What are the material parameters critical for Hydrogen storage materials efficiency
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage technology spans several decades, evolving from conventional high-pressure gas cylinders to sophisticated materials-based solutions. This technological evolution has been driven by the increasing recognition of hydrogen as a clean energy carrier with the potential to decarbonize various sectors including transportation, power generation, and industrial processes.
The development trajectory of hydrogen storage materials has witnessed significant milestones, from metal hydrides discovered in the 1970s to complex chemical hydrides and nanoporous materials in recent years. Each advancement has contributed to enhancing storage capacity, improving kinetics, and addressing safety concerns. The current technological landscape is characterized by a diverse portfolio of storage solutions, each with specific advantages and limitations depending on the application context.
Material parameters play a pivotal role in determining the efficiency and viability of hydrogen storage systems. Gravimetric capacity, measuring the weight percentage of hydrogen stored relative to the material weight, represents a fundamental parameter for mobile applications where weight considerations are paramount. Equally important is volumetric capacity, which quantifies the amount of hydrogen stored per unit volume, critical for space-constrained applications.
Thermodynamic properties, particularly enthalpy of formation and entropy, govern the energy requirements for hydrogen absorption and desorption processes. These parameters directly influence operating temperatures and pressures, which in turn affect system efficiency and practicality. Kinetic properties, including activation energy and reaction rates, determine how quickly hydrogen can be stored and released, a crucial factor for applications requiring rapid response times.
The technical objectives in hydrogen storage material development are multifaceted. Primary goals include achieving the U.S. Department of Energy's targets for automotive applications: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity, with operating temperatures between -40°C and 60°C and filling times under 3.3 minutes. Beyond these quantitative targets, researchers aim to develop materials with enhanced cycling stability, improved thermal management characteristics, and reduced sensitivity to impurities.
Looking forward, the field is trending toward multi-functional materials that combine high storage capacity with catalytic properties, as well as hybrid systems that leverage the strengths of different storage mechanisms. Computational materials science and high-throughput screening methodologies are increasingly being employed to accelerate the discovery and optimization of novel hydrogen storage materials with superior performance characteristics.
The development trajectory of hydrogen storage materials has witnessed significant milestones, from metal hydrides discovered in the 1970s to complex chemical hydrides and nanoporous materials in recent years. Each advancement has contributed to enhancing storage capacity, improving kinetics, and addressing safety concerns. The current technological landscape is characterized by a diverse portfolio of storage solutions, each with specific advantages and limitations depending on the application context.
Material parameters play a pivotal role in determining the efficiency and viability of hydrogen storage systems. Gravimetric capacity, measuring the weight percentage of hydrogen stored relative to the material weight, represents a fundamental parameter for mobile applications where weight considerations are paramount. Equally important is volumetric capacity, which quantifies the amount of hydrogen stored per unit volume, critical for space-constrained applications.
Thermodynamic properties, particularly enthalpy of formation and entropy, govern the energy requirements for hydrogen absorption and desorption processes. These parameters directly influence operating temperatures and pressures, which in turn affect system efficiency and practicality. Kinetic properties, including activation energy and reaction rates, determine how quickly hydrogen can be stored and released, a crucial factor for applications requiring rapid response times.
The technical objectives in hydrogen storage material development are multifaceted. Primary goals include achieving the U.S. Department of Energy's targets for automotive applications: 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity, with operating temperatures between -40°C and 60°C and filling times under 3.3 minutes. Beyond these quantitative targets, researchers aim to develop materials with enhanced cycling stability, improved thermal management characteristics, and reduced sensitivity to impurities.
Looking forward, the field is trending toward multi-functional materials that combine high storage capacity with catalytic properties, as well as hybrid systems that leverage the strengths of different storage mechanisms. Computational materials science and high-throughput screening methodologies are increasingly being employed to accelerate the discovery and optimization of novel hydrogen storage materials with superior performance characteristics.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. Currently valued at approximately $14.8 billion in 2023, the market is projected to reach $31.4 billion by 2030, representing a compound annual growth rate (CAGR) of 11.3%. This growth trajectory is primarily fueled by governmental policies promoting hydrogen as a key component in achieving carbon neutrality targets across major economies.
