Comparative study of Hydrogen storage materials adsorption versus absorption performance
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The quest for efficient hydrogen storage materials has intensified over the past decades, driven by hydrogen's potential as a clean energy carrier with high energy density by weight (142 MJ/kg). However, its low volumetric energy density under ambient conditions presents significant storage challenges that must be overcome for widespread adoption in various applications, particularly in transportation and portable power systems.
The evolution of hydrogen storage technologies has progressed through several generations, beginning with conventional high-pressure tanks and cryogenic liquid storage, which despite their technological maturity, face inherent limitations in safety, energy efficiency, and volumetric capacity. This has prompted extensive research into solid-state storage materials that can potentially offer higher volumetric densities under more moderate operating conditions.
Two fundamental approaches have emerged in solid-state hydrogen storage: adsorption and absorption. Adsorption-based materials, including metal-organic frameworks (MOFs), carbon nanostructures, and zeolites, store hydrogen molecules on their surfaces through weak van der Waals interactions. In contrast, absorption-based materials, such as metal hydrides and complex hydrides, incorporate hydrogen atoms into their crystal lattice through chemical bonding.
The U.S. Department of Energy has established ambitious targets for hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity by 2025, with ultimate targets of 7.5 wt% and 70 g/L. These benchmarks serve as crucial reference points for evaluating the performance and potential of various storage materials and technologies.
Recent technological breakthroughs in nanomaterial synthesis, computational modeling, and characterization techniques have accelerated the development of novel hydrogen storage materials with enhanced properties. The integration of machine learning approaches has further enabled rapid screening and design of candidate materials with optimized adsorption and absorption characteristics.
The primary objective of this comparative study is to systematically evaluate the performance metrics of adsorption versus absorption-based hydrogen storage materials, including storage capacity, operating conditions, kinetics, cycling stability, and energy efficiency. Additionally, this analysis aims to identify the most promising materials within each category and assess their suitability for specific applications ranging from stationary power generation to mobile transportation systems.
Furthermore, this study seeks to establish a comprehensive framework for benchmarking hydrogen storage materials that accounts for not only technical performance but also practical considerations such as cost, scalability, and environmental impact, thereby providing valuable insights for strategic research and development investments in this critical technology area.
The evolution of hydrogen storage technologies has progressed through several generations, beginning with conventional high-pressure tanks and cryogenic liquid storage, which despite their technological maturity, face inherent limitations in safety, energy efficiency, and volumetric capacity. This has prompted extensive research into solid-state storage materials that can potentially offer higher volumetric densities under more moderate operating conditions.
Two fundamental approaches have emerged in solid-state hydrogen storage: adsorption and absorption. Adsorption-based materials, including metal-organic frameworks (MOFs), carbon nanostructures, and zeolites, store hydrogen molecules on their surfaces through weak van der Waals interactions. In contrast, absorption-based materials, such as metal hydrides and complex hydrides, incorporate hydrogen atoms into their crystal lattice through chemical bonding.
The U.S. Department of Energy has established ambitious targets for hydrogen storage systems: 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity by 2025, with ultimate targets of 7.5 wt% and 70 g/L. These benchmarks serve as crucial reference points for evaluating the performance and potential of various storage materials and technologies.
Recent technological breakthroughs in nanomaterial synthesis, computational modeling, and characterization techniques have accelerated the development of novel hydrogen storage materials with enhanced properties. The integration of machine learning approaches has further enabled rapid screening and design of candidate materials with optimized adsorption and absorption characteristics.
The primary objective of this comparative study is to systematically evaluate the performance metrics of adsorption versus absorption-based hydrogen storage materials, including storage capacity, operating conditions, kinetics, cycling stability, and energy efficiency. Additionally, this analysis aims to identify the most promising materials within each category and assess their suitability for specific applications ranging from stationary power generation to mobile transportation systems.
Furthermore, this study seeks to establish a comprehensive framework for benchmarking hydrogen storage materials that accounts for not only technical performance but also practical considerations such as cost, scalability, and environmental impact, thereby providing valuable insights for strategic research and development investments in this critical technology area.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by increasing investments in hydrogen as a clean energy carrier. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate of 9.7% through 2030. This growth trajectory is primarily fueled by the expanding hydrogen economy and the critical need for efficient storage solutions across various applications.
