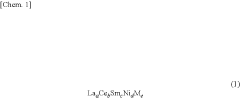What material parameters are essential for Hydrogen storage materials performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The journey of hydrogen storage technologies dates back to the early 20th century, but significant advancements have primarily occurred in the last three decades as environmental concerns and energy security issues have intensified. The evolution of hydrogen storage materials has progressed from conventional methods like high-pressure gas cylinders and cryogenic liquid storage to more sophisticated approaches involving solid-state materials.
The technological trajectory in this field has been driven by the increasing recognition of hydrogen as a clean energy carrier with the potential to decarbonize various sectors including transportation, power generation, and industrial processes. Material science innovations have continuously expanded the portfolio of potential storage solutions, from metal hydrides and complex hydrides to chemical hydrogen carriers and nanoporous materials.
Current technological objectives in hydrogen storage materials research center around addressing the "hydrogen storage trilemma" - simultaneously achieving high gravimetric capacity, high volumetric capacity, and favorable thermodynamics. The U.S. Department of Energy has established benchmarks for automotive applications, targeting 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions.
Material parameters have become increasingly recognized as the fundamental determinants of storage performance. These include crystalline structure, surface area, pore size distribution, binding energy, and catalytic properties. Understanding the relationship between these parameters and hydrogen storage capacity, kinetics, and cycling stability represents the cornerstone of current research efforts.
The technological objectives extend beyond mere storage capacity improvements to encompass practical considerations such as material cost, abundance, safety, and environmental impact. Researchers aim to develop materials that not only demonstrate exceptional storage properties in laboratory settings but also maintain performance under real-world operating conditions including rapid temperature fluctuations, exposure to contaminants, and thousands of absorption-desorption cycles.
Emerging research directions include multi-functional materials that combine storage capabilities with other desirable properties such as thermal management or integrated catalysis. Computational materials science and high-throughput screening methodologies are increasingly employed to accelerate the discovery and optimization of novel hydrogen storage materials, with machine learning approaches showing particular promise in predicting material performance based on fundamental parameters.
The technological trajectory in this field has been driven by the increasing recognition of hydrogen as a clean energy carrier with the potential to decarbonize various sectors including transportation, power generation, and industrial processes. Material science innovations have continuously expanded the portfolio of potential storage solutions, from metal hydrides and complex hydrides to chemical hydrogen carriers and nanoporous materials.
Current technological objectives in hydrogen storage materials research center around addressing the "hydrogen storage trilemma" - simultaneously achieving high gravimetric capacity, high volumetric capacity, and favorable thermodynamics. The U.S. Department of Energy has established benchmarks for automotive applications, targeting 6.5 wt% gravimetric capacity and 50 g/L volumetric capacity under moderate temperature and pressure conditions.
Material parameters have become increasingly recognized as the fundamental determinants of storage performance. These include crystalline structure, surface area, pore size distribution, binding energy, and catalytic properties. Understanding the relationship between these parameters and hydrogen storage capacity, kinetics, and cycling stability represents the cornerstone of current research efforts.
The technological objectives extend beyond mere storage capacity improvements to encompass practical considerations such as material cost, abundance, safety, and environmental impact. Researchers aim to develop materials that not only demonstrate exceptional storage properties in laboratory settings but also maintain performance under real-world operating conditions including rapid temperature fluctuations, exposure to contaminants, and thousands of absorption-desorption cycles.
Emerging research directions include multi-functional materials that combine storage capabilities with other desirable properties such as thermal management or integrated catalysis. Computational materials science and high-throughput screening methodologies are increasingly employed to accelerate the discovery and optimization of novel hydrogen storage materials, with machine learning approaches showing particular promise in predicting material performance based on fundamental parameters.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market is valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030, potentially reaching $28.1 billion by the end of the decade. This growth trajectory is primarily fueled by governmental commitments to carbon neutrality and substantial investments in hydrogen infrastructure worldwide.
The demand for efficient hydrogen storage materials is particularly pronounced in the transportation sector, which currently accounts for nearly 40% of the market share. Automotive manufacturers are increasingly incorporating hydrogen fuel cells into their vehicle designs, with Toyota, Hyundai, and Honda leading commercial deployments. The stationary power generation segment follows closely, representing about 30% of market demand, as industries seek reliable backup power solutions and grid stabilization technologies.
