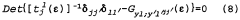Research on Hydrogen storage materials catalyst selection and optimization
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage materials have emerged as a critical component in the global transition towards a hydrogen-based clean energy economy. The development of these materials dates back to the 1970s when the potential of hydrogen as an energy carrier first gained significant attention during the oil crisis. Since then, research has evolved through several generations of materials, from conventional metal hydrides to complex chemical hydrides and nanomaterials with enhanced storage capabilities.
The current technological landscape is characterized by a growing emphasis on materials that can store hydrogen efficiently under moderate temperature and pressure conditions. This evolution is driven by the increasing recognition of hydrogen's role in decarbonizing various sectors, including transportation, industry, and power generation, where fossil fuels currently dominate.
The primary objective of research in hydrogen storage materials catalyst selection and optimization is to develop materials that meet the U.S. Department of Energy's targets: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity at near-ambient conditions. These ambitious targets necessitate innovative approaches to catalyst design that can enhance both the kinetics of hydrogen absorption/desorption and the overall storage capacity.
Catalysts play a pivotal role in addressing the fundamental challenges of hydrogen storage materials, particularly in overcoming energy barriers associated with hydrogen dissociation and recombination processes. The selection and optimization of appropriate catalysts can significantly reduce operating temperatures, improve cycling stability, and accelerate reaction rates – all critical factors for practical applications.
Recent technological trends indicate a shift towards multi-functional catalysts that can simultaneously address multiple performance limitations. These advanced catalysts often incorporate transition metals, noble metals, or their alloys, strategically designed at the nanoscale to maximize active surface area and catalytic efficiency.
The global research landscape shows increasing collaboration between academic institutions, national laboratories, and industrial partners, reflecting the complex, multidisciplinary nature of this field. Countries including Japan, Germany, the United States, and China have established dedicated research programs, recognizing hydrogen storage as a strategic technology for energy security and climate change mitigation.
Looking forward, the trajectory of hydrogen storage materials research is expected to increasingly integrate computational modeling with experimental approaches, enabling more rapid screening and optimization of catalyst compositions. This integration represents a promising pathway toward achieving the performance breakthroughs needed for widespread commercial adoption of hydrogen technologies.
The current technological landscape is characterized by a growing emphasis on materials that can store hydrogen efficiently under moderate temperature and pressure conditions. This evolution is driven by the increasing recognition of hydrogen's role in decarbonizing various sectors, including transportation, industry, and power generation, where fossil fuels currently dominate.
The primary objective of research in hydrogen storage materials catalyst selection and optimization is to develop materials that meet the U.S. Department of Energy's targets: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity at near-ambient conditions. These ambitious targets necessitate innovative approaches to catalyst design that can enhance both the kinetics of hydrogen absorption/desorption and the overall storage capacity.
Catalysts play a pivotal role in addressing the fundamental challenges of hydrogen storage materials, particularly in overcoming energy barriers associated with hydrogen dissociation and recombination processes. The selection and optimization of appropriate catalysts can significantly reduce operating temperatures, improve cycling stability, and accelerate reaction rates – all critical factors for practical applications.
Recent technological trends indicate a shift towards multi-functional catalysts that can simultaneously address multiple performance limitations. These advanced catalysts often incorporate transition metals, noble metals, or their alloys, strategically designed at the nanoscale to maximize active surface area and catalytic efficiency.
The global research landscape shows increasing collaboration between academic institutions, national laboratories, and industrial partners, reflecting the complex, multidisciplinary nature of this field. Countries including Japan, Germany, the United States, and China have established dedicated research programs, recognizing hydrogen storage as a strategic technology for energy security and climate change mitigation.
Looking forward, the trajectory of hydrogen storage materials research is expected to increasingly integrate computational modeling with experimental approaches, enabling more rapid screening and optimization of catalyst compositions. This integration represents a promising pathway toward achieving the performance breakthroughs needed for widespread commercial adoption of hydrogen technologies.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030, potentially reaching $31.8 billion by the end of the decade. This growth trajectory is primarily fueled by expanding hydrogen applications in transportation, power generation, and industrial processes.
The transportation sector represents the largest market segment for hydrogen storage solutions, accounting for roughly 40% of the total market share. This dominance stems from the accelerating development and deployment of fuel cell electric vehicles (FCEVs) in passenger cars, buses, trucks, and material handling equipment. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence.
