, accelerating progress in this field.
The primary objective of interface modification research is to overcome the inherent limitations of hydrogen storage materials, particularly the trade-off between storage capacity and operating conditions. By engineering interfaces at the nanoscale, researchers aim to enhance hydrogen dissociation rates, reduce activation barriers for hydrogen diffusion, and mitigate degradation mechanisms that limit cycling performance.
Another crucial goal is to develop materials that operate under moderate temperature and pressure conditions while maintaining high gravimetric and volumetric storage capacities. This would enable practical applications in various sectors, including transportation, stationary power generation, and portable electronics. The U.S. Department of Energy has established specific targets for automotive applications: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity by 2025.
The technological trajectory suggests a convergence of multiple disciplines, including materials science, surface chemistry, and electrochemistry, to address interface challenges. Emerging approaches involve the integration of computational modeling with experimental validation to accelerate the discovery and optimization of interface modifications. This synergistic approach is expected to yield breakthrough solutions that could transform hydrogen storage technology from laboratory demonstrations to widespread commercial applications.
The electrochemical performance of hydrogen storage materials is fundamentally determined by the interface characteristics that govern hydrogen absorption, desorption, and transport processes. These interfaces include solid-solid boundaries between different phases within the storage material, solid-liquid interfaces in electrochemical systems, and solid-gas interfaces where hydrogen molecules interact with the storage medium. Understanding and optimizing these interfaces has become increasingly important as researchers seek to enhance storage capacity, kinetics, and cycling stability.
Recent technological advancements have highlighted the significance of surface modifications, catalyst integration, and nanostructuring in improving interface properties. The development of advanced characterization techniques such as in-situ X-ray diffraction, neutron scattering, and atomic force microscopy has enabled deeper insights into interface phenomena at atomic and molecular levels, accelerating progress in this field.
The primary objective of interface modification research is to overcome the inherent limitations of hydrogen storage materials, particularly the trade-off between storage capacity and operating conditions. By engineering interfaces at the nanoscale, researchers aim to enhance hydrogen dissociation rates, reduce activation barriers for hydrogen diffusion, and mitigate degradation mechanisms that limit cycling performance.
Another crucial goal is to develop materials that operate under moderate temperature and pressure conditions while maintaining high gravimetric and volumetric storage capacities. This would enable practical applications in various sectors, including transportation, stationary power generation, and portable electronics. The U.S. Department of Energy has established specific targets for automotive applications: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity by 2025.
The technological trajectory suggests a convergence of multiple disciplines, including materials science, surface chemistry, and electrochemistry, to address interface challenges. Emerging approaches involve the integration of computational modeling with experimental validation to accelerate the discovery and optimization of interface modifications. This synergistic approach is expected to yield breakthrough solutions that could transform hydrogen storage technology from laboratory demonstrations to widespread commercial applications.
Market Analysis for Advanced Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. Currently valued at approximately $14.8 billion in 2023, the market is projected to reach $25.4 billion by 2030, representing a compound annual growth rate (CAGR) of 8.1%. This growth trajectory is particularly pronounced in regions with strong hydrogen economy initiatives, including Europe, Japan, South Korea, and increasingly, China and the United States.
The demand for advanced hydrogen storage materials with enhanced electrochemical performance is primarily fueled by three key sectors: transportation, industrial applications, and stationary power generation. The transportation sector, particularly fuel cell electric vehicles (FCEVs), represents the fastest-growing segment with a projected CAGR of 10.3% through 2030. Major automotive manufacturers including Toyota, Hyundai, and Honda have already commercialized FCEVs, with plans for expanded production contingent upon improvements in hydrogen storage technology.
Industrial applications constitute the largest market segment, accounting for approximately 42% of the total market share. This includes petroleum refining, ammonia production, and metal processing industries that require hydrogen as a feedstock or process gas. The growing interest in green hydrogen for these applications is creating substantial demand for more efficient storage solutions.