The market segmentation for hydrogen storage technologies reveals distinct preferences based on application requirements. Industrial applications currently dominate with roughly 45% market share, followed by transportation at 30%, and stationary power at 25%. Within these segments, there is growing demand for both adsorption and absorption-based storage solutions, each serving specific market niches based on their performance characteristics.
Adsorption-based hydrogen storage materials, particularly metal-organic frameworks (MOFs) and carbon-based materials, are gaining traction in applications requiring rapid hydrogen uptake and release. These materials are increasingly favored in portable and mobile applications due to their lower operating pressures and ambient temperature functionality. The market for adsorption materials is growing at approximately 11.2% annually, outpacing the overall hydrogen storage market.
Absorption-based technologies, including metal hydrides and complex hydrides, continue to maintain strong market presence in stationary applications where volumetric efficiency is prioritized over gravimetric capacity. These materials command approximately 38% of the current hydrogen storage materials market, with particular strength in industrial energy storage applications.
Regional analysis indicates that Asia-Pacific leads the hydrogen storage market with 40% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 35%, with significant investments in hydrogen infrastructure as part of its green energy transition. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
Customer demand patterns show increasing preference for storage solutions that balance cost efficiency with performance. While absorption materials traditionally offered higher volumetric capacity, recent advancements in adsorption materials have narrowed this gap, creating more competitive market dynamics. End-users are increasingly evaluating total cost of ownership rather than focusing solely on initial capital expenditure.
Market barriers include high production costs for advanced materials, scaling challenges for novel technologies, and infrastructure limitations. However, these barriers are gradually being addressed through increased R&D funding and supportive government policies worldwide, creating favorable conditions for continued market expansion in both adsorption and absorption hydrogen storage technologies.
The market segmentation for hydrogen storage technologies reveals distinct preferences based on application requirements. Industrial applications currently dominate with roughly 45% market share, followed by transportation at 30%, and stationary power at 25%. Within these segments, there is growing demand for both adsorption and absorption-based storage solutions, each serving specific market niches based on their performance characteristics.
Adsorption-based hydrogen storage materials, particularly metal-organic frameworks (MOFs) and carbon-based materials, are gaining traction in applications requiring rapid hydrogen uptake and release. These materials are increasingly favored in portable and mobile applications due to their lower operating pressures and ambient temperature functionality. The market for adsorption materials is growing at approximately 11.2% annually, outpacing the overall hydrogen storage market.
Absorption-based technologies, including metal hydrides and complex hydrides, continue to maintain strong market presence in stationary applications where volumetric efficiency is prioritized over gravimetric capacity. These materials command approximately 38% of the current hydrogen storage materials market, with particular strength in industrial energy storage applications.
Regional analysis indicates that Asia-Pacific leads the hydrogen storage market with 40% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 35%, with significant investments in hydrogen infrastructure as part of its green energy transition. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
Customer demand patterns show increasing preference for storage solutions that balance cost efficiency with performance. While absorption materials traditionally offered higher volumetric capacity, recent advancements in adsorption materials have narrowed this gap, creating more competitive market dynamics. End-users are increasingly evaluating total cost of ownership rather than focusing solely on initial capital expenditure.
Market barriers include high production costs for advanced materials, scaling challenges for novel technologies, and infrastructure limitations. However, these barriers are gradually being addressed through increased R&D funding and supportive government policies worldwide, creating favorable conditions for continued market expansion in both adsorption and absorption hydrogen storage technologies.
Current Challenges in Adsorption vs Absorption Methods
Despite significant advancements in hydrogen storage technologies, both adsorption and absorption methods face substantial technical challenges that limit their widespread commercial implementation. Adsorption-based systems struggle with insufficient storage capacity at ambient temperatures, typically achieving only 1-2 wt% hydrogen storage under practical conditions, far below the U.S. Department of Energy's target of 6.5 wt% for transportation applications. The fundamental challenge lies in the weak van der Waals interactions between hydrogen molecules and most adsorbent surfaces, necessitating cryogenic temperatures (-196°C) to achieve meaningful capacities.
Material engineering challenges for adsorption systems include optimizing surface area while maintaining appropriate pore size distribution. While materials like MOFs have demonstrated theoretical surface areas exceeding 7000 m²/g, their practical performance is compromised by framework collapse, pore blocking, and limited thermal stability. Additionally, the volumetric storage density remains inadequate for mobile applications due to the inherent porosity of these materials.