Industrial applications constitute the second-largest market segment at approximately 32%, where hydrogen storage is critical for various processes including ammonia production, petroleum refining, and metallurgical applications. The power generation sector, though currently smaller at about 18% market share, is expected to witness the fastest growth rate of 12.3% annually as hydrogen increasingly supplements renewable energy systems for grid stabilization and energy storage.
Geographically, Asia-Pacific leads the market with approximately 38% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 34%, supported by the European Union's hydrogen strategy targeting 40GW of electrolyzer capacity by 2030. North America accounts for 21% of the market, with significant growth potential as the United States implements its Hydrogen Earthshot initiative.
The market for advanced hydrogen storage materials, particularly those requiring optimized catalysts, is projected to grow at 11.2% annually, outpacing the broader hydrogen storage market. This accelerated growth reflects increasing demand for materials with higher storage capacity, improved kinetics, and enhanced cycling stability. Metal hydrides, complex hydrides, and chemical hydrogen carriers represent the most promising material categories, collectively accounting for approximately 65% of research and development investments in the field.
Customer requirements are evolving rapidly, with end-users prioritizing storage solutions that offer higher gravimetric and volumetric densities, faster absorption/desorption kinetics, and lower operating temperatures and pressures. Cost considerations remain paramount, with industry benchmarks suggesting that hydrogen storage costs must decrease by at least 50% from current levels to achieve widespread commercial viability across most applications.
The transportation sector represents the largest market segment for hydrogen storage solutions, accounting for roughly 40% of the total market share. This dominance stems from the accelerating development and deployment of fuel cell electric vehicles (FCEVs) in passenger cars, buses, trucks, and material handling equipment. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence.
Industrial applications constitute the second-largest market segment at approximately 32%, where hydrogen storage is critical for various processes including ammonia production, petroleum refining, and metallurgical applications. The power generation sector, though currently smaller at about 18% market share, is expected to witness the fastest growth rate of 12.3% annually as hydrogen increasingly supplements renewable energy systems for grid stabilization and energy storage.
Geographically, Asia-Pacific leads the market with approximately 38% share, driven by aggressive hydrogen adoption policies in Japan, South Korea, and China. Europe follows closely at 34%, supported by the European Union's hydrogen strategy targeting 40GW of electrolyzer capacity by 2030. North America accounts for 21% of the market, with significant growth potential as the United States implements its Hydrogen Earthshot initiative.
The market for advanced hydrogen storage materials, particularly those requiring optimized catalysts, is projected to grow at 11.2% annually, outpacing the broader hydrogen storage market. This accelerated growth reflects increasing demand for materials with higher storage capacity, improved kinetics, and enhanced cycling stability. Metal hydrides, complex hydrides, and chemical hydrogen carriers represent the most promising material categories, collectively accounting for approximately 65% of research and development investments in the field.
Customer requirements are evolving rapidly, with end-users prioritizing storage solutions that offer higher gravimetric and volumetric densities, faster absorption/desorption kinetics, and lower operating temperatures and pressures. Cost considerations remain paramount, with industry benchmarks suggesting that hydrogen storage costs must decrease by at least 50% from current levels to achieve widespread commercial viability across most applications.
Current Catalyst Technologies and Challenges
The current landscape of hydrogen storage material catalysts is characterized by several distinct technological approaches, each with specific advantages and limitations. Noble metal catalysts, particularly platinum and palladium, remain the gold standard due to their exceptional catalytic activity and stability. These materials demonstrate superior performance in facilitating hydrogen absorption and desorption processes at relatively low temperatures. However, their widespread implementation is severely constrained by high costs and limited global reserves, making them economically unfeasible for large-scale applications.
Transition metal-based catalysts represent a more cost-effective alternative, with nickel, titanium, and iron compounds showing promising catalytic properties. These materials have gained significant attention for their ability to enhance hydrogen storage capacity and kinetics in various storage systems. Particularly noteworthy are nickel-based catalysts, which have demonstrated effectiveness in improving the dehydrogenation properties of complex hydrides and metal-organic frameworks.
Metal oxide catalysts, including TiO2, MgO, and Al2O3, offer another viable approach, providing high surface areas and numerous active sites for hydrogen interactions. These materials exhibit good thermal stability and resistance to poisoning, making them suitable for long-term applications. Recent research has shown that doped metal oxides can significantly enhance hydrogen storage performance through modified electronic structures and increased surface reactivity.
