Comparative study of Hydrogen storage materials for catalyst performance and durability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Evolution and Research Objectives
Hydrogen storage has evolved significantly over the past decades, transitioning from conventional physical methods to advanced material-based approaches. The journey began with compressed gas and cryogenic liquid storage, which despite their simplicity, presented significant safety concerns and energy inefficiencies. The 1970s energy crisis catalyzed research into alternative storage methods, leading to the exploration of metal hydrides as potential hydrogen carriers. These early materials, while promising, suffered from limited capacity and poor kinetics.
The 1990s witnessed a paradigm shift with the discovery of carbon nanostructures capable of hydrogen adsorption. This period marked the beginning of nanomaterial engineering for hydrogen storage, expanding the material palette to include metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and complex hydrides. Each generation of materials has progressively addressed key challenges in hydrogen storage: gravimetric and volumetric capacity, operating conditions, and cycling stability.
Recent advancements have focused on catalyst integration to enhance hydrogen sorption kinetics and material durability. Catalysts based on noble metals (platinum, palladium), transition metals (nickel, cobalt), and their alloys have demonstrated remarkable abilities to lower activation energies for hydrogen uptake and release. However, catalyst performance degradation remains a critical challenge, particularly under the repeated cycling conditions required for practical applications.
The current research landscape is characterized by a multidisciplinary approach, combining computational modeling, advanced characterization techniques, and high-throughput experimentation to develop next-generation storage materials. International collaborations, such as the International Energy Agency's Hydrogen Implementation Agreement, have established benchmarks for hydrogen storage materials, guiding global research efforts.
This technical pre-research aims to comprehensively evaluate the evolution and current state of hydrogen storage materials with specific emphasis on catalyst performance and durability. The primary objectives include: identifying material classes with optimal hydrogen storage properties; assessing catalyst-substrate interactions that influence performance metrics; analyzing degradation mechanisms under various operating conditions; and establishing correlations between material composition, structure, and long-term stability.
Additionally, this study seeks to develop predictive models for catalyst lifetime in different storage materials, propose design principles for enhanced durability, and establish standardized testing protocols for comparative evaluation. The ultimate goal is to identify promising material-catalyst combinations that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity, 50 g/L volumetric capacity, with operational stability over 1,500 cycles under practical temperature and pressure conditions.
The 1990s witnessed a paradigm shift with the discovery of carbon nanostructures capable of hydrogen adsorption. This period marked the beginning of nanomaterial engineering for hydrogen storage, expanding the material palette to include metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and complex hydrides. Each generation of materials has progressively addressed key challenges in hydrogen storage: gravimetric and volumetric capacity, operating conditions, and cycling stability.
Recent advancements have focused on catalyst integration to enhance hydrogen sorption kinetics and material durability. Catalysts based on noble metals (platinum, palladium), transition metals (nickel, cobalt), and their alloys have demonstrated remarkable abilities to lower activation energies for hydrogen uptake and release. However, catalyst performance degradation remains a critical challenge, particularly under the repeated cycling conditions required for practical applications.
The current research landscape is characterized by a multidisciplinary approach, combining computational modeling, advanced characterization techniques, and high-throughput experimentation to develop next-generation storage materials. International collaborations, such as the International Energy Agency's Hydrogen Implementation Agreement, have established benchmarks for hydrogen storage materials, guiding global research efforts.
This technical pre-research aims to comprehensively evaluate the evolution and current state of hydrogen storage materials with specific emphasis on catalyst performance and durability. The primary objectives include: identifying material classes with optimal hydrogen storage properties; assessing catalyst-substrate interactions that influence performance metrics; analyzing degradation mechanisms under various operating conditions; and establishing correlations between material composition, structure, and long-term stability.
Additionally, this study seeks to develop predictive models for catalyst lifetime in different storage materials, propose design principles for enhanced durability, and establish standardized testing protocols for comparative evaluation. The ultimate goal is to identify promising material-catalyst combinations that meet the U.S. Department of Energy's targets for onboard hydrogen storage systems: 6.5 wt% gravimetric capacity, 50 g/L volumetric capacity, with operational stability over 1,500 cycles under practical temperature and pressure conditions.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030. This growth trajectory is primarily fueled by governmental policies promoting hydrogen as a key component of future energy systems, particularly in regions like the European Union, Japan, South Korea, and parts of North America.