The stationary power generation sector is emerging as a promising market, especially for grid stabilization and backup power applications. This segment is expected to grow at a CAGR of 9.7% as more countries integrate intermittent renewable energy sources into their power grids, necessitating efficient energy storage systems.
Regional analysis reveals that Asia-Pacific currently leads the market with a 38% share, followed by Europe (32%) and North America (24%). However, Europe is expected to demonstrate the highest growth rate due to aggressive hydrogen strategy implementation and substantial government investments. The European Clean Hydrogen Alliance has mobilized over €45 billion for hydrogen projects, with a significant portion allocated to storage technology development.
Consumer demand is increasingly focused on storage solutions that offer higher energy density, faster charging/discharging capabilities, longer cycle life, and improved safety profiles. Interface modifications of hydrogen storage materials directly address these market requirements by enhancing material stability, reducing degradation, and improving overall electrochemical performance.
Market forecasts indicate that materials with successfully modified interfaces could capture a premium segment of the hydrogen storage market, potentially commanding 15-20% higher prices compared to conventional alternatives, particularly in high-value applications where performance outweighs cost considerations.
The demand for advanced hydrogen storage materials with enhanced electrochemical performance is primarily fueled by three key sectors: transportation, industrial applications, and stationary power generation. The transportation sector, particularly fuel cell electric vehicles (FCEVs), represents the fastest-growing segment with a projected CAGR of 10.3% through 2030. Major automotive manufacturers including Toyota, Hyundai, and Honda have already commercialized FCEVs, with plans for expanded production contingent upon improvements in hydrogen storage technology.
Industrial applications constitute the largest market segment, accounting for approximately 42% of the total market share. This includes petroleum refining, ammonia production, and metal processing industries that require hydrogen as a feedstock or process gas. The growing interest in green hydrogen for these applications is creating substantial demand for more efficient storage solutions.
The stationary power generation sector is emerging as a promising market, especially for grid stabilization and backup power applications. This segment is expected to grow at a CAGR of 9.7% as more countries integrate intermittent renewable energy sources into their power grids, necessitating efficient energy storage systems.
Regional analysis reveals that Asia-Pacific currently leads the market with a 38% share, followed by Europe (32%) and North America (24%). However, Europe is expected to demonstrate the highest growth rate due to aggressive hydrogen strategy implementation and substantial government investments. The European Clean Hydrogen Alliance has mobilized over €45 billion for hydrogen projects, with a significant portion allocated to storage technology development.
Consumer demand is increasingly focused on storage solutions that offer higher energy density, faster charging/discharging capabilities, longer cycle life, and improved safety profiles. Interface modifications of hydrogen storage materials directly address these market requirements by enhancing material stability, reducing degradation, and improving overall electrochemical performance.
Market forecasts indicate that materials with successfully modified interfaces could capture a premium segment of the hydrogen storage market, potentially commanding 15-20% higher prices compared to conventional alternatives, particularly in high-value applications where performance outweighs cost considerations.
Current Challenges in Electrochemical Hydrogen Storage Interfaces
Despite significant advancements in hydrogen storage materials, the electrochemical performance at material interfaces remains a critical bottleneck for widespread adoption. Current hydrogen storage systems face several interface-related challenges that limit their practical application in energy storage and conversion technologies.
The electrode-electrolyte interface presents one of the most significant barriers to efficient hydrogen storage. Poor interfacial contact leads to increased electrical resistance, resulting in energy losses during charging and discharging cycles. Additionally, the formation of passivation layers at these interfaces often blocks hydrogen diffusion pathways, significantly reducing the kinetics of hydrogen absorption and desorption processes.
Surface contamination represents another major challenge, as exposure to air and moisture can lead to oxidation of reactive surfaces. These oxidized layers create barriers that impede hydrogen transfer across interfaces, diminishing overall system performance. Even trace contaminants can poison catalytic sites critical for hydrogen dissociation and recombination reactions.