Regional analysis reveals that Asia-Pacific dominates the hydrogen storage materials market, holding approximately 45% of the global share. This dominance is attributed to aggressive hydrogen adoption policies in Japan, South Korea, and increasingly China. Europe follows with roughly 30% market share, driven by the European Union's Hydrogen Strategy and substantial funding through the European Green Deal. North America accounts for approximately 20% of the market, with growth accelerating due to recent policy shifts and infrastructure investments.
From a materials perspective, the market is segmented into metal hydrides, chemical hydrides, carbon-based materials, and complex hydrides. Metal hydrides currently lead with approximately 35% market share due to their established technology and relatively high volumetric storage capacity. However, complex hydrides are experiencing the fastest growth rate at 12.3% annually, as research advances improve their performance characteristics.
Customer requirements are increasingly focused on five key performance indicators: gravimetric density (targeting >6 wt%), volumetric density (>40 g/L), operating temperature (<100°C), cycling stability (>1000 cycles), and system cost (<$10/kWh). Market analysis indicates that materials meeting these parameters could capture premium pricing, with potential margins 30-40% higher than conventional solutions.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, materials technology firms such as BASF and Johnson Matthey, and emerging specialized startups focusing on novel storage technologies. Strategic partnerships between material developers and end-users are becoming increasingly common, accelerating commercialization timelines and creating integrated value chains.
The demand for efficient hydrogen storage materials is particularly pronounced in the transportation sector, which currently accounts for nearly 40% of the market share. Automotive manufacturers are increasingly incorporating hydrogen fuel cells into their vehicle designs, with Toyota, Hyundai, and Honda leading commercial deployments. The stationary power generation segment follows closely, representing about 30% of market demand, as industries seek reliable backup power solutions and grid stabilization technologies.
Regional analysis reveals that Asia-Pacific dominates the hydrogen storage materials market, holding approximately 45% of the global share. This dominance is attributed to aggressive hydrogen adoption policies in Japan, South Korea, and increasingly China. Europe follows with roughly 30% market share, driven by the European Union's Hydrogen Strategy and substantial funding through the European Green Deal. North America accounts for approximately 20% of the market, with growth accelerating due to recent policy shifts and infrastructure investments.
From a materials perspective, the market is segmented into metal hydrides, chemical hydrides, carbon-based materials, and complex hydrides. Metal hydrides currently lead with approximately 35% market share due to their established technology and relatively high volumetric storage capacity. However, complex hydrides are experiencing the fastest growth rate at 12.3% annually, as research advances improve their performance characteristics.
Customer requirements are increasingly focused on five key performance indicators: gravimetric density (targeting >6 wt%), volumetric density (>40 g/L), operating temperature (<100°C), cycling stability (>1000 cycles), and system cost (<$10/kWh). Market analysis indicates that materials meeting these parameters could capture premium pricing, with potential margins 30-40% higher than conventional solutions.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, materials technology firms such as BASF and Johnson Matthey, and emerging specialized startups focusing on novel storage technologies. Strategic partnerships between material developers and end-users are becoming increasingly common, accelerating commercialization timelines and creating integrated value chains.
Current State and Challenges in Hydrogen Storage Materials
Hydrogen storage materials research has advanced significantly in recent decades, yet remains at a critical juncture where theoretical potential exceeds practical implementation. Current state-of-the-art materials fall short of the U.S. Department of Energy's 2025 targets for gravimetric capacity (6.5 wt%) and volumetric capacity (50 g/L), with most commercial solutions achieving only 1-2 wt% under practical conditions.
Metal hydrides, particularly complex hydrides like NaAlH4 and LiBH4, demonstrate promising storage capacities but face severe kinetic limitations and unfavorable operating temperatures. Despite theoretical capacities exceeding 10 wt%, practical desorption often requires temperatures above 300°C, rendering them impractical for mobile applications without significant catalytic improvements.
Physisorption-based materials such as metal-organic frameworks (MOFs) and carbon nanostructures offer excellent kinetics and reversibility but struggle with low volumetric capacity at ambient temperatures. The current benchmark MOF-177 achieves only 1.25 wt% at room temperature and moderate pressures, though performance improves dramatically at cryogenic conditions.
