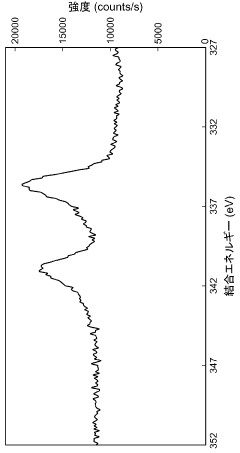
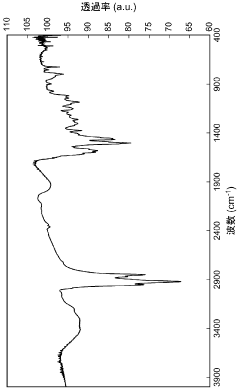
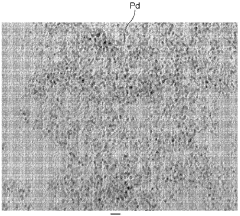
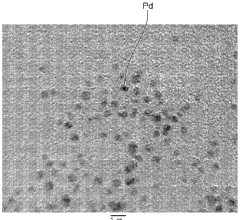
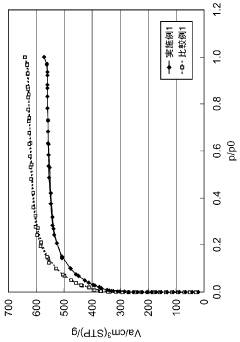
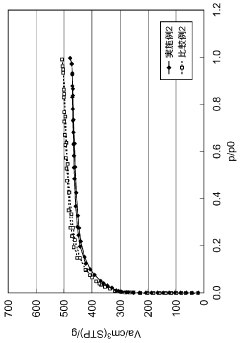
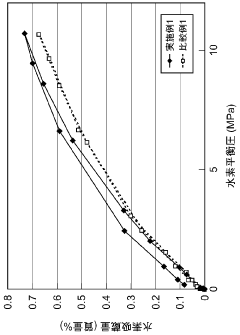
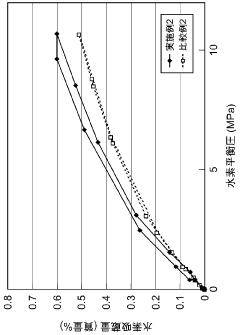
What are the key interface factors affecting Hydrogen storage materials efficiency
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage materials have emerged as a critical component in the global transition towards a hydrogen-based clean energy economy. The development of these materials dates back to the 1970s energy crisis, which prompted intensive research into alternative energy carriers. Over the past five decades, significant advancements have been made in understanding the fundamental mechanisms of hydrogen-material interactions, leading to the development of various storage solutions including metal hydrides, complex hydrides, chemical hydrides, and physisorption materials.
The evolution of hydrogen storage technology has been characterized by progressive improvements in storage capacity, kinetics, and operational conditions. Early research focused primarily on conventional metal hydrides, which offered moderate storage capacities but suffered from slow kinetics and high operating temperatures. Recent technological trends have shifted towards nanomaterials, multi-component systems, and catalyst-enhanced storage materials that demonstrate improved performance metrics.
The primary objective in hydrogen storage material development is to achieve the U.S. Department of Energy's technical targets: a gravimetric capacity of 6.5 wt% and volumetric capacity of 50 g/L, with operating temperatures between -40°C and 60°C, and filling times under 3.3 minutes. These benchmarks are essential for practical implementation in mobile and stationary applications.
Interface factors represent a critical frontier in hydrogen storage research. The hydrogen-material interface governs adsorption/desorption processes, which directly impact storage efficiency. Key interface parameters include surface area, pore structure, surface chemistry, and interfacial energy barriers. Understanding these factors is essential for designing materials with optimized hydrogen uptake and release characteristics.