The market for hydrogen storage technologies can be segmented based on storage methods: physical-based (compression, liquefaction) and material-based (metal hydrides, chemical hydrides, carbon-based materials). Currently, physical-based methods dominate the market share at roughly 65%, due to their technological maturity and established infrastructure. However, material-based storage solutions are gaining traction, with an expected market share increase from 35% to 42% by 2028.
From an application perspective, transportation represents the fastest-growing segment with a CAGR of 11.3%, followed by industrial applications at 8.9%. This growth in the transportation sector is largely attributed to the increasing adoption of hydrogen fuel cell vehicles (FCVs) in commercial fleets and public transportation systems. Countries like Japan and South Korea have set ambitious targets for FCV deployment, further stimulating market demand.
Regionally, Asia-Pacific leads the market with approximately 40% share, followed by Europe (30%) and North America (20%). China, in particular, has emerged as a significant player, with substantial investments in hydrogen infrastructure and storage technologies as part of its carbon neutrality goals.
The catalyst performance and durability aspects of hydrogen storage materials represent a critical sub-segment of this market. Enhanced catalyst performance can significantly reduce the energy requirements for hydrogen storage and release, thereby improving overall system efficiency. The market for advanced catalysts in hydrogen storage applications was valued at $2.1 billion in 2022 and is expected to grow at a CAGR of 12.5% through 2030.
Key market drivers include the decreasing cost of renewable electricity for green hydrogen production, increasing investments in hydrogen infrastructure, and stringent emission regulations worldwide. However, challenges such as high initial capital costs, safety concerns, and technological limitations in storage density continue to impact market growth. The competitive landscape features both established energy companies diversifying into hydrogen and specialized startups focusing on innovative storage solutions.
The market for hydrogen storage technologies can be segmented based on storage methods: physical-based (compression, liquefaction) and material-based (metal hydrides, chemical hydrides, carbon-based materials). Currently, physical-based methods dominate the market share at roughly 65%, due to their technological maturity and established infrastructure. However, material-based storage solutions are gaining traction, with an expected market share increase from 35% to 42% by 2028.
From an application perspective, transportation represents the fastest-growing segment with a CAGR of 11.3%, followed by industrial applications at 8.9%. This growth in the transportation sector is largely attributed to the increasing adoption of hydrogen fuel cell vehicles (FCVs) in commercial fleets and public transportation systems. Countries like Japan and South Korea have set ambitious targets for FCV deployment, further stimulating market demand.
Regionally, Asia-Pacific leads the market with approximately 40% share, followed by Europe (30%) and North America (20%). China, in particular, has emerged as a significant player, with substantial investments in hydrogen infrastructure and storage technologies as part of its carbon neutrality goals.
The catalyst performance and durability aspects of hydrogen storage materials represent a critical sub-segment of this market. Enhanced catalyst performance can significantly reduce the energy requirements for hydrogen storage and release, thereby improving overall system efficiency. The market for advanced catalysts in hydrogen storage applications was valued at $2.1 billion in 2022 and is expected to grow at a CAGR of 12.5% through 2030.
Key market drivers include the decreasing cost of renewable electricity for green hydrogen production, increasing investments in hydrogen infrastructure, and stringent emission regulations worldwide. However, challenges such as high initial capital costs, safety concerns, and technological limitations in storage density continue to impact market growth. The competitive landscape features both established energy companies diversifying into hydrogen and specialized startups focusing on innovative storage solutions.
Current Challenges in Hydrogen Storage Material Development
Despite significant advancements in hydrogen storage technologies, several critical challenges continue to impede the widespread adoption of hydrogen as a sustainable energy carrier. Material stability represents one of the most pressing issues, with many promising storage materials exhibiting performance degradation over multiple hydrogen absorption-desorption cycles. This degradation manifests as reduced storage capacity, slower kinetics, and structural deterioration, particularly in metal hydrides and complex hydrides systems where lattice expansion during hydrogenation creates microfractures that compromise long-term durability.