The transportation sector represents the largest market segment for hydrogen storage solutions, accounting for nearly 40% of the total market share. This is largely due to the expanding adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Japan, South Korea, and Germany are leading this transition with substantial investments in hydrogen refueling infrastructure.
Industrial applications form the second-largest market segment, with hydrogen storage systems being increasingly integrated into manufacturing processes, particularly in steel production, chemical synthesis, and semiconductor fabrication. The demand for efficient hydrogen storage in these sectors is driven by both environmental regulations and economic incentives for reducing carbon emissions.
Regional analysis reveals that Asia-Pacific currently dominates the market with a 42% share, followed by Europe at 31% and North America at 21%. China has emerged as a particularly aggressive player, with its government committing $16 billion toward hydrogen infrastructure development through 2025, focusing heavily on storage technologies.
Market dynamics are significantly influenced by material advancements in hydrogen storage. Traditional compressed gas and cryogenic liquid storage methods are gradually being supplemented by solid-state storage technologies, which offer improved safety profiles and volumetric efficiency. This shift is creating new market opportunities for material science companies and specialized storage solution providers.
Customer segmentation shows distinct requirements across different end-users. While automotive applications prioritize volumetric efficiency and rapid hydrogen release rates, stationary storage applications for grid balancing emphasize cost-effectiveness and long-term stability. This diversification of requirements is driving specialized product development within the market.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, alongside emerging technology-focused firms specializing in advanced material-based storage solutions. Recent market consolidation through strategic acquisitions indicates that industry players are positioning themselves to capture value across the entire hydrogen value chain, with storage technologies representing a critical competitive advantage.
The transportation sector represents the largest market segment for hydrogen storage solutions, accounting for nearly 40% of the total market share. This is largely due to the expanding adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Japan, South Korea, and Germany are leading this transition with substantial investments in hydrogen refueling infrastructure.
Industrial applications form the second-largest market segment, with hydrogen storage systems being increasingly integrated into manufacturing processes, particularly in steel production, chemical synthesis, and semiconductor fabrication. The demand for efficient hydrogen storage in these sectors is driven by both environmental regulations and economic incentives for reducing carbon emissions.
Regional analysis reveals that Asia-Pacific currently dominates the market with a 42% share, followed by Europe at 31% and North America at 21%. China has emerged as a particularly aggressive player, with its government committing $16 billion toward hydrogen infrastructure development through 2025, focusing heavily on storage technologies.
Market dynamics are significantly influenced by material advancements in hydrogen storage. Traditional compressed gas and cryogenic liquid storage methods are gradually being supplemented by solid-state storage technologies, which offer improved safety profiles and volumetric efficiency. This shift is creating new market opportunities for material science companies and specialized storage solution providers.
Customer segmentation shows distinct requirements across different end-users. While automotive applications prioritize volumetric efficiency and rapid hydrogen release rates, stationary storage applications for grid balancing emphasize cost-effectiveness and long-term stability. This diversification of requirements is driving specialized product development within the market.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, alongside emerging technology-focused firms specializing in advanced material-based storage solutions. Recent market consolidation through strategic acquisitions indicates that industry players are positioning themselves to capture value across the entire hydrogen value chain, with storage technologies representing a critical competitive advantage.
Current Challenges in Hydrogen Storage Technology
Despite significant advancements in hydrogen storage technologies, several critical challenges continue to impede widespread adoption of hydrogen as a mainstream energy carrier. The gravimetric and volumetric storage capacities of current materials remain insufficient for practical applications, particularly in mobile and transportation sectors. Most commercial storage systems achieve only 5-6 wt% hydrogen content, falling short of the U.S. Department of Energy's target of 9 wt% for vehicular applications.
Temperature and pressure management presents another substantial hurdle. Many promising materials require either extremely low temperatures (-253°C for liquid hydrogen) or high pressures (350-700 bar for compressed gas), creating significant energy penalties and safety concerns. The energy efficiency of the entire storage-release cycle is compromised when accounting for compression, liquefaction, or thermal management requirements.
Kinetics of hydrogen absorption and desorption remains problematic across multiple material classes. Metal hydrides often exhibit slow loading/unloading rates at ambient conditions, while chemical hydrogen carriers face challenges in reaction completeness and catalyst performance. These kinetic limitations directly impact refueling times and energy delivery rates, critical factors for commercial viability.