Current research aims to elucidate the molecular-level interactions at material interfaces, particularly focusing on how surface defects, dopants, and catalysts influence hydrogen dissociation and recombination. Advanced characterization techniques such as in-situ neutron diffraction, synchrotron X-ray analysis, and computational modeling have become instrumental in probing these interface phenomena.
The technological trajectory indicates a convergence of multiple disciplines—materials science, surface chemistry, nanotechnology, and computational modeling—to address interface challenges. Future developments are expected to focus on hierarchical material architectures that optimize interface properties across multiple length scales, from atomic to macroscopic dimensions.
Achieving breakthrough performance in hydrogen storage materials requires systematic investigation of interface factors, including surface functionalization strategies, catalyst integration methods, and novel material synthesis approaches that can precisely control interface properties to enhance hydrogen storage efficiency.
The evolution of hydrogen storage technology has been characterized by progressive improvements in storage capacity, kinetics, and operational conditions. Early research focused primarily on conventional metal hydrides, which offered moderate storage capacities but suffered from slow kinetics and high operating temperatures. Recent technological trends have shifted towards nanomaterials, multi-component systems, and catalyst-enhanced storage materials that demonstrate improved performance metrics.
The primary objective in hydrogen storage material development is to achieve the U.S. Department of Energy's technical targets: a gravimetric capacity of 6.5 wt% and volumetric capacity of 50 g/L, with operating temperatures between -40°C and 60°C, and filling times under 3.3 minutes. These benchmarks are essential for practical implementation in mobile and stationary applications.
Interface factors represent a critical frontier in hydrogen storage research. The hydrogen-material interface governs adsorption/desorption processes, which directly impact storage efficiency. Key interface parameters include surface area, pore structure, surface chemistry, and interfacial energy barriers. Understanding these factors is essential for designing materials with optimized hydrogen uptake and release characteristics.
Current research aims to elucidate the molecular-level interactions at material interfaces, particularly focusing on how surface defects, dopants, and catalysts influence hydrogen dissociation and recombination. Advanced characterization techniques such as in-situ neutron diffraction, synchrotron X-ray analysis, and computational modeling have become instrumental in probing these interface phenomena.
The technological trajectory indicates a convergence of multiple disciplines—materials science, surface chemistry, nanotechnology, and computational modeling—to address interface challenges. Future developments are expected to focus on hierarchical material architectures that optimize interface properties across multiple length scales, from atomic to macroscopic dimensions.
Achieving breakthrough performance in hydrogen storage materials requires systematic investigation of interface factors, including surface functionalization strategies, catalyst integration methods, and novel material synthesis approaches that can precisely control interface properties to enhance hydrogen storage efficiency.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. Current market valuations place the hydrogen storage sector at approximately $15 billion as of 2023, with projections indicating a compound annual growth rate of 8-12% through 2030, potentially reaching $30-35 billion by decade's end.
Demand for efficient hydrogen storage solutions spans multiple sectors. The transportation industry represents the largest market segment, accounting for roughly 40% of current demand, as automotive manufacturers accelerate development of hydrogen fuel cell vehicles. Industrial applications follow at 30%, with energy storage systems and power generation collectively representing about 25% of market share.
Regional analysis reveals Asia-Pacific as the dominant market, holding approximately 45% of global market share, led by Japan, South Korea, and increasingly China. Europe follows at 30%, with particularly strong growth in Germany, France, and the Nordic countries due to aggressive hydrogen strategy implementations. North America accounts for approximately 20% of the market, with concentrated development in California and the northeastern United States.
Customer requirements are evolving rapidly, with key demands focusing on increased storage density, improved safety profiles, and reduced system costs. End-users consistently cite storage efficiency as the primary concern, with current solutions still falling short of U.S. Department of Energy targets for volumetric and gravimetric capacity.
Market barriers include high infrastructure costs, with hydrogen storage systems typically representing 30-40% of total hydrogen implementation expenses. Technical limitations of current materials also restrict market expansion, particularly in mobile applications where weight and volume constraints are critical. Regulatory frameworks remain inconsistent across regions, creating market fragmentation and impeding standardization efforts.