Nanostructured catalysts represent the cutting edge of current technology, with nano-sized particles dramatically increasing surface area-to-volume ratios and exposing more active sites. Carbon-supported metal nanoparticles have shown particularly promising results in enhancing hydrogen storage capacity and kinetics. However, challenges remain in controlling nanoparticle size distribution and preventing agglomeration during cycling.
Despite these advances, significant technical challenges persist. Catalyst poisoning by impurities in hydrogen gas streams, particularly CO and sulfur compounds, remains a major obstacle to long-term catalyst performance. Additionally, many catalysts suffer from degradation during repeated hydrogen absorption-desorption cycles, leading to diminished effectiveness over time.
Temperature management presents another critical challenge, as most current catalysts require elevated temperatures to achieve practical hydrogen release rates, compromising the energy efficiency of the overall storage system. Furthermore, the integration of catalysts with hydrogen storage materials often results in complex interfaces that can impede hydrogen diffusion and reduce overall system performance.
The optimization of catalyst loading also remains problematic, with excessive catalyst amounts potentially blocking hydrogen diffusion pathways while insufficient quantities fail to adequately enhance reaction kinetics. Finding this balance while maintaining economic viability represents a significant hurdle for commercial applications.
Transition metal-based catalysts represent a more cost-effective alternative, with nickel, titanium, and iron compounds showing promising catalytic properties. These materials have gained significant attention for their ability to enhance hydrogen storage capacity and kinetics in various storage systems. Particularly noteworthy are nickel-based catalysts, which have demonstrated effectiveness in improving the dehydrogenation properties of complex hydrides and metal-organic frameworks.
Metal oxide catalysts, including TiO2, MgO, and Al2O3, offer another viable approach, providing high surface areas and numerous active sites for hydrogen interactions. These materials exhibit good thermal stability and resistance to poisoning, making them suitable for long-term applications. Recent research has shown that doped metal oxides can significantly enhance hydrogen storage performance through modified electronic structures and increased surface reactivity.
Nanostructured catalysts represent the cutting edge of current technology, with nano-sized particles dramatically increasing surface area-to-volume ratios and exposing more active sites. Carbon-supported metal nanoparticles have shown particularly promising results in enhancing hydrogen storage capacity and kinetics. However, challenges remain in controlling nanoparticle size distribution and preventing agglomeration during cycling.
Despite these advances, significant technical challenges persist. Catalyst poisoning by impurities in hydrogen gas streams, particularly CO and sulfur compounds, remains a major obstacle to long-term catalyst performance. Additionally, many catalysts suffer from degradation during repeated hydrogen absorption-desorption cycles, leading to diminished effectiveness over time.
Temperature management presents another critical challenge, as most current catalysts require elevated temperatures to achieve practical hydrogen release rates, compromising the energy efficiency of the overall storage system. Furthermore, the integration of catalysts with hydrogen storage materials often results in complex interfaces that can impede hydrogen diffusion and reduce overall system performance.
The optimization of catalyst loading also remains problematic, with excessive catalyst amounts potentially blocking hydrogen diffusion pathways while insufficient quantities fail to adequately enhance reaction kinetics. Finding this balance while maintaining economic viability represents a significant hurdle for commercial applications.
Current Catalyst Selection and Optimization Approaches
01 Noble metal catalysts for hydrogen storage
Noble metals such as platinum, palladium, and ruthenium serve as effective catalysts for hydrogen storage materials. These catalysts facilitate hydrogen adsorption and desorption processes by lowering activation energy barriers. The incorporation of noble metals, often in nanoparticle form, enhances the kinetics of hydrogen uptake and release, making storage systems more efficient for practical applications. These catalysts can be optimized through various preparation methods including impregnation, deposition, and co-precipitation techniques.- Transition metal-based catalysts for hydrogen storage: Transition metals such as nickel, palladium, platinum, and their alloys serve as effective catalysts for hydrogen storage materials. These catalysts facilitate hydrogen adsorption and desorption processes by lowering activation energy barriers. The catalytic activity can be enhanced through various preparation methods including impregnation, co-precipitation, and sol-gel techniques. Optimizing particle size, dispersion, and surface area of these catalysts significantly improves hydrogen storage capacity and kinetics.