Absorption-based systems, particularly metal hydrides, face different but equally significant challenges. The primary limitation is the unfavorable kinetics of hydrogen absorption/desorption processes, with many promising materials requiring temperatures exceeding 300°C for hydrogen release. This high energy requirement substantially reduces the overall system efficiency. Furthermore, many high-capacity metal hydrides suffer from poor cycling stability, with capacity degradation of 20-30% after just 100 cycles due to particle agglomeration and phase segregation.
The trade-off between thermodynamics and kinetics presents another critical challenge. Materials with favorable thermodynamics (ΔH ≈ 20-40 kJ/mol H₂) often exhibit slow kinetics, while those with rapid kinetics typically have either too stable or too unstable hydride formation enthalpies for practical applications. This fundamental dilemma has proven difficult to resolve despite extensive research efforts.
Heat management represents a significant engineering challenge for both technologies. Absorption processes are highly exothermic during hydrogen uptake, requiring efficient heat removal systems to prevent temperature spikes that would thermodynamically limit further absorption. Conversely, the endothermic desorption process demands substantial heat input, creating complex thermal management requirements that increase system complexity and weight.
Material cost and availability present additional barriers, particularly for absorption systems that often rely on rare earth elements or precious metals as catalysts. The environmental impact of material production and potential toxicity of some promising compounds (e.g., complex borohydrides) further complicate commercial deployment. These multifaceted challenges necessitate innovative approaches that can address the fundamental limitations of both storage mechanisms.
Material engineering challenges for adsorption systems include optimizing surface area while maintaining appropriate pore size distribution. While materials like MOFs have demonstrated theoretical surface areas exceeding 7000 m²/g, their practical performance is compromised by framework collapse, pore blocking, and limited thermal stability. Additionally, the volumetric storage density remains inadequate for mobile applications due to the inherent porosity of these materials.
Absorption-based systems, particularly metal hydrides, face different but equally significant challenges. The primary limitation is the unfavorable kinetics of hydrogen absorption/desorption processes, with many promising materials requiring temperatures exceeding 300°C for hydrogen release. This high energy requirement substantially reduces the overall system efficiency. Furthermore, many high-capacity metal hydrides suffer from poor cycling stability, with capacity degradation of 20-30% after just 100 cycles due to particle agglomeration and phase segregation.
The trade-off between thermodynamics and kinetics presents another critical challenge. Materials with favorable thermodynamics (ΔH ≈ 20-40 kJ/mol H₂) often exhibit slow kinetics, while those with rapid kinetics typically have either too stable or too unstable hydride formation enthalpies for practical applications. This fundamental dilemma has proven difficult to resolve despite extensive research efforts.
Heat management represents a significant engineering challenge for both technologies. Absorption processes are highly exothermic during hydrogen uptake, requiring efficient heat removal systems to prevent temperature spikes that would thermodynamically limit further absorption. Conversely, the endothermic desorption process demands substantial heat input, creating complex thermal management requirements that increase system complexity and weight.
Material cost and availability present additional barriers, particularly for absorption systems that often rely on rare earth elements or precious metals as catalysts. The environmental impact of material production and potential toxicity of some promising compounds (e.g., complex borohydrides) further complicate commercial deployment. These multifaceted challenges necessitate innovative approaches that can address the fundamental limitations of both storage mechanisms.
Comparative Analysis of Current Storage Solutions
01 Metal-organic frameworks (MOFs) for hydrogen storage
Metal-organic frameworks represent a promising class of materials for hydrogen storage due to their high surface area and tunable pore structures. These crystalline porous materials can achieve significant hydrogen adsorption capacities through physisorption mechanisms. MOFs can be designed with specific metal centers and organic linkers to optimize hydrogen binding energy and storage capacity. Their performance in hydrogen storage applications depends on factors such as pore size distribution, specific surface area, and the presence of open metal sites.- Metal-organic frameworks (MOFs) for hydrogen storage: Metal-organic frameworks (MOFs) are promising materials for hydrogen storage due to their high surface area and tunable pore structures. These crystalline porous materials can achieve significant hydrogen adsorption capacities through physisorption mechanisms. MOFs can be designed with specific metal centers and organic linkers to optimize hydrogen binding energy and storage capacity. Their performance in hydrogen adsorption can be enhanced through strategies like introducing open metal sites, functionalization of organic linkers, and creating hierarchical pore structures.