Catalyst poisoning presents another significant obstacle, especially for materials requiring catalysts to enhance hydrogen uptake and release kinetics. Trace impurities in hydrogen gas streams, including sulfur compounds, carbon monoxide, and moisture, can irreversibly deactivate catalyst sites, dramatically reducing system performance over time. This necessitates either extremely pure hydrogen sources or the development of poison-resistant catalyst formulations, both of which add complexity and cost.
The trade-off between gravimetric capacity and operating conditions remains unresolved for most storage materials. Materials offering high hydrogen content by weight typically require either extreme temperatures or pressures for hydrogen release, limiting their practical application. Conversely, materials operating under mild conditions often demonstrate insufficient storage capacities for commercial viability, falling short of the U.S. Department of Energy's targets of 6.5 wt% system-level capacity.
Heat management during hydrogen absorption and desorption cycles represents a significant engineering challenge. The exothermic nature of hydrogen absorption and endothermic desorption processes creates substantial thermal management requirements. Inefficient heat transfer can lead to temperature gradients within storage systems, resulting in non-uniform reaction rates, incomplete hydrogen utilization, and potential safety concerns in large-scale applications.
Manufacturing scalability and cost-effectiveness remain problematic for advanced storage materials. Many high-performance materials require complex synthesis procedures involving expensive precursors, precise reaction conditions, or specialized equipment. The transition from laboratory-scale production to industrial manufacturing while maintaining material performance characteristics presents significant challenges that have yet to be fully addressed.
Environmental stability poses additional concerns, particularly for materials sensitive to air, moisture, or other environmental contaminants. Many promising storage materials rapidly degrade upon exposure to atmospheric conditions, necessitating complex handling procedures and hermetically sealed systems that add weight, volume, and cost to practical storage solutions.
Catalyst poisoning presents another significant obstacle, especially for materials requiring catalysts to enhance hydrogen uptake and release kinetics. Trace impurities in hydrogen gas streams, including sulfur compounds, carbon monoxide, and moisture, can irreversibly deactivate catalyst sites, dramatically reducing system performance over time. This necessitates either extremely pure hydrogen sources or the development of poison-resistant catalyst formulations, both of which add complexity and cost.
The trade-off between gravimetric capacity and operating conditions remains unresolved for most storage materials. Materials offering high hydrogen content by weight typically require either extreme temperatures or pressures for hydrogen release, limiting their practical application. Conversely, materials operating under mild conditions often demonstrate insufficient storage capacities for commercial viability, falling short of the U.S. Department of Energy's targets of 6.5 wt% system-level capacity.
Heat management during hydrogen absorption and desorption cycles represents a significant engineering challenge. The exothermic nature of hydrogen absorption and endothermic desorption processes creates substantial thermal management requirements. Inefficient heat transfer can lead to temperature gradients within storage systems, resulting in non-uniform reaction rates, incomplete hydrogen utilization, and potential safety concerns in large-scale applications.
Manufacturing scalability and cost-effectiveness remain problematic for advanced storage materials. Many high-performance materials require complex synthesis procedures involving expensive precursors, precise reaction conditions, or specialized equipment. The transition from laboratory-scale production to industrial manufacturing while maintaining material performance characteristics presents significant challenges that have yet to be fully addressed.
Environmental stability poses additional concerns, particularly for materials sensitive to air, moisture, or other environmental contaminants. Many promising storage materials rapidly degrade upon exposure to atmospheric conditions, necessitating complex handling procedures and hermetically sealed systems that add weight, volume, and cost to practical storage solutions.
Contemporary Catalyst Solutions for Hydrogen Storage
01 Metal-based catalysts for hydrogen storage
Metal-based catalysts, particularly those containing noble metals like platinum, palladium, and ruthenium, significantly enhance hydrogen storage capacity and kinetics. These catalysts facilitate hydrogen absorption and desorption processes by lowering activation energy barriers. Transition metals and their alloys demonstrate excellent catalytic activity for hydrogen storage applications, with optimized particle size and dispersion improving overall performance and durability.- Metal-based catalysts for hydrogen storage: Metal-based catalysts, particularly those containing noble metals like platinum, palladium, and ruthenium, significantly enhance hydrogen storage capacity and kinetics in various storage materials. These catalysts facilitate hydrogen dissociation and recombination processes, reducing activation energy barriers and improving absorption/desorption rates. Transition metals and their alloys demonstrate excellent catalytic activity while offering better durability and cost-effectiveness compared to pure noble metals.