The geographical distribution of hydrogen storage technology development shows concentration in North America, Europe, and East Asia, with the United States, Germany, Japan, and China leading patent filings. This distribution reflects both research infrastructure investment and strategic national hydrogen economy initiatives.
Key technical challenges persist across material classes. For metal hydrides, the thermodynamic dilemma remains unresolved: materials with suitable enthalpy for ambient operation typically exhibit prohibitively slow kinetics. For physisorption materials, the challenge lies in increasing binding energy without sacrificing reversibility. Additionally, material degradation during cycling represents a universal challenge, with most systems showing 20-30% capacity loss after 1000 cycles.
Manufacturing scalability presents another significant hurdle. Laboratory-scale synthesis methods for advanced materials like MOFs and complex hydrides often involve expensive precursors and energy-intensive processes that prove economically prohibitive at industrial scale. Current production capabilities limit widespread adoption, with estimated costs exceeding $500/kg for high-performance materials compared to DOE targets of $333/kg.
System integration challenges further complicate practical implementation, as thermal management systems, pressure regulation components, and safety features add significant weight and volume, reducing the effective gravimetric and volumetric capacities by 30-50% in real-world applications.
Metal hydrides, particularly complex hydrides like NaAlH4 and LiBH4, demonstrate promising storage capacities but face severe kinetic limitations and unfavorable operating temperatures. Despite theoretical capacities exceeding 10 wt%, practical desorption often requires temperatures above 300°C, rendering them impractical for mobile applications without significant catalytic improvements.
Physisorption-based materials such as metal-organic frameworks (MOFs) and carbon nanostructures offer excellent kinetics and reversibility but struggle with low volumetric capacity at ambient temperatures. The current benchmark MOF-177 achieves only 1.25 wt% at room temperature and moderate pressures, though performance improves dramatically at cryogenic conditions.
The geographical distribution of hydrogen storage technology development shows concentration in North America, Europe, and East Asia, with the United States, Germany, Japan, and China leading patent filings. This distribution reflects both research infrastructure investment and strategic national hydrogen economy initiatives.
Key technical challenges persist across material classes. For metal hydrides, the thermodynamic dilemma remains unresolved: materials with suitable enthalpy for ambient operation typically exhibit prohibitively slow kinetics. For physisorption materials, the challenge lies in increasing binding energy without sacrificing reversibility. Additionally, material degradation during cycling represents a universal challenge, with most systems showing 20-30% capacity loss after 1000 cycles.
Manufacturing scalability presents another significant hurdle. Laboratory-scale synthesis methods for advanced materials like MOFs and complex hydrides often involve expensive precursors and energy-intensive processes that prove economically prohibitive at industrial scale. Current production capabilities limit widespread adoption, with estimated costs exceeding $500/kg for high-performance materials compared to DOE targets of $333/kg.
System integration challenges further complicate practical implementation, as thermal management systems, pressure regulation components, and safety features add significant weight and volume, reducing the effective gravimetric and volumetric capacities by 30-50% in real-world applications.
Current Material Solutions for Hydrogen Storage
01 Metal hydride-based hydrogen storage materials
Metal hydrides are widely used for hydrogen storage due to their high volumetric capacity. These materials form chemical bonds with hydrogen, allowing for reversible storage through absorption and desorption processes. Various metal hydrides including magnesium-based, aluminum-based, and transition metal-based compounds demonstrate different performance characteristics in terms of storage capacity, operating temperature, and cycling stability. Research focuses on improving their kinetics and reducing desorption temperatures to enhance practical applications.- Metal hydride-based hydrogen storage materials: Metal hydrides are compounds formed by hydrogen and metals that can store hydrogen through chemical bonding. These materials offer high volumetric hydrogen storage capacity and can release hydrogen through heating or pressure reduction. Various metal hydride systems include magnesium-based hydrides, complex hydrides like alanates and borohydrides, and intermetallic compounds. Their performance is characterized by storage capacity, operating temperature, cycling stability, and kinetics of hydrogen absorption and desorption.