- Metal hydrides for hydrogen absorption: Metal hydrides store hydrogen through chemical absorption (chemisorption), forming strong chemical bonds with hydrogen atoms. These materials can achieve high volumetric hydrogen densities exceeding that of liquid hydrogen. Various metal hydride systems have been developed, including light metal hydrides (like MgH2, LiH), complex hydrides (like alanates, borohydrides), and intermetallic compounds. The absorption performance depends on factors such as operating temperature, pressure conditions, and kinetics of hydrogen uptake and release. While offering high storage capacity, many metal hydrides face challenges with slow kinetics and high desorption temperatures.
- Carbon-based materials for hydrogen adsorption: Carbon-based materials including activated carbon, carbon nanotubes, graphene, and porous carbon structures are widely studied for hydrogen storage through physical adsorption mechanisms. These materials offer advantages such as light weight, high surface area, and good cycling stability. The hydrogen storage capacity of carbon materials depends primarily on their specific surface area, pore size distribution, and surface functionalization. Various modification strategies, such as doping with heteroatoms (N, B) or metals, can enhance the hydrogen binding energy and improve adsorption performance at ambient conditions.
- Comparative analysis of adsorption versus absorption mechanisms: Hydrogen storage through adsorption (physisorption) involves weak van der Waals interactions between hydrogen molecules and the material surface, typically requiring cryogenic temperatures for significant capacity. In contrast, absorption (chemisorption) involves the formation of chemical bonds, allowing for higher storage densities but often requiring higher temperatures for hydrogen release. Adsorption-based systems generally offer faster kinetics and better cyclability, while absorption-based systems provide higher volumetric capacity. Hybrid materials combining both mechanisms are being developed to leverage the advantages of each approach, aiming to meet the practical requirements for hydrogen storage applications.
- Novel composite and hybrid hydrogen storage materials: Composite and hybrid materials are being developed to overcome the limitations of single-mechanism hydrogen storage systems. These include metal hydride-MOF composites, nanostructured materials with enhanced surface properties, and multi-component systems that combine physisorption and chemisorption mechanisms. Core-shell structures, nanoconfinement approaches, and catalyst-doped systems are being explored to improve kinetics and thermodynamics of hydrogen storage. These hybrid approaches aim to operate at moderate temperatures and pressures while achieving both high gravimetric and volumetric hydrogen storage capacities, addressing the challenges of both adsorption and absorption-based systems.
02 Carbon-based materials for hydrogen adsorption
Carbon-based materials including activated carbon, carbon nanotubes, and graphene derivatives offer advantages for hydrogen storage through surface adsorption. These materials feature high specific surface areas, lightweight properties, and relatively low cost. The hydrogen storage capacity of carbon materials can be enhanced through various modification strategies such as doping with heteroatoms, creating defects, or incorporating metal nanoparticles. The adsorption performance is typically governed by surface area, pore structure, and surface chemistry.Expand Specific Solutions03 Metal hydrides for hydrogen absorption
Metal hydrides store hydrogen through chemical absorption, forming strong bonds between hydrogen and metal atoms. These materials can achieve high volumetric hydrogen densities exceeding that of liquid hydrogen. Various metal hydride systems have been developed, including light metal hydrides (like MgH2, LiH), complex hydrides (like alanates, borohydrides), and intermetallic compounds. The hydrogen absorption performance depends on factors such as operating temperature, pressure conditions, reaction kinetics, and cycling stability. Metal hydrides typically offer higher volumetric storage capacity compared to physisorption materials but often require higher temperatures for hydrogen release.Expand Specific Solutions04 Composite hydrogen storage materials
Composite hydrogen storage materials combine different storage mechanisms or material types to achieve enhanced performance. These may include metal hydride-carbon composites, MOF-polymer composites, or nanostructured multi-component systems. By integrating materials with complementary properties, these composites can overcome limitations of individual components, such as improving kinetics, reducing desorption temperatures, or enhancing cycling stability. The synergistic effects between components can lead to improved hydrogen storage capacity, faster sorption kinetics, and better thermal management.Expand Specific Solutions05 Comparative analysis of adsorption versus absorption mechanisms