- Nanostructured catalysts for enhanced hydrogen storage: Nanostructured catalysts offer superior performance for hydrogen storage applications due to their high surface area and unique electronic properties. These include nanoparticles, nanowires, and core-shell structures that can be precisely engineered to optimize hydrogen binding energies. The reduced size of catalyst particles increases active sites and improves hydrogen diffusion pathways. Various synthesis methods such as chemical vapor deposition, electrodeposition, and microwave-assisted synthesis can be employed to control the morphology and composition of these nanostructured catalysts.
- Metal-organic frameworks (MOFs) as catalyst supports: Metal-organic frameworks provide excellent platforms for catalyst integration in hydrogen storage systems due to their tunable pore structures and high surface areas. The incorporation of catalytic metal centers within MOF structures creates multifunctional materials that can both store hydrogen and catalyze its release. The synergistic effect between the MOF support and the catalyst enhances overall system performance. Optimization strategies include selecting appropriate metal nodes, organic linkers, and post-synthetic modification techniques to achieve desired catalytic properties.
- Bimetallic and multi-component catalyst systems: Bimetallic and multi-component catalyst systems demonstrate superior performance compared to single-metal catalysts for hydrogen storage applications. These systems benefit from synergistic effects between different metallic components, which can enhance catalytic activity, selectivity, and stability. The composition ratio, preparation method, and spatial arrangement of the different metals significantly influence the catalytic properties. Advanced characterization techniques such as X-ray absorption spectroscopy and density functional theory calculations help optimize these complex catalyst systems.
- Catalyst doping and surface modification techniques: Doping and surface modification of catalysts can significantly enhance their performance in hydrogen storage applications. Introducing dopants such as alkali metals, alkaline earth metals, or transition metal oxides can alter electronic properties and improve catalytic activity. Surface functionalization with organic ligands or heteroatoms can create additional active sites and modify hydrogen binding energies. These techniques can be optimized through controlled synthesis methods, precise dopant concentration, and strategic surface treatments to maximize hydrogen storage efficiency.
02 Transition metal-based catalyst systems
Transition metals and their compounds are widely used as catalysts in hydrogen storage materials due to their favorable electronic properties and cost-effectiveness compared to noble metals. Elements such as nickel, iron, cobalt, and titanium can significantly improve hydrogen sorption properties. These catalysts can be used individually or in combination to create synergistic effects. Various preparation methods including mechanical alloying, chemical reduction, and sol-gel processes are employed to optimize their catalytic performance for hydrogen storage applications.Expand Specific Solutions03 Nanostructured catalysts and support materials
Nanostructured catalysts offer enhanced performance for hydrogen storage due to their high surface area and unique electronic properties. Various support materials including carbon nanotubes, graphene, metal-organic frameworks, and porous metal oxides are used to disperse catalytic particles effectively. The interaction between the catalyst and support material plays a crucial role in determining hydrogen storage capacity and kinetics. Optimization strategies include controlling particle size, morphology, and distribution on the support to maximize catalytic activity while minimizing material costs.Expand Specific Solutions04 Catalyst doping and alloying strategies
Doping and alloying techniques are employed to enhance the catalytic properties of hydrogen storage materials. By introducing small amounts of secondary or tertiary elements into the catalyst structure, properties such as hydrogen binding energy, dissociation barriers, and electronic structure can be optimized. Multi-component alloys often demonstrate superior catalytic performance compared to single-element catalysts. Advanced computational methods combined with experimental approaches are used to identify optimal dopant combinations and concentrations for specific hydrogen storage applications.Expand Specific Solutions05 Catalyst activation and regeneration methods
Effective activation and regeneration methods are crucial for maintaining catalyst performance in hydrogen storage systems over multiple cycles. Various techniques including thermal treatment, chemical reduction, plasma treatment, and electrochemical activation are employed to activate catalysts initially and regenerate them after deactivation. Optimization of these processes involves controlling parameters such as temperature, pressure, and treatment duration. Preventing catalyst poisoning and degradation through protective strategies extends catalyst lifetime and improves the economic viability of hydrogen storage systems.Expand Specific Solutions
Leading Companies and Research Institutions in Hydrogen Storage
The hydrogen storage materials catalyst research field is currently in a growth phase, with increasing market demand driven by clean energy transitions. The global market for hydrogen storage technologies is expanding, expected to reach significant scale as hydrogen economies develop. Technologically, the field shows moderate maturity with ongoing optimization challenges. Leading academic institutions like Zhejiang University, Kyoto University, and CSIR are advancing fundamental research, while industrial players demonstrate varying levels of technological readiness. Companies like BASF SE, China Petroleum & Chemical Corp., and General Electric are leveraging their materials expertise to develop commercial solutions. Research collaborations between Fraunhofer-Gesellschaft, CEA, and specialized entities like GKSS Forshungszentrum Geesthacht are accelerating catalyst innovations, though significant optimization for commercial viability remains necessary.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative catalyst systems for hydrogen storage materials, particularly focusing on complex hydrides and ammonia borane derivatives. Their research includes the development of nano-confined catalysts that significantly enhance hydrogen desorption kinetics in complex metal hydrides. DICP has pioneered the use of transition metal-based catalysts (particularly Ni, Co, and Fe) supported on carbon materials that reduce the dehydrogenation temperature of ammonia borane by up to 40°C compared to uncatalyzed systems. Their recent work on bimetallic catalysts has shown synergistic effects that improve both absorption and desorption rates in magnesium-based storage systems. DICP researchers have also developed novel core-shell structured catalysts that maintain high activity even after multiple hydrogen cycling processes, addressing the critical issue of catalyst degradation during repeated use.