Cycling stability represents a persistent challenge, with many materials showing significant capacity degradation after repeated hydrogen loading-unloading cycles. This degradation stems from structural changes, surface contamination, or chemical decomposition during cycling, severely limiting operational lifetimes of storage systems.
Material cost and availability create economic barriers to scalability. Rare earth elements and platinum group metals, often essential for high-performance storage materials, face supply constraints and price volatility. The complex synthesis processes required for advanced nanomaterials further increase production costs.
System integration challenges exist at the engineering level, where heat management, mechanical stability, and safety considerations must be addressed simultaneously. The volumetric expansion of many storage materials during hydrogen loading creates mechanical stress that must be accommodated in system design.
Environmental and safety concerns persist, particularly regarding potential toxicity of some storage materials and their degradation products. Pyrophoric tendencies of certain metal hydrides and the potential for uncontrolled hydrogen release present safety risks that require robust engineering solutions.
Standardization and regulatory frameworks remain underdeveloped, creating uncertainty for technology developers and potential adopters. The lack of unified testing protocols and safety standards complicates comparative assessment of different storage technologies and slows commercial deployment.
Temperature and pressure management presents another substantial hurdle. Many promising materials require either extremely low temperatures (-253°C for liquid hydrogen) or high pressures (350-700 bar for compressed gas), creating significant energy penalties and safety concerns. The energy efficiency of the entire storage-release cycle is compromised when accounting for compression, liquefaction, or thermal management requirements.
Kinetics of hydrogen absorption and desorption remains problematic across multiple material classes. Metal hydrides often exhibit slow loading/unloading rates at ambient conditions, while chemical hydrogen carriers face challenges in reaction completeness and catalyst performance. These kinetic limitations directly impact refueling times and energy delivery rates, critical factors for commercial viability.
Cycling stability represents a persistent challenge, with many materials showing significant capacity degradation after repeated hydrogen loading-unloading cycles. This degradation stems from structural changes, surface contamination, or chemical decomposition during cycling, severely limiting operational lifetimes of storage systems.
Material cost and availability create economic barriers to scalability. Rare earth elements and platinum group metals, often essential for high-performance storage materials, face supply constraints and price volatility. The complex synthesis processes required for advanced nanomaterials further increase production costs.
System integration challenges exist at the engineering level, where heat management, mechanical stability, and safety considerations must be addressed simultaneously. The volumetric expansion of many storage materials during hydrogen loading creates mechanical stress that must be accommodated in system design.
Environmental and safety concerns persist, particularly regarding potential toxicity of some storage materials and their degradation products. Pyrophoric tendencies of certain metal hydrides and the potential for uncontrolled hydrogen release present safety risks that require robust engineering solutions.
Standardization and regulatory frameworks remain underdeveloped, creating uncertainty for technology developers and potential adopters. The lack of unified testing protocols and safety standards complicates comparative assessment of different storage technologies and slows commercial deployment.
Current Material Solutions for Hydrogen Storage
01 Metal hydride-based hydrogen storage materials
Metal hydrides are compounds formed by metals or metal alloys that can absorb and release hydrogen under specific temperature and pressure conditions. These materials offer high volumetric hydrogen storage capacity and can be designed with various compositions to optimize storage efficiency. Metal hydrides typically operate through a reversible chemical reaction where hydrogen molecules dissociate at the metal surface and form chemical bonds with the metal atoms, allowing for controlled hydrogen storage and release.- Metal hydride-based hydrogen storage materials: Metal hydrides are compounds formed by hydrogen and metals or metal alloys that can store hydrogen through chemical bonding. These materials offer high volumetric hydrogen storage capacity and can release hydrogen through thermal desorption. Various metal hydride systems including magnesium-based, aluminum-based, and transition metal-based hydrides have been developed to improve storage efficiency, with research focusing on reducing desorption temperatures and improving cycling stability.