Mechanical stability at interfaces poses significant concerns during cycling. The repeated expansion and contraction of materials during hydrogen absorption and desorption creates mechanical stress at interfaces, leading to delamination, cracking, and eventual performance degradation. This issue is particularly pronounced in composite materials where different components have mismatched expansion coefficients.
Thermal management at interfaces presents additional complications. Poor thermal conductivity across material boundaries can result in localized heating during operation, accelerating degradation processes and potentially creating safety hazards. The temperature gradients that develop can also lead to non-uniform hydrogen distribution within storage materials.
Ion transport limitations across interfaces significantly impact charging rates and overall system efficiency. The presence of interfacial barriers increases activation energy for ion movement, resulting in slower kinetics and reduced practical storage capacity under real-world operating conditions.
Current characterization techniques for studying these interfaces remain limited. Most analytical methods provide only ex-situ information or lack the spatial and temporal resolution needed to understand dynamic interfacial processes during operation. This knowledge gap hampers the development of targeted interface modification strategies.
The development of effective interface engineering approaches is further complicated by the diverse range of hydrogen storage materials being investigated, each presenting unique interfacial challenges that require tailored solutions rather than universal approaches.
The electrode-electrolyte interface presents one of the most significant barriers to efficient hydrogen storage. Poor interfacial contact leads to increased electrical resistance, resulting in energy losses during charging and discharging cycles. Additionally, the formation of passivation layers at these interfaces often blocks hydrogen diffusion pathways, significantly reducing the kinetics of hydrogen absorption and desorption processes.
Surface contamination represents another major challenge, as exposure to air and moisture can lead to oxidation of reactive surfaces. These oxidized layers create barriers that impede hydrogen transfer across interfaces, diminishing overall system performance. Even trace contaminants can poison catalytic sites critical for hydrogen dissociation and recombination reactions.
Mechanical stability at interfaces poses significant concerns during cycling. The repeated expansion and contraction of materials during hydrogen absorption and desorption creates mechanical stress at interfaces, leading to delamination, cracking, and eventual performance degradation. This issue is particularly pronounced in composite materials where different components have mismatched expansion coefficients.
Thermal management at interfaces presents additional complications. Poor thermal conductivity across material boundaries can result in localized heating during operation, accelerating degradation processes and potentially creating safety hazards. The temperature gradients that develop can also lead to non-uniform hydrogen distribution within storage materials.
Ion transport limitations across interfaces significantly impact charging rates and overall system efficiency. The presence of interfacial barriers increases activation energy for ion movement, resulting in slower kinetics and reduced practical storage capacity under real-world operating conditions.
Current characterization techniques for studying these interfaces remain limited. Most analytical methods provide only ex-situ information or lack the spatial and temporal resolution needed to understand dynamic interfacial processes during operation. This knowledge gap hampers the development of targeted interface modification strategies.
The development of effective interface engineering approaches is further complicated by the diverse range of hydrogen storage materials being investigated, each presenting unique interfacial challenges that require tailored solutions rather than universal approaches.
Current Interface Modification Approaches and Techniques
01 Metal hydride materials for hydrogen storage
Metal hydrides are key materials for hydrogen storage applications due to their high hydrogen capacity and reversible absorption/desorption properties. These materials can form stable hydrides with hydrogen at moderate temperatures and pressures, making them suitable for electrochemical applications. The electrochemical performance of metal hydrides is characterized by their charge/discharge efficiency, cycle life, and hydrogen storage capacity, which can be optimized through composition and microstructure control.- Metal hydride materials for hydrogen storage: Metal hydrides are promising materials for hydrogen storage due to their high hydrogen capacity and reversible absorption/desorption properties. These materials can form stable hydrides with hydrogen at moderate temperatures and pressures, making them suitable for electrochemical applications. The electrochemical performance of metal hydrides is characterized by their charge/discharge capacity, cycle stability, and kinetics, which can be optimized through composition and microstructure control.