- Carbon-based hydrogen storage materials: Carbon-based materials such as activated carbon, carbon nanotubes, graphene, and carbon aerogels can store hydrogen through physisorption mechanisms. These materials feature high surface area and porosity that enable hydrogen molecules to adsorb onto their surfaces. The performance of carbon-based hydrogen storage materials depends on their specific surface area, pore structure, surface functionality, and operating conditions including temperature and pressure. They typically offer advantages in terms of lightweight properties and fast kinetics.
- Metal-organic frameworks for hydrogen storage: Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. Their exceptional porosity, tunable pore size, and high surface area make them promising candidates for hydrogen storage. MOFs can store hydrogen through physisorption mechanisms, with performance metrics including gravimetric and volumetric capacity, adsorption enthalpy, and structural stability during cycling. Their modular nature allows for design optimization through metal center and organic linker selection.
- Composite hydrogen storage materials: Composite hydrogen storage materials combine different types of storage mechanisms or materials to overcome limitations of individual components. These may include metal hydride-carbon composites, catalyst-doped systems, or core-shell structures. By integrating multiple materials, composites can achieve improved hydrogen storage capacity, enhanced kinetics, better thermal management, and reduced operating temperatures. The synergistic effects between components often result in performance characteristics superior to those of the individual constituents.
- Performance enhancement techniques for hydrogen storage materials: Various techniques can enhance the performance of hydrogen storage materials, including nanostructuring, catalyst addition, defect engineering, and surface modification. Nanostructuring reduces diffusion distances and increases active surface area. Catalysts improve hydrogen dissociation and recombination kinetics. Defect engineering creates additional binding sites for hydrogen. Surface modifications can prevent degradation and improve cycling stability. These approaches collectively address key performance metrics such as storage capacity, operating conditions, kinetics, and long-term durability.
02 Carbon-based hydrogen storage materials
Carbon-based materials such as activated carbon, carbon nanotubes, and graphene offer promising hydrogen storage capabilities through physical adsorption mechanisms. These materials feature high surface areas and tunable pore structures that can be optimized for hydrogen uptake. The performance of carbon-based storage materials is characterized by fast kinetics and good reversibility, though they typically require low temperatures to achieve significant storage capacities. Surface functionalization and doping strategies are employed to enhance their hydrogen affinity and overall storage performance.Expand Specific Solutions03 Complex hydrides for high-capacity hydrogen storage
Complex hydrides, including borohydrides, alanates, and amides, represent advanced hydrogen storage materials with exceptionally high theoretical capacities. These materials store hydrogen through complex chemical bonds and can achieve gravimetric capacities exceeding conventional metal hydrides. Their performance is characterized by high energy density but often limited by slow kinetics and high operating temperatures. Research focuses on catalyst development, nanostructuring, and compositional modifications to address these challenges and improve cycling stability and reversibility for practical applications.Expand Specific Solutions04 Composite and nanostructured hydrogen storage materials
Composite and nanostructured materials combine different storage mechanisms to achieve enhanced performance. These include metal-organic frameworks, nanoconfined hydrides, and multi-component systems that leverage synergistic effects between different materials. Nanostructuring improves hydrogen diffusion pathways and reaction kinetics, while reducing desorption temperatures. These advanced materials demonstrate improved cycling stability, faster kinetics, and tunable operating conditions compared to their bulk counterparts, making them promising candidates for next-generation hydrogen storage applications.Expand Specific Solutions05 Performance evaluation and testing methods for hydrogen storage materials