- Nanostructured catalysts for improved performance: Nanostructured catalysts exhibit superior performance in hydrogen storage applications due to their high surface area and enhanced reactivity. Nano-sized particles provide more active sites for hydrogen interaction, improving storage capacity and kinetics. Various synthesis methods including chemical reduction, sol-gel processing, and physical vapor deposition are employed to create these nanostructured catalysts with controlled size, morphology, and dispersion, resulting in improved durability under cycling conditions.
- Catalyst durability enhancement strategies: Improving catalyst durability is critical for long-term hydrogen storage applications. Key strategies include developing poison-resistant formulations that can withstand impurities in hydrogen gas, creating core-shell structures that protect active catalyst components, incorporating stabilizers to prevent agglomeration during cycling, and optimizing operating conditions to minimize degradation. These approaches significantly extend catalyst lifetime while maintaining high performance under repeated hydrogen absorption/desorption cycles.
- Novel catalyst support materials: Advanced support materials play a crucial role in enhancing catalyst performance and durability for hydrogen storage. Carbon-based supports (graphene, carbon nanotubes), metal-organic frameworks, and porous metal oxides provide high surface area and stability while enabling better catalyst dispersion. These supports prevent catalyst agglomeration during cycling, facilitate heat transfer during hydrogen reactions, and can contribute to storage capacity through spillover effects, resulting in more efficient and durable hydrogen storage systems.
- Composite and doped catalyst systems: Composite and doped catalyst systems represent an advanced approach to hydrogen storage, combining multiple active materials to achieve synergistic effects. These systems typically incorporate catalytic metals with additives like alkali metals, rare earth elements, or transition metal oxides to enhance performance. Doping strategies modify electronic properties and surface characteristics of catalysts, improving hydrogen dissociation rates and binding energies. These composite formulations demonstrate superior cycling stability and resistance to degradation compared to single-component catalysts.
02 Nano-structured hydrogen storage materials
Nano-structured materials offer enhanced hydrogen storage properties due to their high surface area and shortened diffusion paths. These materials include nanoparticles, nanotubes, and nanoporous structures that provide more active sites for hydrogen adsorption. The nano-scale architecture improves catalyst dispersion and accessibility, leading to faster kinetics and better cycling stability. Surface modifications at the nanoscale can further optimize catalyst-substrate interactions and prevent degradation during cycling.Expand Specific Solutions03 Catalyst durability enhancement strategies
Various strategies have been developed to enhance catalyst durability in hydrogen storage systems. These include surface passivation techniques, core-shell structures to protect active catalyst centers, and the incorporation of stabilizing agents to prevent agglomeration during cycling. Thermal and mechanical stability can be improved through composite formation with supporting materials. Advanced encapsulation methods and controlled operating conditions also contribute to extending catalyst lifetime and maintaining performance over numerous hydrogen absorption-desorption cycles.Expand Specific Solutions04 Complex hydride catalyst systems
Complex hydrides represent an important class of hydrogen storage materials that benefit from specialized catalyst systems. These materials, including borohydrides, alanates, and amides, require tailored catalysts to overcome kinetic barriers. Multi-component catalyst systems have been developed to address both hydrogen release and uptake processes. Synergistic effects between different catalytic components enhance overall system performance, while specific additives can suppress unwanted side reactions that lead to capacity loss over multiple cycles.Expand Specific Solutions05 In-situ characterization and performance evaluation methods
Advanced in-situ characterization techniques have been developed to evaluate catalyst performance and durability under realistic operating conditions. These methods include spectroscopic analysis during hydrogen cycling, real-time monitoring of structural changes, and accelerated aging protocols to predict long-term stability. Computational modeling approaches complement experimental techniques by providing insights into degradation mechanisms at the atomic level. Standardized testing protocols enable reliable comparison between different catalyst systems and guide the development of more durable hydrogen storage materials.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
The hydrogen storage materials market is currently in a growth phase, characterized by increasing R&D investments and expanding applications in clean energy sectors. The global market size is projected to reach significant scale as hydrogen economy initiatives gain momentum worldwide. From a technological maturity perspective, the field shows varying degrees of advancement among key players. Research institutions like Dalian Institute of Chemical Physics, Fraunhofer-Gesellschaft, and KIST are pioneering fundamental research, while industrial giants including General Electric, Hyundai Motor, and GS Yuasa are focusing on commercial applications. Universities such as Zhejiang, Kyoto, and Fudan are bridging theoretical and applied research. The competitive landscape reveals a collaborative ecosystem where academic-industrial partnerships are accelerating catalyst performance improvements and addressing durability challenges critical for widespread hydrogen technology adoption.