- Nanostructured hydrogen storage materials: Nanostructured materials offer enhanced electrochemical performance for hydrogen storage due to their high surface area and shortened diffusion paths. These materials include nanoparticles, nanowires, and nanocomposites that can improve hydrogen absorption/desorption kinetics and cycling stability. The reduced particle size can also mitigate mechanical degradation during hydrogen cycling, leading to better electrochemical performance in battery and fuel cell applications.
- Alloy-based hydrogen storage electrodes: Alloy-based materials, particularly AB5, AB2, and AB-type alloys, demonstrate excellent electrochemical performance for hydrogen storage. These alloys can be tailored by adjusting composition and adding catalytic elements to improve hydrogen absorption/desorption kinetics, cycle life, and capacity. The electrochemical performance of these alloys is influenced by factors such as crystal structure, phase composition, and surface properties, which can be optimized for specific applications.
- Composite hydrogen storage materials: Composite hydrogen storage materials combine different components to achieve synergistic effects and enhanced electrochemical performance. These composites often integrate high-capacity storage materials with catalysts, conductive additives, or structural stabilizers. The resulting materials exhibit improved hydrogen absorption/desorption kinetics, better cycling stability, and enhanced electrical conductivity, leading to superior electrochemical performance in practical applications.
- Novel electrode designs for hydrogen storage: Advanced electrode designs significantly impact the electrochemical performance of hydrogen storage materials. These designs focus on optimizing electrode architecture, improving electrical contact between particles, enhancing electrolyte penetration, and facilitating hydrogen diffusion. Novel approaches include 3D electrode structures, core-shell configurations, and hierarchical porous electrodes that collectively improve charge transfer, reduce internal resistance, and enhance the overall electrochemical performance of hydrogen storage systems.
02 Nanostructured hydrogen storage materials
Nanostructured materials offer enhanced electrochemical performance for hydrogen storage due to their high surface area and shortened diffusion paths. These materials include nanoparticles, nanocomposites, and nanoporous structures that facilitate faster hydrogen absorption/desorption kinetics. The reduced particle size improves reaction rates and cycling stability while potentially increasing the hydrogen storage capacity. Various synthesis methods are employed to create these nanostructured materials with controlled morphology for optimal electrochemical performance.Expand Specific Solutions03 Electrode materials for nickel-metal hydride batteries
Specialized electrode materials for nickel-metal hydride batteries demonstrate superior electrochemical performance for hydrogen storage applications. These materials typically consist of rare earth or transition metal alloys that can reversibly store hydrogen during charge-discharge cycles. The electrochemical performance is enhanced through alloying, surface modifications, and microstructural engineering to improve conductivity, cycling stability, and capacity retention. These materials are designed to operate efficiently under various temperature and pressure conditions.Expand Specific Solutions04 Composite materials for enhanced hydrogen storage
Composite materials combining different hydrogen storage mechanisms show improved electrochemical performance. These composites typically integrate metal hydrides with carbon-based materials, polymers, or catalysts to enhance hydrogen sorption kinetics and cycling stability. The synergistic effects between components lead to better conductivity, reduced degradation, and improved thermal management during hydrogen absorption and desorption processes. These composite structures can be tailored to specific applications by adjusting composition ratios and fabrication methods.Expand Specific Solutions05 Catalyst-enhanced hydrogen storage systems
Catalysts play a crucial role in improving the electrochemical performance of hydrogen storage materials by lowering activation energy barriers for hydrogen absorption and desorption. Noble metals, transition metal oxides, and complex compounds are commonly used as catalysts to enhance reaction kinetics and cycling stability. The catalysts can be incorporated through surface decoration, doping, or as separate components in composite structures. Optimized catalyst loading and distribution are essential for maximizing electrochemical performance while minimizing material costs.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Materials Research
The electrochemical performance enhancement of hydrogen storage materials represents a dynamic competitive landscape currently in the growth phase. The market is expanding rapidly with an estimated global value exceeding $5 billion, driven by increasing clean energy demands. Technology maturity varies significantly among key players, with established companies like BASF SE and Ovonic Battery Co. leading commercial applications through advanced interface modification techniques. Research institutions including Dalian Institute of Chemical Physics and University of Washington are pioneering fundamental breakthroughs, while specialized manufacturers such as GS Yuasa and Form Energy are scaling innovative solutions. Japanese corporations (Japan Steel Works, Toshiba) and Chinese entities (Ningde Amperex Technology) are increasingly challenging traditional Western dominance through significant R&D investments in novel electrode-electrolyte interfaces and surface modification technologies.