Emerging market opportunities exist in integrating hydrogen storage with renewable energy systems, particularly for grid stabilization and seasonal energy storage. The materials science sector is experiencing increased investment, with venture capital funding for advanced hydrogen storage materials research growing by approximately 65% between 2020 and 2023.
Price sensitivity analysis indicates that breakthrough materials improving interface efficiency could potentially reduce overall system costs by 25-35%, representing a critical threshold for widespread commercial adoption across multiple sectors.
Demand for efficient hydrogen storage solutions spans multiple sectors. The transportation industry represents the largest market segment, accounting for roughly 40% of current demand, as automotive manufacturers accelerate development of hydrogen fuel cell vehicles. Industrial applications follow at 30%, with energy storage systems and power generation collectively representing about 25% of market share.
Regional analysis reveals Asia-Pacific as the dominant market, holding approximately 45% of global market share, led by Japan, South Korea, and increasingly China. Europe follows at 30%, with particularly strong growth in Germany, France, and the Nordic countries due to aggressive hydrogen strategy implementations. North America accounts for approximately 20% of the market, with concentrated development in California and the northeastern United States.
Customer requirements are evolving rapidly, with key demands focusing on increased storage density, improved safety profiles, and reduced system costs. End-users consistently cite storage efficiency as the primary concern, with current solutions still falling short of U.S. Department of Energy targets for volumetric and gravimetric capacity.
Market barriers include high infrastructure costs, with hydrogen storage systems typically representing 30-40% of total hydrogen implementation expenses. Technical limitations of current materials also restrict market expansion, particularly in mobile applications where weight and volume constraints are critical. Regulatory frameworks remain inconsistent across regions, creating market fragmentation and impeding standardization efforts.
Emerging market opportunities exist in integrating hydrogen storage with renewable energy systems, particularly for grid stabilization and seasonal energy storage. The materials science sector is experiencing increased investment, with venture capital funding for advanced hydrogen storage materials research growing by approximately 65% between 2020 and 2023.
Price sensitivity analysis indicates that breakthrough materials improving interface efficiency could potentially reduce overall system costs by 25-35%, representing a critical threshold for widespread commercial adoption across multiple sectors.
Interface Challenges in Current Hydrogen Storage Technologies
The interface between hydrogen storage materials and hydrogen molecules represents a critical frontier in advancing hydrogen storage technologies. Current hydrogen storage systems face significant challenges at these interfaces, limiting overall system efficiency and practical application. The primary interface challenge lies in the hydrogen adsorption/desorption kinetics, where the interaction between hydrogen molecules and storage material surfaces determines the rate and efficiency of hydrogen uptake and release.
Surface area accessibility presents another major interface challenge. Many promising materials with high theoretical storage capacities suffer from limited practical performance due to inadequate surface exposure to hydrogen molecules. This is particularly evident in metal-organic frameworks (MOFs) and porous carbon materials, where pore blockage and collapse under operating conditions can significantly reduce accessible interface area.
Interfacial heat transfer represents a substantial technical barrier, especially in metal hydride systems. The exothermic nature of hydrogen absorption and endothermic desorption processes creates thermal management challenges at material interfaces. Insufficient heat dissipation during charging can lead to temperature increases that inhibit further hydrogen uptake, while inadequate heat supply during discharge slows hydrogen release rates.
Chemical stability at interfaces poses long-term durability concerns. Many storage materials undergo surface degradation through oxidation, contamination, or structural reorganization after multiple hydrogen cycling events. This degradation progressively alters the interface properties, resulting in diminished storage capacity and slower kinetics over time.
Catalyst integration at interfaces presents both opportunities and challenges. While catalysts can dramatically improve hydrogen dissociation and recombination rates, achieving uniform catalyst distribution and maintaining catalyst activity at interfaces remains problematic. Catalyst agglomeration and poisoning at material interfaces frequently lead to performance deterioration.
