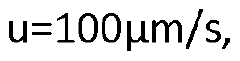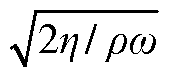Compare FTIR vs HPLC: Solvent Separation Efficiency
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
FTIR and HPLC Technology Background and Objectives
Fourier Transform Infrared Spectroscopy (FTIR) and High-Performance Liquid Chromatography (HPLC) represent two fundamental analytical techniques that have revolutionized chemical analysis across multiple industries. FTIR technology emerged in the 1960s as an advancement over dispersive IR spectroscopy, utilizing mathematical Fourier transforms to convert raw data into actual spectra. This innovation dramatically improved signal-to-noise ratios and reduced analysis time from hours to seconds. HPLC, meanwhile, evolved from traditional column chromatography in the 1970s, with significant improvements in pressure systems, column materials, and detection methods.
The technological evolution of both systems has followed distinct but complementary paths. FTIR has progressed from large benchtop instruments to portable, handheld devices with enhanced spectral resolution and sensitivity. Modern FTIR systems incorporate advanced interferometers, more sensitive detectors, and sophisticated software algorithms for spectral analysis. HPLC has similarly advanced with the development of ultra-high-performance liquid chromatography (UHPLC), which operates at significantly higher pressures, allowing for faster analyses with improved resolution.
When examining solvent separation efficiency specifically, these technologies serve different but interconnected purposes. HPLC directly separates components in a mixture based on their differential interactions with the stationary and mobile phases, making it a separation technique first and an analytical method second. FTIR, conversely, is purely an analytical technique that identifies compounds based on their absorption of infrared radiation at specific wavelengths, providing molecular structural information.
The primary technical objective in comparing these technologies for solvent separation efficiency is to determine their respective capabilities, limitations, and complementary applications. HPLC excels in quantitative analysis, offering superior separation of complex mixtures with detection limits in the parts-per-billion range. FTIR provides rapid, non-destructive analysis with minimal sample preparation and the ability to identify functional groups and molecular structures.
Current technological trends point toward integration of these techniques, with hyphenated methods like HPLC-FTIR becoming increasingly important. These combined approaches leverage the separation power of HPLC with the identification capabilities of FTIR, offering more comprehensive analytical solutions. Additionally, miniaturization efforts are advancing for both technologies, with microfluidic HPLC systems and compact FTIR spectrometers expanding their application range.
The ultimate goal of this technical assessment is to evaluate how these technologies can be optimally deployed—either independently or in combination—to achieve maximum solvent separation efficiency across various industrial applications, including pharmaceutical development, environmental monitoring, food safety, and materials science.
The technological evolution of both systems has followed distinct but complementary paths. FTIR has progressed from large benchtop instruments to portable, handheld devices with enhanced spectral resolution and sensitivity. Modern FTIR systems incorporate advanced interferometers, more sensitive detectors, and sophisticated software algorithms for spectral analysis. HPLC has similarly advanced with the development of ultra-high-performance liquid chromatography (UHPLC), which operates at significantly higher pressures, allowing for faster analyses with improved resolution.
When examining solvent separation efficiency specifically, these technologies serve different but interconnected purposes. HPLC directly separates components in a mixture based on their differential interactions with the stationary and mobile phases, making it a separation technique first and an analytical method second. FTIR, conversely, is purely an analytical technique that identifies compounds based on their absorption of infrared radiation at specific wavelengths, providing molecular structural information.
The primary technical objective in comparing these technologies for solvent separation efficiency is to determine their respective capabilities, limitations, and complementary applications. HPLC excels in quantitative analysis, offering superior separation of complex mixtures with detection limits in the parts-per-billion range. FTIR provides rapid, non-destructive analysis with minimal sample preparation and the ability to identify functional groups and molecular structures.
Current technological trends point toward integration of these techniques, with hyphenated methods like HPLC-FTIR becoming increasingly important. These combined approaches leverage the separation power of HPLC with the identification capabilities of FTIR, offering more comprehensive analytical solutions. Additionally, miniaturization efforts are advancing for both technologies, with microfluidic HPLC systems and compact FTIR spectrometers expanding their application range.
The ultimate goal of this technical assessment is to evaluate how these technologies can be optimally deployed—either independently or in combination—to achieve maximum solvent separation efficiency across various industrial applications, including pharmaceutical development, environmental monitoring, food safety, and materials science.