Hydrogen storage through physisorption (adsorption) typically operates at low temperatures and offers fast kinetics but limited capacity, while chemisorption (absorption) provides higher storage densities but often requires elevated temperatures and exhibits slower kinetics. Physisorption materials like MOFs and carbon-based materials bind hydrogen through weak van der Waals forces, while absorption materials like metal hydrides form chemical bonds with hydrogen. The choice between adsorption and absorption depends on application requirements including operating conditions, gravimetric and volumetric capacity needs, cycling performance, and system complexity. Hybrid systems combining both mechanisms are being developed to leverage the advantages of each approach.Expand Specific Solutions
Leading Research Institutions and Industrial Players
Hydrogen storage materials market is in a growth phase, with increasing demand driven by clean energy transitions. The market size is expanding rapidly, particularly in automotive and energy sectors. Technologically, both adsorption and absorption methods show varying maturity levels. Toyota, Hyundai, and Honda lead in automotive applications with significant R&D investments in metal hydride systems. Academic institutions like EPFL and University of Tokyo contribute fundamental research, while specialized companies such as Santoku, GRZ Technologies, and Ilika focus on material innovations. The competition landscape shows automotive manufacturers partnering with research institutions to overcome density and efficiency challenges in hydrogen storage solutions.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered metal hydride-based hydrogen storage systems that primarily utilize absorption mechanisms. Their technology employs specialized alloys (typically Ti-Cr-V based) that chemically bond with hydrogen atoms within their crystal lattice structure. Toyota's approach achieves volumetric densities exceeding 40 g/L at moderate pressures (35-70 MPa), significantly higher than compressed gas storage. Their integrated thermal management systems address the exothermic absorption and endothermic desorption challenges, using vehicle cooling systems to regulate temperatures during refueling and operation. Toyota has also developed hybrid storage systems that combine absorption-based metal hydrides with adsorption materials to optimize both gravimetric and volumetric efficiency across different operating conditions, particularly for their fuel cell vehicles like the Mirai.
Strengths: Superior volumetric hydrogen density compared to compressed gas; operates at lower pressures than high-pressure tanks; excellent safety profile due to stable chemical bonding. Weaknesses: Higher system weight reducing gravimetric density; requires thermal management systems; relatively slow kinetics during rapid refueling scenarios; higher production costs compared to simpler storage methods.
Hyundai Motor Co., Ltd.
Technical Solution: Hyundai has developed a comprehensive hydrogen storage portfolio comparing both adsorption and absorption technologies. Their absorption-based approach centers on complex metal hydrides (primarily aluminum-based) that achieve hydrogen densities of 7.6 wt% at operating pressures below 10 MPa. These systems incorporate proprietary catalysts that reduce desorption temperatures to approximately 80-120°C, compatible with PEM fuel cell waste heat. For adsorption-based storage, Hyundai has pioneered carbon-based nanostructured materials including graphene-derived frameworks with surface areas exceeding 3500 m²/g. Their comparative analysis demonstrates that while absorption systems provide 30-40% higher volumetric density, their adsorption materials offer significantly faster kinetics with complete charging possible in under 5 minutes. Hyundai's research has particularly focused on the temperature-pressure relationships between the two storage mechanisms, developing predictive models that optimize storage efficiency across various operating conditions for their NEXO fuel cell vehicle platform.
Strengths: Balanced approach addressing both volumetric and gravimetric density requirements; carbon-based adsorption materials offer cost advantages over exotic MOFs; integrated thermal management systems leverage vehicle waste heat. Weaknesses: Complex metal hydrides face degradation after repeated cycling; adsorption materials require cryogenic conditions for maximum capacity; system complexity increases with hybrid approaches.
Key Patents and Scientific Breakthroughs
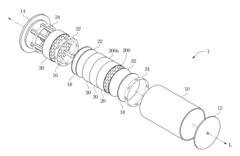
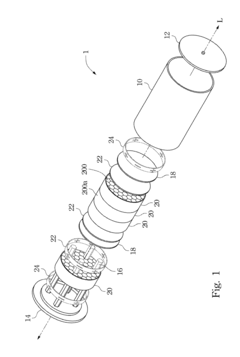
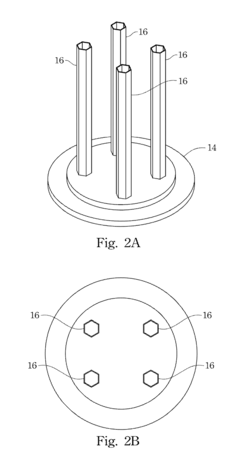
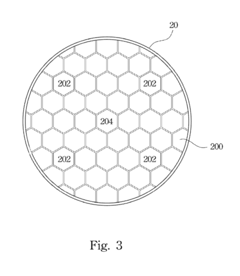
Hydrogen storage device
PatentInactiveUS20120132545A1
Innovation
- A hydrogen storage device design that includes a hydrogen storage can divided by partitions to manage stress and equipped with heat-conducting bars and through-holes to enhance heat transfer and hydrogen flow efficiency, preventing material degradation and improving the efficiency of hydrogen absorption and desorption.