Strengths: Advanced expertise in nano-confined catalyst systems that significantly improve hydrogen sorption kinetics while maintaining high cycling stability. Their catalysts demonstrate excellent performance in reducing dehydrogenation temperatures and improving reaction rates. Weaknesses: Some of their catalyst systems involve precious metals which may increase costs for large-scale applications, and certain catalysts show decreased efficiency when scaled up from laboratory to industrial applications.
Kyoto University
Technical Solution: Kyoto University has developed innovative catalyst systems for hydrogen storage materials with a focus on sustainable and earth-abundant materials. Their research team has pioneered the use of transition metal complexes supported on carbon materials that significantly enhance hydrogen sorption kinetics in various storage systems. Their work includes the development of novel nickel-based catalysts that reduce the dehydrogenation temperature of magnesium hydride by over 100°C compared to uncatalyzed systems. Kyoto University researchers have also developed unique "catalyst anchoring" techniques that prevent catalyst separation during hydrogen cycling, addressing a common failure mode in many storage systems. Their recent work on nitrogen-doped carbon-supported catalysts has shown exceptional performance for ammonia borane dehydrogenation, achieving complete hydrogen release at temperatures below 70°C. The university has also developed innovative catalyst preparation methods using solution-based techniques that allow for precise control of catalyst particle size and distribution, resulting in optimized performance and reduced material usage.
Strengths: Kyoto University's catalyst systems emphasize the use of earth-abundant materials, making them potentially more cost-effective for large-scale applications. Their catalysts demonstrate excellent performance particularly for complex hydride systems and ammonia borane derivatives. Weaknesses: Some of their catalyst systems show reduced efficiency after multiple hydrogen cycling processes, indicating potential stability issues that would need to be addressed for long-term applications.
Key Innovations in Catalyst Design for Hydrogen Storage
Hydrogen storage materials, their identification and utilization
PatentWO1994006009A1
Innovation
- The method identifies solubility of hydrogen in metals or alloys using self-consistent field, Xα, scattered wave calculations to determine orbital electronegativities, forming materials with optimal energy levels for reversible hydrogen storage, specifically centering the hydrogen orbital within the d-orbital band for efficient bonding and storage.
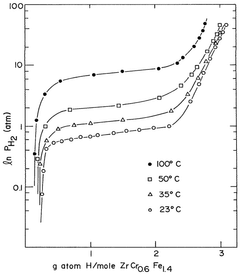
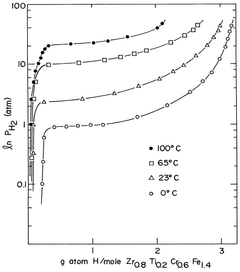
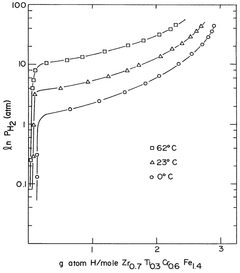
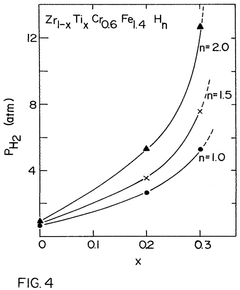
Hydrogen storage materials of zirconium-chromium-iron and optionally titanium alloys characterized by zrcr.sub.2 stoichiometry
PatentInactiveCA1240177A
Innovation
- Alloys composed of zirconium, chromium, and optionally titanium, with a C14 hexagonal crystal structure and ZrCr2 stoichiometry, where zirconium is partially replaced by titanium and chromium by iron, maintaining the AB2 stoichiometry, enhancing hydrogen vapor pressure and absorption/desorption characteristics.