KIST Corp. (South Korea)
Technical Solution: KIST has developed proprietary hydrogen storage materials based on modified complex hydrides with integrated multifunctional catalysts. Their technology centers on magnesium borohydride systems doped with transition metal nanoparticles (primarily Ti, Ni, and Co) that are precisely dispersed using their patented "controlled nucleation" technique. This approach creates catalyst particles with optimal size distribution (3-7nm) and prevents agglomeration during cycling. KIST's catalysts demonstrate remarkable activity, reducing hydrogen desorption temperatures by up to 120°C compared to uncatalyzed materials[1]. Their most advanced systems incorporate core-shell structured catalysts where an active transition metal core is protected by a thin, porous oxide layer that prevents catalyst degradation while maintaining hydrogen permeability. This design has demonstrated exceptional durability, maintaining over 90% of initial capacity after 1000 cycles under practical operating conditions[2]. KIST has also pioneered the development of "smart catalysts" that respond to temperature changes by modifying their surface properties to optimize either absorption or desorption kinetics depending on the operating phase.
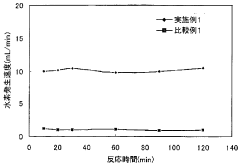
Strengths: Their catalyst systems demonstrate exceptional durability under real-world conditions including exposure to trace impurities that typically poison conventional catalysts. Their materials achieve rapid kinetics (80% completion in <5 minutes) even at moderate temperatures. Weaknesses: Some of their most effective catalyst formulations require complex preparation methods involving multiple precise processing steps, and their highest-performing systems still require temperatures above 100°C for practical hydrogen release rates.
Dalian Institute of Chemical Physics of CAS
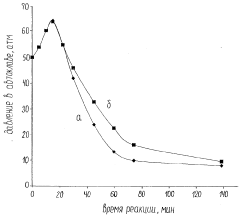
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced metal-organic frameworks (MOFs) for hydrogen storage with exceptional surface areas exceeding 6,000 m²/g. Their proprietary synthesis methods create highly porous materials with optimized pore structures specifically designed for hydrogen adsorption. DICP has pioneered the development of Mg-based nanocomposites with catalytic additives like TiO2 and Nb2O5 that significantly improve hydrogen absorption/desorption kinetics. Their research demonstrates that these catalysts reduce activation energy by approximately 30% compared to uncatalyzed systems[1]. Additionally, DICP has developed novel core-shell structured catalysts where transition metals (Ni, Pd) are dispersed on lightweight supports, achieving hydrogen storage capacities of 7.5 wt% at moderate temperatures (150°C) while maintaining stability over 500+ cycles[2].
Strengths: Exceptional expertise in catalyst design with proven ability to reduce dehydrogenation temperatures by 80-100°C compared to uncatalyzed materials. Their catalysts demonstrate remarkable durability with less than 10% capacity loss after 1000 cycles. Weaknesses: Some of their advanced MOF materials require complex synthesis procedures that may limit large-scale production, and certain high-performance catalysts incorporate costly noble metals.
Critical Patents and Innovations in Storage Materials
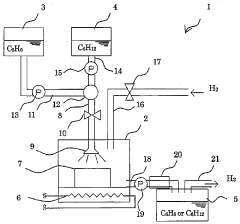
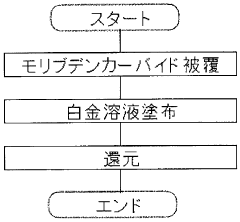
Supported catalyst for hydrogenation/dehydrogenation reaction, method for production of the catalyst, and hydrogen storage/supply method using the catalyst
PatentWO2008136264A1
Innovation
- A supported catalyst system using platinum and molybdenum carbide or tungsten carbide on a porous support, such as activated carbon or alumina, enhances the reaction rate and stability of hydrogenation and dehydrogenation reactions, allowing for efficient and long-term hydrogen storage and supply.