Environmental Impact and Sustainability Assessment
The environmental impact of hydrogen storage technologies is a critical consideration in the transition toward a hydrogen-based economy. When comparing adsorption and absorption storage methods, their respective environmental footprints differ significantly throughout their lifecycle. Adsorption materials, such as carbon-based structures and metal-organic frameworks (MOFs), generally require less energy-intensive manufacturing processes compared to metal hydrides used in absorption systems.
Production of absorption materials, particularly complex metal hydrides, often involves energy-intensive metallurgical processes and rare earth elements that may present sustainability challenges. Mining these materials can lead to habitat disruption, water pollution, and significant carbon emissions. Conversely, carbon-based adsorption materials can be derived from more sustainable sources, including biomass, potentially offering a lower environmental impact during the production phase.
During operational use, absorption systems typically require higher temperatures for hydrogen release, resulting in greater energy consumption compared to adsorption systems that operate at more moderate conditions. This energy requirement directly correlates with increased greenhouse gas emissions if the energy source is not renewable. However, absorption materials generally demonstrate longer cycling stability, potentially reducing the environmental burden associated with material replacement and disposal.
End-of-life considerations reveal that many metal hydride materials used in absorption systems can be recycled, though the processes may be complex and energy-intensive. Adsorption materials, particularly those based on carbon, may offer simpler recycling pathways or biodegradability options, depending on their composition and manufacturing processes.
Water consumption presents another important environmental metric. Absorption systems often require cooling during hydrogenation due to exothermic reactions, potentially increasing water usage in regions where this resource is scarce. Adsorption systems typically have lower cooling requirements, presenting an advantage in water-stressed environments.
From a sustainability perspective, both technologies must be evaluated within the broader context of a hydrogen economy's development. The environmental benefits of either storage method ultimately depend on the source of hydrogen itself. If hydrogen is produced through electrolysis powered by renewable energy, the overall environmental impact is significantly reduced compared to hydrogen derived from fossil fuel reforming processes.
Life cycle assessment (LCA) studies indicate that while both technologies have environmental trade-offs, adsorption materials generally demonstrate lower embodied energy and reduced global warming potential when considering the complete lifecycle. However, continuous improvements in material science are narrowing this gap, with newer absorption materials showing enhanced environmental performance through reduced energy requirements and improved recyclability.
Production of absorption materials, particularly complex metal hydrides, often involves energy-intensive metallurgical processes and rare earth elements that may present sustainability challenges. Mining these materials can lead to habitat disruption, water pollution, and significant carbon emissions. Conversely, carbon-based adsorption materials can be derived from more sustainable sources, including biomass, potentially offering a lower environmental impact during the production phase.
During operational use, absorption systems typically require higher temperatures for hydrogen release, resulting in greater energy consumption compared to adsorption systems that operate at more moderate conditions. This energy requirement directly correlates with increased greenhouse gas emissions if the energy source is not renewable. However, absorption materials generally demonstrate longer cycling stability, potentially reducing the environmental burden associated with material replacement and disposal.
End-of-life considerations reveal that many metal hydride materials used in absorption systems can be recycled, though the processes may be complex and energy-intensive. Adsorption materials, particularly those based on carbon, may offer simpler recycling pathways or biodegradability options, depending on their composition and manufacturing processes.
Water consumption presents another important environmental metric. Absorption systems often require cooling during hydrogenation due to exothermic reactions, potentially increasing water usage in regions where this resource is scarce. Adsorption systems typically have lower cooling requirements, presenting an advantage in water-stressed environments.
From a sustainability perspective, both technologies must be evaluated within the broader context of a hydrogen economy's development. The environmental benefits of either storage method ultimately depend on the source of hydrogen itself. If hydrogen is produced through electrolysis powered by renewable energy, the overall environmental impact is significantly reduced compared to hydrogen derived from fossil fuel reforming processes.