Standardized testing protocols and performance metrics are essential for evaluating hydrogen storage materials. Key performance parameters include gravimetric and volumetric capacity, operating temperature and pressure ranges, cycling stability, kinetics of absorption/desorption, and thermal management characteristics. Advanced characterization techniques such as pressure-composition-temperature measurements, thermogravimetric analysis, and in-situ diffraction methods provide critical insights into material behavior. These evaluation methods enable meaningful comparisons between different storage technologies and guide material optimization strategies for specific applications.Expand Specific Solutions
Leading Organizations in Hydrogen Storage Research
Hydrogen storage materials technology is currently in a transitional phase from research to early commercialization, with a global market expected to reach significant growth as hydrogen economies develop. Key material parameters essential for performance include gravimetric and volumetric capacity, thermodynamics (enthalpy of formation), kinetics (absorption/desorption rates), cycling stability, and operating conditions (temperature/pressure requirements). The competitive landscape features academic institutions like Zhejiang University and University of Tokyo conducting fundamental research, while companies demonstrate varying levels of technological maturity. Established players like Santoku Corp. and Toyota Central R&D Labs focus on metal hydrides, while startups like GRZ Technologies and Hydrexia develop novel storage solutions. Automotive manufacturers including Mercedes-Benz and Nissan are increasingly investing in hydrogen technologies to support their clean mobility strategies.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced metal-organic frameworks (MOFs) for hydrogen storage, focusing on optimizing surface area, pore size distribution, and binding energy. Their research emphasizes materials with high gravimetric capacity (>7 wt%) and volumetric density (>40 g/L) at moderate pressures. DICP has pioneered the development of Mg-based materials with enhanced kinetics through catalytic additives and nanostructuring techniques. Their approach includes systematic investigation of thermodynamic parameters (ΔH ≈ 20-40 kJ/mol H₂) to achieve reversible hydrogen storage at practical temperatures. Recent breakthroughs include novel composite materials combining chemical and physical storage mechanisms, achieving improved cycling stability over 1000 cycles with minimal capacity degradation.
Strengths: World-leading expertise in MOF synthesis and characterization; strong integration of theoretical modeling with experimental validation; extensive facilities for in-situ characterization. Weaknesses: Some materials still require high desorption temperatures; challenges in scaling production methods from laboratory to industrial scale.
GRZ Technologies SA
Technical Solution: GRZ Technologies has developed proprietary metal alloy-based hydrogen storage materials with optimized thermodynamic and kinetic properties. Their approach focuses on tuning the plateau pressure through precise control of alloy composition, achieving equilibrium pressures of 1-10 bar at ambient temperature. GRZ's materials feature carefully engineered microstructure with grain sizes below 50 nm to enhance hydrogen diffusion pathways, resulting in charging rates up to 5 times faster than conventional materials. The company has pioneered a unique manufacturing process that creates controlled porosity (40-60%) and specific surface area (>500 m²/g), critical parameters for rapid hydrogen uptake and release. Their materials demonstrate exceptional cycling stability with less than 10% capacity degradation after 1000 cycles, achieved through specialized surface treatments that minimize oxidation and contamination effects. GRZ's technology emphasizes the relationship between crystal structure defects and hydrogen trapping sites, optimizing binding energies in the 15-25 kJ/mol range for room temperature operation.
Strengths: Highly practical focus on system integration; materials designed specifically for stationary storage applications; excellent cycling stability and resistance to poisoning. Weaknesses: Lower gravimetric capacity compared to some experimental materials; production costs remain higher than conventional storage technologies.
Critical Material Parameters Analysis and Impact
Hydrogen storage material, hydrogen storage container, and hydrogen supply apparatus
PatentPendingUS20240208807A1
Innovation
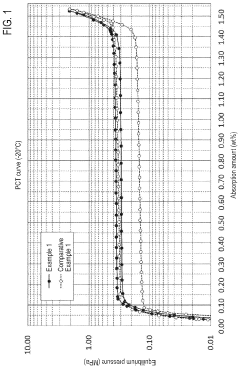
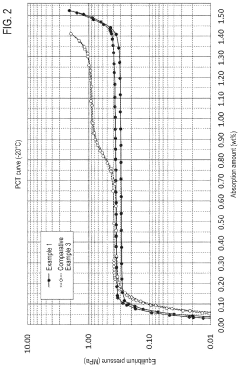
- Development of hydrogen storage materials with specific rare earth and transition metal compositions, such as LaNi5-based alloys, optimized for -20°C operation, featuring reduced hysteresis and stable hydrogen desorption with minimal pressure fluctuations, as represented by the elemental composition formula (M, a, b, c, d, e) ensuring large hydrogen storage and desorption capacity.