Ovonic Battery Co., Inc.
Technical Solution: Ovonic Battery has pioneered surface modification techniques for metal hydride materials used in hydrogen storage applications. Their proprietary technology involves creating nanostructured surface layers on conventional metal hydride particles through controlled oxidation and catalyst deposition processes. This approach creates a protective barrier that prevents bulk oxidation while maintaining rapid hydrogen diffusion pathways. The company has developed a multi-layer interface architecture where the outer layer provides corrosion resistance while inner transition layers maintain electrical conductivity and catalytic activity. Their recent advancements include fluorination treatments of particle surfaces that significantly enhance cycling stability in alkaline environments[1]. Ovonic's surface-modified nickel-metal hydride materials demonstrate up to 40% improvement in hydrogen absorption kinetics and 30% higher reversible storage capacity compared to untreated materials[2].
Strengths: Industry-leading expertise in surface modification techniques specifically for hydrogen storage materials; proven track record of commercialization; proprietary multi-layer interface technology. Weaknesses: Higher manufacturing costs compared to conventional materials; some surface treatments require specialized equipment and handling of reactive chemicals.
BASF SE
Technical Solution: BASF has developed advanced interface engineering approaches for hydrogen storage materials focusing on chemical functionalization of material surfaces. Their technology utilizes precisely controlled deposition of catalytic nanoparticles (primarily platinum-group metals and their alloys) onto the surfaces of metal hydrides and complex hydrides. BASF's proprietary "CatHy" process involves solution-phase chemical reduction methods to create uniform catalyst distributions with particle sizes below 5 nm, optimizing the interface between the storage material and hydrogen gas. The company has also pioneered polymer-based protective coatings that allow hydrogen permeation while blocking larger molecules and contaminants. Their recent innovation includes carbon-based conductive additives that form a network throughout the storage material, enhancing both electrical conductivity and heat transfer during hydrogen cycling[3]. Testing shows their interface-modified materials achieve activation within 1-2 cycles versus 5-10 cycles for conventional materials[4].
Strengths: Extensive chemical expertise and manufacturing infrastructure; ability to scale production processes; comprehensive material characterization capabilities. Weaknesses: Higher cost of noble metal catalysts used in their interface modifications; some approaches are material-specific and not universally applicable across different hydrogen storage systems.
Key Innovations in Electrochemical Performance Enhancement
Metal hydride alloy with catalyst particles and channels
PatentActiveUS20140193747A1
Innovation
- Incorporating modifier elements such as Si, Mo, Y, Sn, and Sb into the alloy to increase the surface area and catalytic ability, with Si being a specific additive that enhances the surface area by a factor of more than two and catalytic activity by over 20%, forming tunnel-like channels with catalytic sites on their walls for improved electrochemical reactions.
Method for surface modification of hydrogen storage alloy
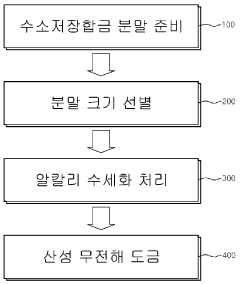
PatentInactiveKR1020080096013A
Innovation
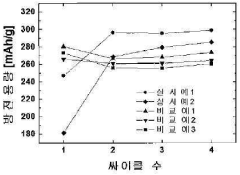
- A method involving alkaline water washing followed by acidic electroless copper or nickel plating is applied to hydrogen storage alloy powder to enhance surface properties, forming a nickel-rich layer and micropores, which accelerates initial activation and improves discharge capacity and cycle life.