- Carbon-based hydrogen storage materials: Carbon-based materials such as activated carbon, carbon nanotubes, graphene, and carbon aerogels can store hydrogen through physisorption mechanisms. These materials offer advantages including lightweight properties, high surface area, and tunable pore structures. Research focuses on enhancing the hydrogen adsorption capacity by surface modification, doping with metals, and optimizing pore size distribution to increase storage efficiency at practical temperatures and pressures.
- Chemical hydrogen storage systems: Chemical hydrogen storage systems store hydrogen in chemical compounds that release hydrogen through controlled chemical reactions. These include ammonia borane, sodium borohydride, formic acid derivatives, and liquid organic hydrogen carriers (LOHCs). These systems can achieve high gravimetric hydrogen storage capacities and operate at near-ambient conditions. Research focuses on catalyst development to improve hydrogen release kinetics and developing efficient regeneration processes for spent materials.
- Complex hydride hydrogen storage materials: Complex hydrides, including alanates, borohydrides, and amides, offer high hydrogen storage capacities through strong chemical bonds. These materials can store multiple hydrogen atoms per metal atom, resulting in high gravimetric storage densities. Research focuses on reducing desorption temperatures, improving reversibility, and enhancing reaction kinetics through catalysts and nanostructuring techniques to make these materials practical for commercial applications.
- Hybrid and composite hydrogen storage systems: Hybrid and composite hydrogen storage systems combine different storage mechanisms or materials to overcome limitations of individual approaches. These include metal-organic frameworks (MOFs), nanostructured composites, and multi-component systems that integrate physisorption and chemisorption properties. By combining materials with complementary properties, these systems can achieve enhanced storage capacities, improved kinetics, and better thermal management, leading to higher overall system efficiency.
02 Carbon-based hydrogen storage materials
Carbon-based materials such as carbon nanotubes, graphene, and activated carbon provide effective hydrogen storage through adsorption mechanisms. These materials offer advantages including lightweight structure, high surface area, and tunable pore sizes that enhance hydrogen uptake efficiency. The hydrogen molecules are typically stored through physisorption on the material surface, with storage capacity dependent on surface area, pore structure, and operating conditions. Various modifications and functionalization techniques can be applied to improve the hydrogen storage capacity of these carbon-based materials.Expand Specific Solutions03 Complex hydride hydrogen storage systems
Complex hydrides, including borohydrides, alanates, and amides, offer high gravimetric hydrogen storage capacity. These materials store hydrogen through chemical bonds within complex structures and can release hydrogen through thermal decomposition or catalytic reactions. The efficiency of complex hydride systems can be enhanced through catalyst addition, nanostructuring, and compositional optimization. Research focuses on improving the kinetics of hydrogen absorption and desorption while maintaining high storage capacity and addressing challenges related to thermal management and system reversibility.Expand Specific Solutions04 Hybrid and composite hydrogen storage materials
Hybrid and composite materials combine different hydrogen storage mechanisms to overcome limitations of single-material systems. These materials typically integrate metal hydrides with carbon structures, polymers, or other materials to create synergistic effects that enhance overall storage efficiency. The composite approach allows for improved heat management, faster kinetics, and better cycling stability. By combining materials with complementary properties, these systems can achieve higher volumetric and gravimetric capacities while operating under more moderate temperature and pressure conditions.Expand Specific Solutions05 Efficiency enhancement techniques for hydrogen storage
Various techniques can be employed to enhance the efficiency of hydrogen storage materials, including catalyst incorporation, nanostructuring, and surface modification. Catalysts reduce energy barriers for hydrogen absorption and desorption, while nanostructuring increases surface area and shortens diffusion paths. Temperature and pressure management systems optimize operating conditions, and doping with specific elements can modify electronic properties to improve hydrogen binding. These enhancement techniques collectively address key challenges in hydrogen storage efficiency including kinetics, capacity, and operational stability.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
Hydrogen storage materials technology is currently in a transitional phase from early commercialization to broader market adoption, with a global market expected to reach $5-7 billion by 2030. The competitive landscape features academic institutions (Zhejiang University, Fudan University) focusing on fundamental material parameters like binding energy and surface area, while industrial players demonstrate varying levels of technical maturity. Companies like Santoku Corp. and GRZ Technologies have achieved commercial-ready hydrogen-absorbing alloys, while Fraunhofer-Gesellschaft and AIST lead in metal-organic frameworks research. GKN Hydrogen and Intelligent Energy represent the application-focused segment, developing integrated storage systems. The field is characterized by international collaboration between research institutions and industry, with increasing focus on lightweight materials with higher gravimetric capacity and improved thermodynamics.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced metal-organic frameworks (MOFs) for hydrogen storage with exceptional surface areas exceeding 6000 m²/g. Their research focuses on optimizing critical parameters including pore size distribution (optimal 0.6-0.7 nm for H₂ adsorption), binding energy (ideal range of 15-25 kJ/mol), and gravimetric/volumetric capacity. DICP has pioneered MOF-based materials with integrated metal nanoparticles that enhance hydrogen binding through spillover mechanisms. Their materials demonstrate hydrogen storage capacities approaching 7.0 wt% at moderate pressures (50 bar) and ambient temperatures, significantly outperforming conventional storage methods. The institute has also developed computational screening methods to predict hydrogen storage performance based on material parameters, accelerating the discovery process.