Interface engineering challenges extend to composite storage systems, where different materials with complementary properties are combined. The boundaries between these materials often create resistance to hydrogen diffusion and heat transfer, limiting the theoretical benefits of such hybrid approaches.
Nanoscale interface phenomena introduce additional complexities, as quantum effects and surface energetics at the nanoscale can significantly alter hydrogen binding energies and diffusion barriers. Understanding and controlling these nanoscale interface effects remains an ongoing scientific challenge that requires advanced characterization techniques and computational modeling approaches.
Surface area accessibility presents another major interface challenge. Many promising materials with high theoretical storage capacities suffer from limited practical performance due to inadequate surface exposure to hydrogen molecules. This is particularly evident in metal-organic frameworks (MOFs) and porous carbon materials, where pore blockage and collapse under operating conditions can significantly reduce accessible interface area.
Interfacial heat transfer represents a substantial technical barrier, especially in metal hydride systems. The exothermic nature of hydrogen absorption and endothermic desorption processes creates thermal management challenges at material interfaces. Insufficient heat dissipation during charging can lead to temperature increases that inhibit further hydrogen uptake, while inadequate heat supply during discharge slows hydrogen release rates.
Chemical stability at interfaces poses long-term durability concerns. Many storage materials undergo surface degradation through oxidation, contamination, or structural reorganization after multiple hydrogen cycling events. This degradation progressively alters the interface properties, resulting in diminished storage capacity and slower kinetics over time.
Catalyst integration at interfaces presents both opportunities and challenges. While catalysts can dramatically improve hydrogen dissociation and recombination rates, achieving uniform catalyst distribution and maintaining catalyst activity at interfaces remains problematic. Catalyst agglomeration and poisoning at material interfaces frequently lead to performance deterioration.
Interface engineering challenges extend to composite storage systems, where different materials with complementary properties are combined. The boundaries between these materials often create resistance to hydrogen diffusion and heat transfer, limiting the theoretical benefits of such hybrid approaches.
Nanoscale interface phenomena introduce additional complexities, as quantum effects and surface energetics at the nanoscale can significantly alter hydrogen binding energies and diffusion barriers. Understanding and controlling these nanoscale interface effects remains an ongoing scientific challenge that requires advanced characterization techniques and computational modeling approaches.
Current Interface Engineering Approaches
01 Metal hydride-based hydrogen storage materials
Metal hydrides are compounds that can store hydrogen through chemical bonding. These materials can absorb hydrogen under pressure and release it when heated, making them effective for hydrogen storage. Various metal hydrides, including those based on magnesium, aluminum, and transition metals, offer different storage capacities and operating conditions. Research focuses on improving their hydrogen storage capacity, kinetics, and cycling stability to enhance overall efficiency.- Metal hydride-based hydrogen storage materials: Metal hydrides are compounds formed by hydrogen and various metals or alloys that can store hydrogen through chemical bonding. These materials offer high volumetric hydrogen storage capacity and can release hydrogen through heating or pressure reduction. The efficiency of metal hydride storage systems depends on factors such as operating temperature, pressure conditions, and the specific metal or alloy composition used. Research focuses on improving absorption/desorption kinetics and reducing the weight of these systems to enhance overall efficiency.
- Carbon-based hydrogen storage materials: Carbon-based materials including carbon nanotubes, graphene, and activated carbon are being developed for hydrogen storage applications. These materials store hydrogen through adsorption mechanisms, where hydrogen molecules adhere to the surface of the carbon structure. The efficiency of carbon-based storage systems is determined by their specific surface area, pore structure, and operating conditions. Researchers are working on surface modifications and doping techniques to enhance the hydrogen binding energy and improve storage capacity at practical temperatures and pressures.