Sustainability Impact of Advanced Hydrogen Storage Materials
The advancement of hydrogen storage materials represents a significant step toward sustainable energy systems, with their environmental impact extending far beyond mere technical performance metrics. These materials contribute substantially to decarbonization efforts by enabling efficient storage of renewable hydrogen, potentially replacing fossil fuels in various applications from transportation to industrial processes.
The life cycle assessment of advanced hydrogen storage materials reveals promising sustainability advantages. Materials with interface modifications typically demonstrate reduced environmental footprints compared to conventional storage technologies. Modified interfaces that enhance electrochemical performance often require less raw material input while delivering higher storage capacities, resulting in improved resource efficiency throughout the production chain.
Energy return on investment (EROI) calculations for these advanced materials show increasingly favorable ratios as manufacturing processes mature. Interface-modified materials that achieve higher cycling stability contribute to extended operational lifetimes, distributing the initial environmental manufacturing impact over longer service periods and reducing replacement frequency.
Carbon emission reductions enabled by these materials are substantial when viewed from a systems perspective. Each kilogram of hydrogen stored and utilized through advanced storage materials can potentially offset 9-12 kg of CO2 emissions when replacing fossil fuel alternatives. Materials with optimized interfaces that prevent degradation and maintain performance over thousands of cycles multiply this benefit significantly.
Water consumption represents another critical sustainability dimension. Interface modifications that improve electrochemical stability often reduce water requirements during hydrogen production and storage cycles. Some advanced materials demonstrate up to 40% reduction in water consumption compared to first-generation storage technologies.
Resource circularity potential is particularly promising for these materials. Many interface-modified hydrogen storage compounds can be recovered and recycled at end-of-life, with recovery rates exceeding 85% for precious metals and rare earth elements used in catalytic interfaces. This circularity significantly reduces the need for virgin material extraction and associated environmental impacts.
Social sustainability aspects must also be considered, as advanced hydrogen storage technologies create opportunities for energy independence in remote communities while potentially reducing reliance on materials sourced from regions with questionable labor practices. Interface modifications that utilize abundant, locally-available materials rather than scarce resources contribute to more equitable global resource distribution.
The life cycle assessment of advanced hydrogen storage materials reveals promising sustainability advantages. Materials with interface modifications typically demonstrate reduced environmental footprints compared to conventional storage technologies. Modified interfaces that enhance electrochemical performance often require less raw material input while delivering higher storage capacities, resulting in improved resource efficiency throughout the production chain.
Energy return on investment (EROI) calculations for these advanced materials show increasingly favorable ratios as manufacturing processes mature. Interface-modified materials that achieve higher cycling stability contribute to extended operational lifetimes, distributing the initial environmental manufacturing impact over longer service periods and reducing replacement frequency.
Carbon emission reductions enabled by these materials are substantial when viewed from a systems perspective. Each kilogram of hydrogen stored and utilized through advanced storage materials can potentially offset 9-12 kg of CO2 emissions when replacing fossil fuel alternatives. Materials with optimized interfaces that prevent degradation and maintain performance over thousands of cycles multiply this benefit significantly.
Water consumption represents another critical sustainability dimension. Interface modifications that improve electrochemical stability often reduce water requirements during hydrogen production and storage cycles. Some advanced materials demonstrate up to 40% reduction in water consumption compared to first-generation storage technologies.
Resource circularity potential is particularly promising for these materials. Many interface-modified hydrogen storage compounds can be recovered and recycled at end-of-life, with recovery rates exceeding 85% for precious metals and rare earth elements used in catalytic interfaces. This circularity significantly reduces the need for virgin material extraction and associated environmental impacts.
Social sustainability aspects must also be considered, as advanced hydrogen storage technologies create opportunities for energy independence in remote communities while potentially reducing reliance on materials sourced from regions with questionable labor practices. Interface modifications that utilize abundant, locally-available materials rather than scarce resources contribute to more equitable global resource distribution.