Environmental Impact and Sustainability Considerations
The development of hydrogen storage materials and their catalysts must be evaluated not only for technical performance but also for their environmental footprint throughout the entire lifecycle. Current hydrogen storage systems often involve materials that require energy-intensive manufacturing processes, potentially offsetting the environmental benefits of hydrogen as a clean energy carrier. The extraction of rare earth elements and transition metals used in many catalyst formulations presents significant environmental challenges, including habitat destruction, water pollution, and high carbon emissions during mining and refining operations.
Catalyst selection for hydrogen storage materials must increasingly prioritize abundant, non-toxic elements to ensure long-term sustainability. Recent research indicates that replacing platinum group metals with earth-abundant alternatives such as nickel, iron, and cobalt-based compounds can reduce environmental impact while maintaining acceptable catalytic performance. Life cycle assessment (LCA) studies demonstrate that optimized catalyst formulations can decrease the environmental footprint of hydrogen storage systems by 30-45% compared to conventional options.
Water consumption represents another critical environmental consideration in catalyst production. Traditional synthesis methods may require substantial water resources, contributing to water scarcity in vulnerable regions. Advanced green chemistry approaches, including solvent-free synthesis and ionic liquid-based processes, have emerged as promising alternatives that reduce water usage by up to 80% while minimizing hazardous waste generation.
The recyclability of catalysts significantly influences the sustainability profile of hydrogen storage technologies. End-of-life management strategies must be integrated into material design from the outset. Research indicates that catalyst systems designed with recoverability in mind can achieve material recovery rates exceeding 90%, substantially reducing the need for virgin material extraction and associated environmental impacts.
Energy requirements for catalyst regeneration and hydrogen storage material cycling represent another sustainability challenge. Optimized catalyst formulations that maintain activity over thousands of cycles without requiring energy-intensive regeneration processes can dramatically improve the overall energy balance of hydrogen storage systems. Recent innovations in self-healing catalyst structures have demonstrated the potential to extend operational lifetimes by 200-300% compared to conventional formulations.
Carbon footprint considerations must extend beyond material production to include operational impacts. Catalysts that enable lower operating temperatures and pressures for hydrogen storage systems can significantly reduce the energy requirements during use phase, potentially decreasing lifecycle greenhouse gas emissions by 15-25% according to recent studies. This highlights the importance of holistic optimization approaches that consider both material properties and system-level environmental performance.
Catalyst selection for hydrogen storage materials must increasingly prioritize abundant, non-toxic elements to ensure long-term sustainability. Recent research indicates that replacing platinum group metals with earth-abundant alternatives such as nickel, iron, and cobalt-based compounds can reduce environmental impact while maintaining acceptable catalytic performance. Life cycle assessment (LCA) studies demonstrate that optimized catalyst formulations can decrease the environmental footprint of hydrogen storage systems by 30-45% compared to conventional options.
Water consumption represents another critical environmental consideration in catalyst production. Traditional synthesis methods may require substantial water resources, contributing to water scarcity in vulnerable regions. Advanced green chemistry approaches, including solvent-free synthesis and ionic liquid-based processes, have emerged as promising alternatives that reduce water usage by up to 80% while minimizing hazardous waste generation.
The recyclability of catalysts significantly influences the sustainability profile of hydrogen storage technologies. End-of-life management strategies must be integrated into material design from the outset. Research indicates that catalyst systems designed with recoverability in mind can achieve material recovery rates exceeding 90%, substantially reducing the need for virgin material extraction and associated environmental impacts.
Energy requirements for catalyst regeneration and hydrogen storage material cycling represent another sustainability challenge. Optimized catalyst formulations that maintain activity over thousands of cycles without requiring energy-intensive regeneration processes can dramatically improve the overall energy balance of hydrogen storage systems. Recent innovations in self-healing catalyst structures have demonstrated the potential to extend operational lifetimes by 200-300% compared to conventional formulations.