- Chemical hydrogen storage systems: Chemical hydrogen storage involves materials that release hydrogen through chemical reactions. Examples include ammonia borane, sodium borohydride, and formic acid. These systems can achieve high hydrogen storage densities but often face challenges with regeneration efficiency. Research is focused on developing catalysts to improve hydrogen release rates at lower temperatures and creating more energy-efficient regeneration processes. The overall system efficiency depends on both the hydrogen release reaction and the energy required for regeneration of the spent material.
- Composite and hybrid hydrogen storage materials: Composite and hybrid materials combine different hydrogen storage mechanisms to overcome limitations of single-material approaches. These may include metal hydride-carbon composites, metal-organic frameworks (MOFs), or systems that integrate physical and chemical storage methods. By combining complementary materials, these systems can achieve improved kinetics, reduced operating temperatures, and enhanced cycling stability. The efficiency of composite systems depends on the synergistic effects between components and optimized material interfaces that facilitate hydrogen transfer and storage.
- System design and engineering for hydrogen storage efficiency: Beyond material properties, the overall efficiency of hydrogen storage systems depends significantly on engineering design factors. These include thermal management systems, pressure control mechanisms, and integration with fuel cells or other hydrogen utilization technologies. Advanced designs incorporate heat recovery systems to capture and utilize the heat generated during hydrogen absorption, reducing energy losses. Modular and scalable designs are being developed to optimize efficiency across different applications, from portable devices to stationary energy storage and transportation.
02 Carbon-based hydrogen storage materials
Carbon-based materials such as carbon nanotubes, graphene, and activated carbon are being investigated for hydrogen storage applications. These materials can store hydrogen through adsorption mechanisms, where hydrogen molecules are attracted to the surface of the carbon structure. The high surface area and tunable pore structure of carbon materials make them promising candidates for efficient hydrogen storage, though challenges remain in achieving high storage capacity at ambient conditions.Expand Specific Solutions03 Complex hydride systems for hydrogen storage
Complex hydrides, including borohydrides, alanates, and amides, offer high theoretical hydrogen storage capacities. These materials store hydrogen through chemical bonds and can release hydrogen through thermal decomposition or catalytic reactions. Research is focused on improving their reversibility, reducing desorption temperatures, and enhancing kinetics through catalysts and nanostructuring to make them more efficient for practical hydrogen storage applications.Expand Specific Solutions04 Hydrogen storage system design and efficiency
The design of hydrogen storage systems significantly impacts overall efficiency. This includes considerations of heat management during absorption and desorption processes, pressure control systems, and integration with fuel cells or other hydrogen utilization technologies. Advanced system designs incorporate thermal management solutions, pressure regulation mechanisms, and optimized container geometries to maximize volumetric and gravimetric efficiency while ensuring safe and reliable operation.Expand Specific Solutions05 Novel composite materials for enhanced hydrogen storage
Composite materials that combine different hydrogen storage mechanisms offer improved performance compared to single-material systems. These composites may integrate metal hydrides with carbon materials, incorporate catalysts to enhance kinetics, or utilize nanoscale engineering to optimize hydrogen diffusion pathways. By synergistically combining materials with complementary properties, these composites can achieve higher storage capacities, faster kinetics, and improved cycling stability, addressing multiple challenges in hydrogen storage efficiency.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
The hydrogen storage materials efficiency landscape is evolving rapidly, currently transitioning from early-stage research to commercial applications. The market is projected to grow significantly as hydrogen gains importance in clean energy transitions. Technologically, the field shows varying maturity levels across different storage approaches. Leading players include automotive manufacturers like Hyundai, Kia, and Nissan, who are integrating hydrogen technologies into vehicle platforms. Research institutions such as Japan Science & Technology Agency and Deutsches Zentrum für Luft- und Raumfahrt are advancing fundamental science, while specialized companies like GRZ Technologies and GKN Hydrogen are developing commercial storage solutions with proprietary technologies addressing key interface challenges. Academic institutions including Fudan University and University of Houston contribute significant research to overcome material interface limitations.
Hyundai Motor Co., Ltd.