Market Demand Analysis for Solvent Separation Technologies
The global market for solvent separation technologies has witnessed substantial growth in recent years, driven by increasing demands across pharmaceutical, chemical, environmental, and food industries. The combined market value for analytical instruments used in solvent separation exceeded $12 billion in 2022, with a projected CAGR of 5.8% through 2028, highlighting the critical importance of these technologies in various industrial applications.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 35% of the total demand. These industries require precise solvent separation techniques for drug development, quality control, and research applications. The stringent regulatory requirements imposed by FDA, EMA, and other regulatory bodies have further accelerated the adoption of advanced analytical methods like FTIR and HPLC for solvent analysis and separation.
Environmental monitoring and testing laboratories constitute another significant market segment, driven by increasing environmental regulations and growing concerns about industrial pollutants. Government initiatives worldwide to monitor water quality, air pollution, and soil contamination have created substantial demand for efficient solvent separation technologies that can detect trace contaminants with high accuracy.
The chemical manufacturing industry represents the third-largest market segment, where solvent separation technologies are essential for process optimization, quality control, and product development. As manufacturers focus on developing greener processes and reducing environmental impact, the demand for more efficient separation technologies continues to rise.
Regional analysis indicates North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate, primarily driven by rapid industrialization in China and India, increasing R&D investments, and growing environmental concerns.
Customer surveys reveal that end-users prioritize accuracy, sensitivity, analysis speed, and cost-effectiveness when selecting solvent separation technologies. HPLC systems are preferred in applications requiring quantitative analysis and high throughput, while FTIR spectroscopy is favored for qualitative analysis and rapid screening applications.
Market trends indicate growing demand for hybrid systems that combine multiple analytical techniques, offering comprehensive analysis capabilities. Additionally, there is increasing interest in portable and miniaturized systems that enable on-site analysis, particularly in environmental monitoring and point-of-care diagnostics.
The COVID-19 pandemic temporarily disrupted supply chains but simultaneously accelerated digitalization in analytical laboratories, creating new opportunities for remote monitoring and automated systems. Post-pandemic recovery has been strong, with renewed focus on research infrastructure development and technological advancements in separation sciences.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for approximately 35% of the total demand. These industries require precise solvent separation techniques for drug development, quality control, and research applications. The stringent regulatory requirements imposed by FDA, EMA, and other regulatory bodies have further accelerated the adoption of advanced analytical methods like FTIR and HPLC for solvent analysis and separation.
Environmental monitoring and testing laboratories constitute another significant market segment, driven by increasing environmental regulations and growing concerns about industrial pollutants. Government initiatives worldwide to monitor water quality, air pollution, and soil contamination have created substantial demand for efficient solvent separation technologies that can detect trace contaminants with high accuracy.
The chemical manufacturing industry represents the third-largest market segment, where solvent separation technologies are essential for process optimization, quality control, and product development. As manufacturers focus on developing greener processes and reducing environmental impact, the demand for more efficient separation technologies continues to rise.
Regional analysis indicates North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate, primarily driven by rapid industrialization in China and India, increasing R&D investments, and growing environmental concerns.
Customer surveys reveal that end-users prioritize accuracy, sensitivity, analysis speed, and cost-effectiveness when selecting solvent separation technologies. HPLC systems are preferred in applications requiring quantitative analysis and high throughput, while FTIR spectroscopy is favored for qualitative analysis and rapid screening applications.
Market trends indicate growing demand for hybrid systems that combine multiple analytical techniques, offering comprehensive analysis capabilities. Additionally, there is increasing interest in portable and miniaturized systems that enable on-site analysis, particularly in environmental monitoring and point-of-care diagnostics.
The COVID-19 pandemic temporarily disrupted supply chains but simultaneously accelerated digitalization in analytical laboratories, creating new opportunities for remote monitoring and automated systems. Post-pandemic recovery has been strong, with renewed focus on research infrastructure development and technological advancements in separation sciences.
Current Status and Challenges in Separation Science
Separation science has witnessed significant advancements in recent decades, with various analytical techniques being developed and refined for solvent separation applications. Currently, the field faces several challenges related to efficiency, accuracy, and applicability across different sample types. High-Performance Liquid Chromatography (HPLC) remains the gold standard for quantitative analysis of complex mixtures, offering excellent resolution and sensitivity for a wide range of compounds.
The current HPLC technology landscape features ultra-high-performance systems capable of operating at pressures exceeding 15,000 psi, enabling faster analyses with superior resolution. However, these systems face challenges including high operational costs, complex method development requirements, and limitations in analyzing certain compound classes without derivatization. Additionally, the environmental impact of solvent consumption presents a significant sustainability concern.
Fourier Transform Infrared Spectroscopy (FTIR) has emerged as a complementary technique with distinct advantages in certain separation applications. Modern FTIR systems feature improved signal-to-noise ratios and can be coupled with attenuated total reflection (ATR) accessories, eliminating the need for extensive sample preparation. The non-destructive nature of FTIR analysis represents a significant advantage over traditional HPLC methods.