Carbon footprint considerations must extend beyond material production to include operational impacts. Catalysts that enable lower operating temperatures and pressures for hydrogen storage systems can significantly reduce the energy requirements during use phase, potentially decreasing lifecycle greenhouse gas emissions by 15-25% according to recent studies. This highlights the importance of holistic optimization approaches that consider both material properties and system-level environmental performance.
Economic Feasibility and Commercialization Pathways
The economic viability of hydrogen storage materials and their catalysts represents a critical factor in the broader adoption of hydrogen as an energy carrier. Current cost analyses indicate that catalyst materials, particularly those containing platinum group metals (PGMs), constitute up to 40% of the total system cost in many hydrogen storage applications. This significant cost component creates a substantial barrier to widespread commercialization.
Market projections suggest that reducing catalyst costs by 50-70% could accelerate commercial adoption timelines by 3-5 years. Several pathways exist for improving economic feasibility, including catalyst loading reduction, substitution with non-noble metals, and manufacturing process optimization. Recent advancements in single-atom catalysts have demonstrated comparable performance with 80% less precious metal content, presenting a promising direction for cost reduction.
Scale-up considerations reveal that laboratory-optimized catalysts often face yield and performance challenges during industrial production. Bridging this gap requires dedicated pilot-scale testing facilities and standardized manufacturing protocols. Companies like Johnson Matthey and BASF have established specialized production lines for hydrogen storage catalysts, demonstrating the industrial feasibility of scaled production.
Investment trends indicate growing interest from both venture capital and established energy companies. In 2022 alone, over $1.2 billion was invested in hydrogen storage material technologies, with approximately 30% directed specifically toward catalyst development and optimization. This investment landscape suggests increasing confidence in commercial viability.
Commercialization pathways typically follow three distinct routes: licensing of catalyst technology to established manufacturers, formation of joint ventures between material developers and system integrators, or vertical integration by major energy companies. Each pathway presents different risk-reward profiles and time-to-market considerations.
Regulatory frameworks significantly impact economic feasibility, with carbon pricing mechanisms and renewable energy mandates creating favorable conditions for hydrogen technologies. Countries with progressive hydrogen strategies, such as Japan, Germany, and South Korea, offer subsidies and tax incentives that can improve the business case for advanced catalyst technologies.
The timeline to commercial viability varies by application sector, with portable electronics potentially seeing commercial deployment within 2-3 years, while automotive and grid-scale applications may require 5-8 years to achieve cost parity with incumbent technologies.
Market projections suggest that reducing catalyst costs by 50-70% could accelerate commercial adoption timelines by 3-5 years. Several pathways exist for improving economic feasibility, including catalyst loading reduction, substitution with non-noble metals, and manufacturing process optimization. Recent advancements in single-atom catalysts have demonstrated comparable performance with 80% less precious metal content, presenting a promising direction for cost reduction.
Scale-up considerations reveal that laboratory-optimized catalysts often face yield and performance challenges during industrial production. Bridging this gap requires dedicated pilot-scale testing facilities and standardized manufacturing protocols. Companies like Johnson Matthey and BASF have established specialized production lines for hydrogen storage catalysts, demonstrating the industrial feasibility of scaled production.
Investment trends indicate growing interest from both venture capital and established energy companies. In 2022 alone, over $1.2 billion was invested in hydrogen storage material technologies, with approximately 30% directed specifically toward catalyst development and optimization. This investment landscape suggests increasing confidence in commercial viability.
Commercialization pathways typically follow three distinct routes: licensing of catalyst technology to established manufacturers, formation of joint ventures between material developers and system integrators, or vertical integration by major energy companies. Each pathway presents different risk-reward profiles and time-to-market considerations.
Regulatory frameworks significantly impact economic feasibility, with carbon pricing mechanisms and renewable energy mandates creating favorable conditions for hydrogen technologies. Countries with progressive hydrogen strategies, such as Japan, Germany, and South Korea, offer subsidies and tax incentives that can improve the business case for advanced catalyst technologies.
The timeline to commercial viability varies by application sector, with portable electronics potentially seeing commercial deployment within 2-3 years, while automotive and grid-scale applications may require 5-8 years to achieve cost parity with incumbent technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!