Strengths: World-leading expertise in MOF synthesis with precise control over pore architecture and surface chemistry; integrated computational and experimental approaches for rapid material screening. Weaknesses: Some materials require complex synthesis procedures that may challenge industrial scalability; performance degradation after multiple adsorption-desorption cycles remains a challenge for long-term applications.
GRZ Technologies SA
Technical Solution: GRZ Technologies has developed a proprietary metal hydride-based hydrogen storage system that focuses on optimizing critical material parameters for commercial applications. Their technology centers on specially formulated Ti-Mn based alloys with modified crystal structures that achieve hydrogen storage densities exceeding 150 kg H₂/m³ - significantly higher than compressed hydrogen at 700 bar (approximately 40 kg/m³). GRZ's materials feature carefully engineered plateau pressures (0.5-30 bar) and enthalpy values (25-35 kJ/mol H₂) that enable hydrogen absorption/desorption at near-ambient temperatures (0-80°C) without requiring extreme heating or cooling. The company has optimized particle size distribution (typically 10-100 μm) and incorporated thermal conductivity enhancers to address heat management challenges during hydrogen charging/discharging, achieving thermal conductivity values of 10-15 W/m·K compared to 1-2 W/m·K for conventional metal hydrides.
Strengths: Commercially viable system with practical operating conditions; excellent volumetric storage density exceeding DOE targets; integrated thermal management solutions for rapid refueling. Weaknesses: Lower gravimetric capacity (typically 1.5-2.5 wt%) compared to some experimental materials; requires high-purity hydrogen to prevent degradation of storage capacity over multiple cycles.
Critical Material Parameters Analysis
Hydrogen storage materials and hydrogen fuel cells
PatentActiveUS8883371B2
Innovation
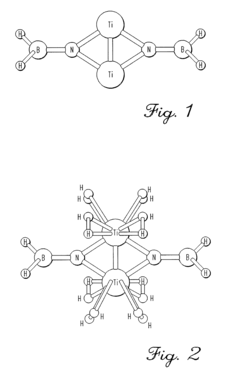
- Development of solid-state hydrogen storage materials comprising transition metal atoms bonded to elements from period 2 of the periodic table, which form stable hydrogenated states capable of adsorbing and desorbing hydrogen at temperatures below 200°C and pressures of 1 atm or less, achieving over 6.5% hydrogen weight percentage.
Hydrogen storage materials
PatentInactiveUS9133025B2
Innovation
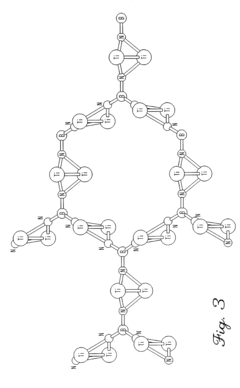
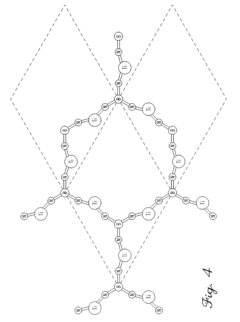
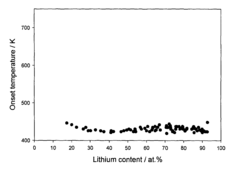
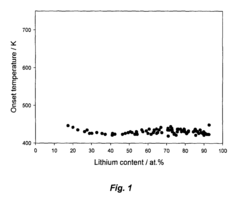
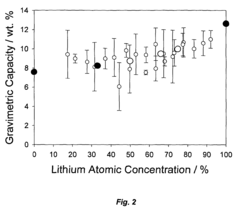
- Development of hydrogen storage materials with a specific composition of lithium and magnesium hydrides (LixMgyHn) within defined ranges of x, y, and n, allowing for reversible dehydriding and rehydriding under mild conditions, utilizing methods like vapor deposition in the presence of a hydrogen source to create an amorphous structure for enhanced hydrogen diffusion.