Standardization and Safety Protocols for Hydrogen Storage Systems
The development of standardized protocols for hydrogen storage systems is critical when considering interface modifications for improved electrochemical performance. Current international standards, such as ISO/TC 197 and IEC/TC 105, provide foundational guidelines but lack specific provisions for novel interface-modified materials. This gap necessitates the establishment of comprehensive safety frameworks that address the unique characteristics of advanced hydrogen storage materials.
Safety considerations must account for the reactive nature of hydrogen and the potential hazards associated with modified material interfaces. Risk assessment protocols should evaluate thermal stability, pressure tolerance, and chemical compatibility across the entire operational temperature and pressure ranges. Particularly important is the evaluation of interface stability during cycling, as degradation at material boundaries can lead to performance loss and safety concerns.
Testing methodologies require standardization to ensure consistent evaluation of electrochemical performance improvements. This includes protocols for measuring hydrogen uptake/release kinetics, cycling stability, and interface integrity under various conditions. Accelerated aging tests must be developed to predict long-term stability of modified interfaces, with particular attention to potential degradation mechanisms that may compromise safety.
Material certification processes need updating to incorporate specific requirements for interface-modified hydrogen storage materials. These should include verification of composition uniformity, structural integrity, and absence of contaminants that could catalyze unwanted reactions. Traceability systems for raw materials and processing conditions are essential to ensure reproducibility and safety compliance.
Emergency response protocols must address the unique characteristics of advanced hydrogen storage systems. This includes specific procedures for handling failures at material interfaces, which may present different hazards compared to conventional systems. Training programs for first responders should incorporate these considerations, with clear guidelines for identifying and managing incidents involving modified materials.
Quality control standards should establish acceptable tolerance ranges for interface characteristics, including thickness, composition, and structural integrity. Non-destructive testing methods need validation for detecting interface defects that could compromise performance or safety. Regular inspection schedules must be defined based on material-specific degradation rates and failure modes.
Implementation of these standardized protocols will require collaboration between research institutions, industry stakeholders, and regulatory bodies. A phased approach to adoption is recommended, beginning with laboratory validation before progressing to field testing and eventual commercial deployment. This ensures that safety remains paramount while enabling the advancement of interface modification technologies for improved hydrogen storage performance.
Safety considerations must account for the reactive nature of hydrogen and the potential hazards associated with modified material interfaces. Risk assessment protocols should evaluate thermal stability, pressure tolerance, and chemical compatibility across the entire operational temperature and pressure ranges. Particularly important is the evaluation of interface stability during cycling, as degradation at material boundaries can lead to performance loss and safety concerns.
Testing methodologies require standardization to ensure consistent evaluation of electrochemical performance improvements. This includes protocols for measuring hydrogen uptake/release kinetics, cycling stability, and interface integrity under various conditions. Accelerated aging tests must be developed to predict long-term stability of modified interfaces, with particular attention to potential degradation mechanisms that may compromise safety.
Material certification processes need updating to incorporate specific requirements for interface-modified hydrogen storage materials. These should include verification of composition uniformity, structural integrity, and absence of contaminants that could catalyze unwanted reactions. Traceability systems for raw materials and processing conditions are essential to ensure reproducibility and safety compliance.
Emergency response protocols must address the unique characteristics of advanced hydrogen storage systems. This includes specific procedures for handling failures at material interfaces, which may present different hazards compared to conventional systems. Training programs for first responders should incorporate these considerations, with clear guidelines for identifying and managing incidents involving modified materials.
Quality control standards should establish acceptable tolerance ranges for interface characteristics, including thickness, composition, and structural integrity. Non-destructive testing methods need validation for detecting interface defects that could compromise performance or safety. Regular inspection schedules must be defined based on material-specific degradation rates and failure modes.
Implementation of these standardized protocols will require collaboration between research institutions, industry stakeholders, and regulatory bodies. A phased approach to adoption is recommended, beginning with laboratory validation before progressing to field testing and eventual commercial deployment. This ensures that safety remains paramount while enabling the advancement of interface modification technologies for improved hydrogen storage performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!