Technical Solution: Hyundai has developed advanced hydrogen storage systems for their fuel cell vehicles that address multiple interface factors affecting efficiency. Their technology employs Type IV carbon fiber-reinforced polymer tanks with specialized hydrogen-compatible polymer liners that minimize permeation losses at the material interface. Hyundai's systems operate at 700 bar pressure and incorporate precisely engineered pressure-temperature interfaces to optimize hydrogen density while maintaining safety parameters. The company has developed proprietary interface materials between the storage system and fuel cell that regulate hydrogen flow rates based on demand, improving overall system efficiency. Their latest storage systems feature advanced thermal management interfaces that maintain optimal operating temperatures (typically -40°C to 85°C) across varying ambient conditions and usage patterns. Hyundai has also addressed the critical gas-solid interface in their refueling systems, developing pre-cooling technology that manages the heat generated during rapid refueling, allowing their vehicles to achieve a full refuel in under 5 minutes while preventing temperature-related efficiency losses. Their NEXO fuel cell vehicle incorporates three hydrogen tanks with a total capacity of 6.33 kg, providing a driving range of approximately 666 km.
Strengths: High-pressure system provides excellent gravimetric storage density (around 5.7 wt%); rapid refueling capability; proven durability in commercial applications with over 10,000 vehicles deployed. Weaknesses: Requires expensive carbon fiber reinforcement for high-pressure containment; energy penalty for compression; limited volumetric efficiency compared to some advanced material-based storage systems.
GRZ Technologies SA
Technical Solution: GRZ Technologies has developed a proprietary metal hydride-based hydrogen storage system that addresses key interface factors affecting efficiency. Their technology utilizes specially designed metal alloys with optimized surface properties to enhance hydrogen adsorption and desorption kinetics. The system incorporates advanced heat management interfaces that efficiently distribute and remove heat during hydrogen charging and discharging processes, which is critical as the hydrogen absorption process is exothermic while desorption is endothermic. GRZ's innovation includes precisely engineered porous structures with controlled particle sizes (typically in the nanometer range) that maximize the surface area for hydrogen interaction while minimizing diffusion distances. Their systems also feature specialized surface treatments that prevent oxidation and contamination of the metal hydride interfaces, which can significantly degrade storage capacity over time. The company has demonstrated storage densities exceeding 100 kg H₂/m³, substantially higher than compressed gas storage at comparable pressures.
Strengths: Superior volumetric storage density compared to compressed hydrogen; operates at moderate pressures (30-50 bar) enhancing safety; excellent thermal management system integration. Weaknesses: Higher system weight compared to some alternatives; requires precise temperature control for optimal operation; potential for performance degradation if exposed to certain contaminants.
Critical Interface Mechanisms Analysis
Hydrogen storage material
PatentWO2009154200A1
Innovation
- A hydrogen storage material comprising fine metal particles bonded with an organic compound having characteristic groups, which increases the surface area and forms voids for enhanced hydrogen absorption, both chemically and physically, using metals like Pd, V, and Ti, and organic compounds with aromatic rings and specific functional groups for improved bonding and rigidity.
Hydrogen storage material
PatentWO2011046001A1
Innovation
- A hydrogen storage material comprising a porous carbon material with oxygen-containing functional groups and Li bonded to its surface, specifically on the inner walls of its pores, which enhances hydrogen storage capacity through strong adsorption.
Regulatory Framework for Hydrogen Storage Systems
The regulatory landscape for hydrogen storage systems has evolved significantly in response to the growing importance of hydrogen as a clean energy carrier. International standards such as ISO/TC 197 provide comprehensive guidelines for hydrogen technologies, including specific requirements for storage materials and interface factors that affect efficiency. These standards address critical safety parameters, material compatibility, and performance metrics that manufacturers must adhere to.