Safety and Regulatory Considerations
The implementation of hydrogen storage systems necessitates rigorous safety protocols and adherence to regulatory frameworks due to hydrogen's flammability and potential hazards. Material parameters that enhance storage efficiency must be balanced with safety considerations. For instance, high-pressure storage materials require robust containment systems certified to withstand specific pressure thresholds, typically regulated by standards such as ISO 16111 for hydrogen absorbed in reversible metal hydrides.
Temperature management represents another critical safety parameter, as many hydrogen storage materials exhibit exothermic reactions during absorption processes. Materials with lower heat of adsorption values (typically below 15-20 kJ/mol) present reduced thermal management challenges and consequently fewer safety risks during rapid charging cycles. Regulatory bodies like the International Organization for Standardization (ISO) and the Society of Automotive Engineers (SAE) have established specific temperature operation ranges for different storage technologies.
Material stability under cycling conditions directly impacts safety profiles. Degradation mechanisms such as pulverization in metal hydrides or structural collapse in MOFs can create fine particles that increase ignition risks. Regulations increasingly require materials to maintain structural integrity for a minimum number of cycles (typically 1,000+ for automotive applications) before significant performance degradation occurs.
Impurity tolerance represents another parameter with significant safety implications. Materials that can withstand common contaminants without producing toxic byproducts or experiencing catastrophic performance degradation are preferred from a regulatory standpoint. The SAE J2719 standard specifies hydrogen purity requirements (99.97%) for fuel cell vehicles, which indirectly influences material selection criteria.
Regulatory frameworks vary globally, creating challenges for technology deployment. The European Union's Regulation (EC) No 79/2009 concerning hydrogen-powered vehicles establishes specific requirements for materials used in hydrogen storage systems, while the United States Department of Energy has established performance targets including safety parameters such as loss of hydrogen during normal operation (<0.05 g/h/kg H₂ stored). Japan's High Pressure Gas Safety Act imposes additional requirements specific to their market.
Material toxicity and environmental impact have gained increasing regulatory attention. Materials containing toxic elements like beryllium or cadmium face significant regulatory hurdles despite potentially favorable storage parameters. Life cycle assessment requirements are becoming standard in regulatory frameworks, with particular emphasis on end-of-life disposal or recycling pathways for storage materials.
Temperature management represents another critical safety parameter, as many hydrogen storage materials exhibit exothermic reactions during absorption processes. Materials with lower heat of adsorption values (typically below 15-20 kJ/mol) present reduced thermal management challenges and consequently fewer safety risks during rapid charging cycles. Regulatory bodies like the International Organization for Standardization (ISO) and the Society of Automotive Engineers (SAE) have established specific temperature operation ranges for different storage technologies.
Material stability under cycling conditions directly impacts safety profiles. Degradation mechanisms such as pulverization in metal hydrides or structural collapse in MOFs can create fine particles that increase ignition risks. Regulations increasingly require materials to maintain structural integrity for a minimum number of cycles (typically 1,000+ for automotive applications) before significant performance degradation occurs.
Impurity tolerance represents another parameter with significant safety implications. Materials that can withstand common contaminants without producing toxic byproducts or experiencing catastrophic performance degradation are preferred from a regulatory standpoint. The SAE J2719 standard specifies hydrogen purity requirements (99.97%) for fuel cell vehicles, which indirectly influences material selection criteria.