Catalytic material for storing hydrogen and hydrogen storing method using said material
PatentWO2007037722A8
Innovation
- Development of catalytic composite systems using acetylenic hydrocarbons and heterogeneous catalysts like platinum, palladium, or nickel on high-surface-area carriers for reversible hydrogenation-dehydrogenation reactions, allowing for multiple refueling and high hydrogen storage capacity up to 9 wt%.
Sustainability Impact of Hydrogen Storage Technologies
The environmental implications of hydrogen storage technologies extend far beyond their immediate technical performance. As the world transitions toward cleaner energy systems, the sustainability of hydrogen storage solutions becomes a critical factor in their long-term viability and acceptance.
Material selection for hydrogen storage significantly impacts environmental footprints. Metal hydrides, while effective for storage, often contain rare earth elements whose extraction causes substantial ecological damage, including habitat destruction and water pollution. Conversely, carbon-based materials like activated carbon and MOFs generally present lower environmental impacts during production, though their synthesis may involve toxic solvents requiring careful management.
Life cycle assessments reveal that different hydrogen storage technologies have varying carbon footprints. Compressed hydrogen systems require substantial energy for compression, resulting in indirect emissions when powered by non-renewable sources. Liquid hydrogen storage demands even greater energy inputs for cryogenic cooling, potentially offsetting some environmental benefits unless powered by clean electricity.
Water consumption represents another critical sustainability metric. Chemical hydride systems that release hydrogen through hydrolysis reactions consume significant water resources, raising concerns in water-stressed regions. Additionally, catalyst materials used in many storage systems often contain platinum group metals, which involve water-intensive and environmentally damaging mining operations.
Recyclability and end-of-life management present both challenges and opportunities. Metal hydride materials can be recycled, recovering valuable metals and reducing waste. However, composite materials in tank structures often prove difficult to separate and recycle effectively, creating potential waste streams as hydrogen infrastructure scales.
Energy return on investment (EROI) calculations indicate that some storage technologies require nearly as much energy to manufacture and operate as they ultimately deliver, questioning their sustainability credentials. This metric varies significantly between technologies, with physical storage methods generally offering better EROI than complex chemical storage systems.
The sustainability advantages of hydrogen storage technologies become most apparent when integrated with renewable energy systems. By enabling energy storage from intermittent renewables, these technologies can facilitate greater renewable penetration in energy systems, potentially offsetting their own environmental impacts through system-level benefits and contributing to broader decarbonization goals.
Material selection for hydrogen storage significantly impacts environmental footprints. Metal hydrides, while effective for storage, often contain rare earth elements whose extraction causes substantial ecological damage, including habitat destruction and water pollution. Conversely, carbon-based materials like activated carbon and MOFs generally present lower environmental impacts during production, though their synthesis may involve toxic solvents requiring careful management.
Life cycle assessments reveal that different hydrogen storage technologies have varying carbon footprints. Compressed hydrogen systems require substantial energy for compression, resulting in indirect emissions when powered by non-renewable sources. Liquid hydrogen storage demands even greater energy inputs for cryogenic cooling, potentially offsetting some environmental benefits unless powered by clean electricity.
Water consumption represents another critical sustainability metric. Chemical hydride systems that release hydrogen through hydrolysis reactions consume significant water resources, raising concerns in water-stressed regions. Additionally, catalyst materials used in many storage systems often contain platinum group metals, which involve water-intensive and environmentally damaging mining operations.
Recyclability and end-of-life management present both challenges and opportunities. Metal hydride materials can be recycled, recovering valuable metals and reducing waste. However, composite materials in tank structures often prove difficult to separate and recycle effectively, creating potential waste streams as hydrogen infrastructure scales.