National regulatory frameworks vary considerably across regions, with countries like Japan, Germany, and the United States leading in establishing robust hydrogen storage regulations. The European Union's Renewable Energy Directive II and the Alternative Fuels Infrastructure Directive contain specific provisions related to hydrogen storage systems, emphasizing material efficiency and interface optimization. Similarly, the U.S. Department of Energy has established technical targets for hydrogen storage systems that directly address interface factors affecting material efficiency.
Safety regulations constitute a significant portion of the regulatory framework, focusing on pressure management, thermal stability, and material degradation prevention. These regulations often mandate specific testing protocols to evaluate how interface factors such as surface contamination, catalyst distribution, and boundary layer phenomena affect overall storage efficiency. Compliance with these safety standards necessitates sophisticated material design that optimizes hydrogen uptake and release kinetics.
Environmental regulations increasingly influence hydrogen storage material development, with requirements for lifecycle assessment and recyclability becoming more stringent. Regulations now commonly address the environmental impact of rare earth elements and catalysts used at material interfaces to enhance hydrogen storage capacity. These environmental considerations directly impact material selection and interface engineering decisions.
Certification processes for hydrogen storage systems require rigorous testing of material interfaces under various operating conditions. Testing protocols typically evaluate how temperature fluctuations, pressure cycling, and impurity exposure affect interface properties and overall system efficiency. Material manufacturers must demonstrate compliance with these protocols to obtain necessary certifications for commercial deployment.
Emerging regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing greater innovation in interface engineering while maintaining safety. Regulatory harmonization efforts are underway internationally to create consistent standards for hydrogen storage materials, with particular attention to interface factors that determine efficiency across different applications from stationary storage to mobile applications in the transportation sector.
National regulatory frameworks vary considerably across regions, with countries like Japan, Germany, and the United States leading in establishing robust hydrogen storage regulations. The European Union's Renewable Energy Directive II and the Alternative Fuels Infrastructure Directive contain specific provisions related to hydrogen storage systems, emphasizing material efficiency and interface optimization. Similarly, the U.S. Department of Energy has established technical targets for hydrogen storage systems that directly address interface factors affecting material efficiency.
Safety regulations constitute a significant portion of the regulatory framework, focusing on pressure management, thermal stability, and material degradation prevention. These regulations often mandate specific testing protocols to evaluate how interface factors such as surface contamination, catalyst distribution, and boundary layer phenomena affect overall storage efficiency. Compliance with these safety standards necessitates sophisticated material design that optimizes hydrogen uptake and release kinetics.
Environmental regulations increasingly influence hydrogen storage material development, with requirements for lifecycle assessment and recyclability becoming more stringent. Regulations now commonly address the environmental impact of rare earth elements and catalysts used at material interfaces to enhance hydrogen storage capacity. These environmental considerations directly impact material selection and interface engineering decisions.
Certification processes for hydrogen storage systems require rigorous testing of material interfaces under various operating conditions. Testing protocols typically evaluate how temperature fluctuations, pressure cycling, and impurity exposure affect interface properties and overall system efficiency. Material manufacturers must demonstrate compliance with these protocols to obtain necessary certifications for commercial deployment.
Emerging regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing greater innovation in interface engineering while maintaining safety. Regulatory harmonization efforts are underway internationally to create consistent standards for hydrogen storage materials, with particular attention to interface factors that determine efficiency across different applications from stationary storage to mobile applications in the transportation sector.
Safety Considerations in Material Interface Design
Safety considerations in hydrogen storage material interface design are paramount due to hydrogen's high flammability and potential for embrittlement. The interface between hydrogen and storage materials presents unique challenges that must be addressed to ensure operational safety and system integrity.
Material degradation at interfaces represents a critical safety concern. Hydrogen can cause embrittlement in many metals, particularly at interface boundaries where stress concentrations exist. This phenomenon occurs when hydrogen atoms penetrate the material structure, reducing ductility and tensile strength, potentially leading to catastrophic failure under normal operating conditions. Advanced interface designs must incorporate materials resistant to hydrogen embrittlement or protective barrier layers to mitigate this risk.