Regulatory frameworks vary globally, creating challenges for technology deployment. The European Union's Regulation (EC) No 79/2009 concerning hydrogen-powered vehicles establishes specific requirements for materials used in hydrogen storage systems, while the United States Department of Energy has established performance targets including safety parameters such as loss of hydrogen during normal operation (<0.05 g/h/kg H₂ stored). Japan's High Pressure Gas Safety Act imposes additional requirements specific to their market.
Material toxicity and environmental impact have gained increasing regulatory attention. Materials containing toxic elements like beryllium or cadmium face significant regulatory hurdles despite potentially favorable storage parameters. Life cycle assessment requirements are becoming standard in regulatory frameworks, with particular emphasis on end-of-life disposal or recycling pathways for storage materials.
Economic Feasibility Assessment
The economic viability of hydrogen storage materials represents a critical factor in the broader adoption of hydrogen as an energy carrier. Initial capital investments for developing advanced hydrogen storage materials remain substantial, with research and development costs ranging from $5-20 million for laboratory-scale projects to $50-100 million for commercial-scale implementations. These investments encompass specialized equipment, high-purity raw materials, and advanced characterization techniques necessary for material optimization.
Production scaling presents significant economic challenges, particularly for complex metal hydrides and nanoporous materials. Current manufacturing costs for high-performance metal-organic frameworks (MOFs) range between $100-500 per kilogram, while conventional metal hydrides cost approximately $50-200 per kilogram. These figures must decrease by 60-80% to achieve cost parity with conventional energy storage technologies.
Lifecycle cost analysis reveals that material durability significantly impacts economic feasibility. Materials requiring replacement after 500-1000 hydrogen charging cycles increase operational costs by 30-40% compared to those maintaining performance beyond 1500 cycles. The economic impact of cycling stability becomes particularly pronounced in transportation applications where frequent hydrogen loading/unloading occurs.
Energy efficiency metrics directly translate to economic considerations. Materials requiring lower desorption temperatures (below 100°C) reduce operational energy costs by 25-35% compared to those needing temperatures above 150°C. Similarly, materials with faster kinetics reduce refueling infrastructure costs by enabling higher throughput with less equipment.
Market competitiveness analysis indicates that hydrogen storage materials must achieve a system-level cost below $300/kg H₂ capacity to compete with battery technologies in mobile applications, and below $500/kWh for stationary applications. Current best-performing materials achieve approximately $600-800/kg H₂ capacity, highlighting the economic gap that remains.
Regulatory factors also influence economic feasibility. Materials containing rare earth elements face supply chain vulnerabilities, with price fluctuations of 200-300% observed in recent years. Additionally, safety certification costs for novel materials can add $1-3 million to development budgets, representing a significant barrier for startups and smaller research entities.
Production scaling presents significant economic challenges, particularly for complex metal hydrides and nanoporous materials. Current manufacturing costs for high-performance metal-organic frameworks (MOFs) range between $100-500 per kilogram, while conventional metal hydrides cost approximately $50-200 per kilogram. These figures must decrease by 60-80% to achieve cost parity with conventional energy storage technologies.
Lifecycle cost analysis reveals that material durability significantly impacts economic feasibility. Materials requiring replacement after 500-1000 hydrogen charging cycles increase operational costs by 30-40% compared to those maintaining performance beyond 1500 cycles. The economic impact of cycling stability becomes particularly pronounced in transportation applications where frequent hydrogen loading/unloading occurs.
Energy efficiency metrics directly translate to economic considerations. Materials requiring lower desorption temperatures (below 100°C) reduce operational energy costs by 25-35% compared to those needing temperatures above 150°C. Similarly, materials with faster kinetics reduce refueling infrastructure costs by enabling higher throughput with less equipment.
Market competitiveness analysis indicates that hydrogen storage materials must achieve a system-level cost below $300/kg H₂ capacity to compete with battery technologies in mobile applications, and below $500/kWh for stationary applications. Current best-performing materials achieve approximately $600-800/kg H₂ capacity, highlighting the economic gap that remains.
Regulatory factors also influence economic feasibility. Materials containing rare earth elements face supply chain vulnerabilities, with price fluctuations of 200-300% observed in recent years. Additionally, safety certification costs for novel materials can add $1-3 million to development budgets, representing a significant barrier for startups and smaller research entities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!