Life cycle assessment (LCA) studies indicate that while both technologies have environmental trade-offs, adsorption materials generally demonstrate lower embodied energy and reduced global warming potential when considering the complete lifecycle. However, continuous improvements in material science are narrowing this gap, with newer absorption materials showing enhanced environmental performance through reduced energy requirements and improved recyclability.
Policy Framework and Standardization Requirements
The development of hydrogen storage technologies requires robust policy frameworks and standardization to ensure safety, interoperability, and market adoption. Currently, policies governing hydrogen storage materials vary significantly across regions, creating challenges for global technology deployment. The European Union has established the Hydrogen Strategy with specific regulations for storage materials, emphasizing safety parameters for both adsorption and absorption technologies. Similarly, the United States Department of Energy has implemented the Hydrogen Program Plan with technical targets for storage materials, including gravimetric capacity (7-9 wt% for system-level storage) and volumetric density requirements.
International standardization efforts are primarily led by the International Organization for Standardization (ISO) through its Technical Committee 197, which has developed standards such as ISO 16111 for hydrogen absorbed in reversible metal hydrides. However, comprehensive standards specifically addressing the comparative performance metrics between adsorption and absorption materials remain underdeveloped, creating barriers to technology comparison and market entry.
Safety regulations represent a critical component of the policy framework, with distinct requirements for different storage mechanisms. Absorption materials, particularly metal hydrides, face stringent thermal management regulations due to exothermic hydrogen uptake processes. Conversely, adsorption materials, especially MOFs and carbon-based materials, encounter regulations focused on pressure containment and material stability under cycling conditions.
Certification protocols for hydrogen storage materials differ substantially between regions, necessitating harmonization efforts. The development of universal testing methodologies for comparing adsorption versus absorption performance would significantly accelerate commercialization pathways. Current certification gaps include standardized cycling durability tests, impurity tolerance assessments, and system-level performance validation under varied environmental conditions.
Financial incentives and research funding mechanisms also play a crucial role in advancing hydrogen storage technologies. Several countries have implemented targeted funding programs for material development, with the EU's Horizon Europe and Japan's Green Innovation Fund allocating substantial resources specifically for advanced storage materials research. These programs increasingly require standardized performance reporting to facilitate technology comparison and investment decisions.
Future policy development should focus on creating technology-neutral frameworks that establish performance-based standards rather than prescriptive requirements. This approach would enable fair comparison between adsorption and absorption technologies while fostering innovation. Additionally, international collaboration on certification mutual recognition would reduce market entry barriers and accelerate global deployment of optimal hydrogen storage solutions regardless of the underlying material technology.
International standardization efforts are primarily led by the International Organization for Standardization (ISO) through its Technical Committee 197, which has developed standards such as ISO 16111 for hydrogen absorbed in reversible metal hydrides. However, comprehensive standards specifically addressing the comparative performance metrics between adsorption and absorption materials remain underdeveloped, creating barriers to technology comparison and market entry.
Safety regulations represent a critical component of the policy framework, with distinct requirements for different storage mechanisms. Absorption materials, particularly metal hydrides, face stringent thermal management regulations due to exothermic hydrogen uptake processes. Conversely, adsorption materials, especially MOFs and carbon-based materials, encounter regulations focused on pressure containment and material stability under cycling conditions.
Certification protocols for hydrogen storage materials differ substantially between regions, necessitating harmonization efforts. The development of universal testing methodologies for comparing adsorption versus absorption performance would significantly accelerate commercialization pathways. Current certification gaps include standardized cycling durability tests, impurity tolerance assessments, and system-level performance validation under varied environmental conditions.
Financial incentives and research funding mechanisms also play a crucial role in advancing hydrogen storage technologies. Several countries have implemented targeted funding programs for material development, with the EU's Horizon Europe and Japan's Green Innovation Fund allocating substantial resources specifically for advanced storage materials research. These programs increasingly require standardized performance reporting to facilitate technology comparison and investment decisions.
Future policy development should focus on creating technology-neutral frameworks that establish performance-based standards rather than prescriptive requirements. This approach would enable fair comparison between adsorption and absorption technologies while fostering innovation. Additionally, international collaboration on certification mutual recognition would reduce market entry barriers and accelerate global deployment of optimal hydrogen storage solutions regardless of the underlying material technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!