Thermal management at material interfaces requires careful consideration. The adsorption and desorption of hydrogen are typically exothermic and endothermic processes respectively, creating significant temperature gradients at interfaces. Inadequate thermal management can lead to hotspots, thermal runaway, or material degradation. Interface designs must incorporate efficient heat transfer mechanisms to maintain temperature uniformity and prevent localized overheating.
Leakage prevention represents another crucial safety aspect. Hydrogen molecules are extremely small and can diffuse through many conventional materials, particularly at interface junctions. This necessitates specialized sealing technologies and interface designs that minimize potential escape pathways. Composite interfaces with multiple barrier layers have shown promise in reducing permeation rates while maintaining operational efficiency.
Chemical stability between hydrogen and interface materials must be ensured throughout the storage system's lifecycle. Some materials may catalyze unwanted side reactions or decompose when exposed to hydrogen, particularly under pressure or temperature fluctuations. Interface designs should incorporate chemically compatible materials that remain stable under all anticipated operating conditions, preventing the formation of potentially hazardous compounds.
Monitoring and detection systems integrated at critical interfaces provide an additional safety layer. Advanced sensor technologies capable of detecting hydrogen leakage, material stress, or temperature anomalies at interfaces allow for early intervention before safety-critical situations develop. These systems should be designed with redundancy and positioned at vulnerable interface points throughout the storage system.
Standardized safety protocols for interface design and testing are essential for industry-wide implementation. These should include accelerated aging tests that simulate long-term exposure effects at interfaces, cyclic loading to evaluate fatigue resistance, and extreme condition testing to determine safety margins. Such comprehensive testing regimes ensure that interface designs maintain their integrity throughout the expected service life of hydrogen storage systems.
Material degradation at interfaces represents a critical safety concern. Hydrogen can cause embrittlement in many metals, particularly at interface boundaries where stress concentrations exist. This phenomenon occurs when hydrogen atoms penetrate the material structure, reducing ductility and tensile strength, potentially leading to catastrophic failure under normal operating conditions. Advanced interface designs must incorporate materials resistant to hydrogen embrittlement or protective barrier layers to mitigate this risk.
Thermal management at material interfaces requires careful consideration. The adsorption and desorption of hydrogen are typically exothermic and endothermic processes respectively, creating significant temperature gradients at interfaces. Inadequate thermal management can lead to hotspots, thermal runaway, or material degradation. Interface designs must incorporate efficient heat transfer mechanisms to maintain temperature uniformity and prevent localized overheating.
Leakage prevention represents another crucial safety aspect. Hydrogen molecules are extremely small and can diffuse through many conventional materials, particularly at interface junctions. This necessitates specialized sealing technologies and interface designs that minimize potential escape pathways. Composite interfaces with multiple barrier layers have shown promise in reducing permeation rates while maintaining operational efficiency.
Chemical stability between hydrogen and interface materials must be ensured throughout the storage system's lifecycle. Some materials may catalyze unwanted side reactions or decompose when exposed to hydrogen, particularly under pressure or temperature fluctuations. Interface designs should incorporate chemically compatible materials that remain stable under all anticipated operating conditions, preventing the formation of potentially hazardous compounds.
Monitoring and detection systems integrated at critical interfaces provide an additional safety layer. Advanced sensor technologies capable of detecting hydrogen leakage, material stress, or temperature anomalies at interfaces allow for early intervention before safety-critical situations develop. These systems should be designed with redundancy and positioned at vulnerable interface points throughout the storage system.
Standardized safety protocols for interface design and testing are essential for industry-wide implementation. These should include accelerated aging tests that simulate long-term exposure effects at interfaces, cyclic loading to evaluate fatigue resistance, and extreme condition testing to determine safety margins. Such comprehensive testing regimes ensure that interface designs maintain their integrity throughout the expected service life of hydrogen storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!