Hydrogen storage materials
PatentInactiveUS9133025B2
Innovation
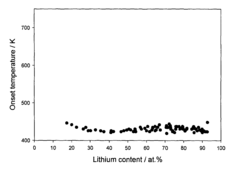
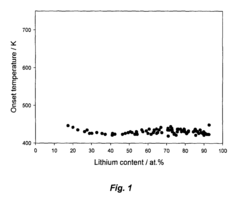
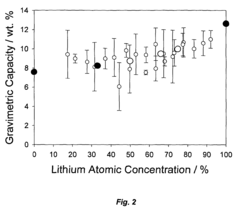
- Development of hydrogen storage materials with a specific composition of lithium and magnesium hydrides (LixMgyHn) within defined ranges of x, y, and n, allowing for reversible dehydriding and rehydriding under mild conditions, utilizing methods like vapor deposition in the presence of a hydrogen source to create an amorphous structure for enhanced hydrogen diffusion.
Safety and Regulatory Framework for Hydrogen Storage
The regulatory landscape for hydrogen storage materials is complex and evolving rapidly as hydrogen gains prominence in clean energy strategies worldwide. International standards such as ISO/TC 197 and IEC/TC 105 establish fundamental safety requirements for hydrogen storage systems, covering aspects from material compatibility to pressure vessel design. These standards are continuously updated to incorporate new material developments and safety insights from research and industry experience.
National regulations vary significantly across regions, with countries like Japan, Germany, and the United States implementing distinct frameworks. The U.S. Department of Energy's technical targets for hydrogen storage systems include specific safety parameters that directly influence material selection, such as operational temperature ranges, pressure limitations, and toxicity thresholds. Material parameters must comply with these regulatory requirements to be commercially viable.
Risk assessment methodologies for hydrogen storage materials have become increasingly sophisticated, focusing on material-specific properties like embrittlement resistance, thermal stability, and reaction kinetics during charging/discharging cycles. These assessments inform both regulatory development and material design parameters, creating a feedback loop between innovation and safety governance.
Certification processes for hydrogen storage materials require extensive testing protocols that evaluate performance under normal and extreme conditions. Materials must demonstrate resilience against environmental factors, mechanical stress, and cycling degradation while maintaining safety margins. These certification requirements directly influence which material parameters researchers prioritize during development phases.
Insurance considerations also play a significant role in material parameter selection, as insurers require quantifiable safety data before underwriting hydrogen storage installations. This has led to the development of standardized testing methodologies that evaluate material failure modes and establish safety thresholds for different application environments.
The transportation of hydrogen storage materials faces particularly stringent regulations due to the combined challenges of pressure, potential reactivity, and public safety concerns. UN regulations for dangerous goods transport establish specific requirements for material containment systems that directly impact material selection parameters, especially for mobile applications.
As hydrogen infrastructure expands globally, regulatory harmonization efforts are underway to establish consistent safety standards across jurisdictions. This harmonization process is gradually defining a common set of essential material parameters that balance safety requirements with performance needs, creating clearer development targets for materials scientists and engineers working in this field.
National regulations vary significantly across regions, with countries like Japan, Germany, and the United States implementing distinct frameworks. The U.S. Department of Energy's technical targets for hydrogen storage systems include specific safety parameters that directly influence material selection, such as operational temperature ranges, pressure limitations, and toxicity thresholds. Material parameters must comply with these regulatory requirements to be commercially viable.
Risk assessment methodologies for hydrogen storage materials have become increasingly sophisticated, focusing on material-specific properties like embrittlement resistance, thermal stability, and reaction kinetics during charging/discharging cycles. These assessments inform both regulatory development and material design parameters, creating a feedback loop between innovation and safety governance.
Certification processes for hydrogen storage materials require extensive testing protocols that evaluate performance under normal and extreme conditions. Materials must demonstrate resilience against environmental factors, mechanical stress, and cycling degradation while maintaining safety margins. These certification requirements directly influence which material parameters researchers prioritize during development phases.
Insurance considerations also play a significant role in material parameter selection, as insurers require quantifiable safety data before underwriting hydrogen storage installations. This has led to the development of standardized testing methodologies that evaluate material failure modes and establish safety thresholds for different application environments.
The transportation of hydrogen storage materials faces particularly stringent regulations due to the combined challenges of pressure, potential reactivity, and public safety concerns. UN regulations for dangerous goods transport establish specific requirements for material containment systems that directly impact material selection parameters, especially for mobile applications.