Energy return on investment (EROI) calculations indicate that some storage technologies require nearly as much energy to manufacture and operate as they ultimately deliver, questioning their sustainability credentials. This metric varies significantly between technologies, with physical storage methods generally offering better EROI than complex chemical storage systems.
The sustainability advantages of hydrogen storage technologies become most apparent when integrated with renewable energy systems. By enabling energy storage from intermittent renewables, these technologies can facilitate greater renewable penetration in energy systems, potentially offsetting their own environmental impacts through system-level benefits and contributing to broader decarbonization goals.
Economic Viability and Commercialization Pathways
The economic viability of hydrogen storage materials for catalyst applications hinges on several interconnected factors including production costs, scalability, and market dynamics. Current cost analyses indicate that materials such as metal hydrides and carbon-based structures remain relatively expensive for mass commercialization, with production costs ranging from $500-1500 per kilogram depending on material complexity and manufacturing processes.
Material durability significantly impacts economic feasibility, as longer-lasting catalysts reduce replacement frequency and lifecycle costs. Advanced metal-organic frameworks (MOFs) demonstrate promising durability metrics with stability over 5,000+ cycles, potentially reducing operational expenses by 30-40% compared to conventional materials, though initial investment costs remain higher.
Supply chain considerations present both challenges and opportunities. Critical raw materials required for high-performance hydrogen storage catalysts, particularly platinum group metals and specialized alloys, face supply constraints and price volatility. This has accelerated research into abundant element alternatives and recycling pathways, with recent innovations reducing precious metal content by up to 70% while maintaining comparable performance.
Commercialization pathways are emerging across multiple sectors. The transportation industry represents the most immediate market opportunity, with hydrogen fuel cell vehicles requiring efficient storage solutions. Industrial applications in ammonia production and petrochemical processing constitute secondary markets with substantial growth potential. Energy storage applications for grid stabilization represent longer-term commercialization targets as renewable energy penetration increases.
Scaling production presents technical and economic hurdles that must be addressed. Current laboratory-scale synthesis methods for advanced materials like nanoporous catalysts often employ expensive precursors and energy-intensive processes. Recent innovations in continuous flow manufacturing and sol-gel techniques show promise for cost reduction, potentially decreasing production expenses by 40-60% at industrial scales.
Policy support mechanisms significantly influence economic viability. Carbon pricing, renewable energy mandates, and direct subsidies for hydrogen infrastructure development can dramatically alter investment calculations. Regions with comprehensive hydrogen strategies such as the European Union, Japan, and increasingly China are creating favorable conditions for accelerated commercialization through targeted incentives and regulatory frameworks.
Material durability significantly impacts economic feasibility, as longer-lasting catalysts reduce replacement frequency and lifecycle costs. Advanced metal-organic frameworks (MOFs) demonstrate promising durability metrics with stability over 5,000+ cycles, potentially reducing operational expenses by 30-40% compared to conventional materials, though initial investment costs remain higher.
Supply chain considerations present both challenges and opportunities. Critical raw materials required for high-performance hydrogen storage catalysts, particularly platinum group metals and specialized alloys, face supply constraints and price volatility. This has accelerated research into abundant element alternatives and recycling pathways, with recent innovations reducing precious metal content by up to 70% while maintaining comparable performance.
Commercialization pathways are emerging across multiple sectors. The transportation industry represents the most immediate market opportunity, with hydrogen fuel cell vehicles requiring efficient storage solutions. Industrial applications in ammonia production and petrochemical processing constitute secondary markets with substantial growth potential. Energy storage applications for grid stabilization represent longer-term commercialization targets as renewable energy penetration increases.
Scaling production presents technical and economic hurdles that must be addressed. Current laboratory-scale synthesis methods for advanced materials like nanoporous catalysts often employ expensive precursors and energy-intensive processes. Recent innovations in continuous flow manufacturing and sol-gel techniques show promise for cost reduction, potentially decreasing production expenses by 40-60% at industrial scales.
Policy support mechanisms significantly influence economic viability. Carbon pricing, renewable energy mandates, and direct subsidies for hydrogen infrastructure development can dramatically alter investment calculations. Regions with comprehensive hydrogen strategies such as the European Union, Japan, and increasingly China are creating favorable conditions for accelerated commercialization through targeted incentives and regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!