As hydrogen infrastructure expands globally, regulatory harmonization efforts are underway to establish consistent safety standards across jurisdictions. This harmonization process is gradually defining a common set of essential material parameters that balance safety requirements with performance needs, creating clearer development targets for materials scientists and engineers working in this field.
Sustainability Assessment of Storage Materials
Sustainability assessment of hydrogen storage materials requires a comprehensive evaluation framework that considers environmental, economic, and social impacts throughout the material's lifecycle. The assessment must balance the material's hydrogen storage performance with its broader sustainability implications.
Environmental considerations form the cornerstone of sustainability assessment. Materials requiring energy-intensive extraction processes or rare earth elements often present significant environmental challenges despite excellent storage properties. Life Cycle Assessment (LCA) methodologies provide quantitative measures of environmental impacts, including carbon footprint, water usage, and land use changes associated with material production and disposal.
Resource availability represents another critical dimension in sustainability evaluation. Materials dependent on scarce elements face long-term viability concerns regardless of their technical performance. The geographical distribution of raw materials introduces geopolitical considerations that may affect supply chain resilience and material accessibility, potentially limiting widespread adoption of certain storage solutions.
Energy efficiency across the complete hydrogen storage cycle significantly impacts overall sustainability. The energy required for hydrogen loading and release must be considered alongside storage capacity metrics. Materials requiring high temperatures or pressures for hydrogen desorption may demonstrate excellent gravimetric capacity but poor overall energy efficiency, reducing their sustainability value.
Manufacturing scalability presents both technical and sustainability challenges. Production processes that can be scaled while maintaining environmental performance are essential for commercial viability. Materials requiring complex synthesis routes or toxic precursors may face regulatory barriers and increased production costs, limiting their market potential despite promising laboratory performance.
End-of-life considerations complete the sustainability assessment framework. Recyclability and reusability of storage materials significantly impact their long-term environmental footprint. Materials designed with circular economy principles offer advantages through reduced waste generation and resource conservation, even when their initial performance metrics may be slightly lower than alternatives.
Economic viability remains inseparable from sustainability assessment. Cost-effective materials that balance performance with reasonable production expenses are more likely to achieve market penetration and deliver real-world sustainability benefits. The total cost of ownership, including maintenance and operational expenses, provides a more comprehensive economic sustainability metric than material cost alone.
Environmental considerations form the cornerstone of sustainability assessment. Materials requiring energy-intensive extraction processes or rare earth elements often present significant environmental challenges despite excellent storage properties. Life Cycle Assessment (LCA) methodologies provide quantitative measures of environmental impacts, including carbon footprint, water usage, and land use changes associated with material production and disposal.
Resource availability represents another critical dimension in sustainability evaluation. Materials dependent on scarce elements face long-term viability concerns regardless of their technical performance. The geographical distribution of raw materials introduces geopolitical considerations that may affect supply chain resilience and material accessibility, potentially limiting widespread adoption of certain storage solutions.
Energy efficiency across the complete hydrogen storage cycle significantly impacts overall sustainability. The energy required for hydrogen loading and release must be considered alongside storage capacity metrics. Materials requiring high temperatures or pressures for hydrogen desorption may demonstrate excellent gravimetric capacity but poor overall energy efficiency, reducing their sustainability value.
Manufacturing scalability presents both technical and sustainability challenges. Production processes that can be scaled while maintaining environmental performance are essential for commercial viability. Materials requiring complex synthesis routes or toxic precursors may face regulatory barriers and increased production costs, limiting their market potential despite promising laboratory performance.
End-of-life considerations complete the sustainability assessment framework. Recyclability and reusability of storage materials significantly impact their long-term environmental footprint. Materials designed with circular economy principles offer advantages through reduced waste generation and resource conservation, even when their initial performance metrics may be slightly lower than alternatives.
Economic viability remains inseparable from sustainability assessment. Cost-effective materials that balance performance with reasonable production expenses are more likely to achieve market penetration and deliver real-world sustainability benefits. The total cost of ownership, including maintenance and operational expenses, provides a more comprehensive economic sustainability metric than material cost alone.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!