A major challenge in the field is achieving optimal separation efficiency while minimizing analysis time and solvent consumption. HPLC typically requires substantial method development to optimize mobile phase composition, flow rate, and column selection for each specific application. In contrast, FTIR offers rapid analysis but struggles with complex mixture resolution compared to chromatographic techniques.
Miniaturization represents another significant trend, with microfluidic separation systems gaining prominence. These systems promise reduced solvent consumption and faster analyses but face challenges in scaling and reproducibility. The integration of FTIR with microfluidic platforms remains in early developmental stages, presenting both opportunities and technical hurdles.
Data processing capabilities have become increasingly important, with advanced chemometric approaches being applied to both HPLC and FTIR data. Machine learning algorithms are being developed to enhance spectral interpretation and chromatographic peak identification, though standardization of these approaches remains a challenge.
Regulatory considerations also impact the adoption of different separation techniques. HPLC methods are well-established in regulatory frameworks, while FTIR applications for quantitative analysis in regulated environments require additional validation steps. This regulatory landscape influences technology adoption rates across different industries.
The geographical distribution of separation science expertise shows concentration in North America, Europe, and increasingly in Asia, particularly China and Japan. Research output in this field has grown substantially, with publications related to novel separation techniques increasing by approximately 8% annually over the past five years.
The current HPLC technology landscape features ultra-high-performance systems capable of operating at pressures exceeding 15,000 psi, enabling faster analyses with superior resolution. However, these systems face challenges including high operational costs, complex method development requirements, and limitations in analyzing certain compound classes without derivatization. Additionally, the environmental impact of solvent consumption presents a significant sustainability concern.
Fourier Transform Infrared Spectroscopy (FTIR) has emerged as a complementary technique with distinct advantages in certain separation applications. Modern FTIR systems feature improved signal-to-noise ratios and can be coupled with attenuated total reflection (ATR) accessories, eliminating the need for extensive sample preparation. The non-destructive nature of FTIR analysis represents a significant advantage over traditional HPLC methods.
A major challenge in the field is achieving optimal separation efficiency while minimizing analysis time and solvent consumption. HPLC typically requires substantial method development to optimize mobile phase composition, flow rate, and column selection for each specific application. In contrast, FTIR offers rapid analysis but struggles with complex mixture resolution compared to chromatographic techniques.
Miniaturization represents another significant trend, with microfluidic separation systems gaining prominence. These systems promise reduced solvent consumption and faster analyses but face challenges in scaling and reproducibility. The integration of FTIR with microfluidic platforms remains in early developmental stages, presenting both opportunities and technical hurdles.
Data processing capabilities have become increasingly important, with advanced chemometric approaches being applied to both HPLC and FTIR data. Machine learning algorithms are being developed to enhance spectral interpretation and chromatographic peak identification, though standardization of these approaches remains a challenge.
Regulatory considerations also impact the adoption of different separation techniques. HPLC methods are well-established in regulatory frameworks, while FTIR applications for quantitative analysis in regulated environments require additional validation steps. This regulatory landscape influences technology adoption rates across different industries.
The geographical distribution of separation science expertise shows concentration in North America, Europe, and increasingly in Asia, particularly China and Japan. Research output in this field has grown substantially, with publications related to novel separation techniques increasing by approximately 8% annually over the past five years.
Comparative Analysis of FTIR and HPLC Separation Solutions
01 FTIR analysis techniques for solvent separation
Fourier Transform Infrared Spectroscopy (FTIR) is used to analyze and characterize solvents in separation processes. This technique helps identify functional groups and molecular structures in various solvents, allowing for the assessment of separation efficiency. FTIR can detect impurities and monitor the purity of separated solvents in real-time, providing valuable data for optimizing separation processes.- FTIR analysis techniques for solvent separation: Fourier Transform Infrared Spectroscopy (FTIR) is utilized to analyze and characterize solvents during separation processes. This technique helps identify functional groups and molecular structures in different solvents, allowing for the assessment of separation efficiency. FTIR provides spectral fingerprints that can be used to monitor the purity of separated solvents and detect impurities or contaminants that might affect separation efficiency.
- HPLC methodologies for enhanced solvent separation: High-Performance Liquid Chromatography (HPLC) methodologies are employed to achieve efficient solvent separation. These methods involve optimizing mobile phase composition, column selection, and detection parameters to enhance separation efficiency. Advanced HPLC techniques can separate complex solvent mixtures with high resolution, allowing for precise quantification and characterization of individual components. The efficiency of separation can be measured through parameters such as theoretical plates, resolution, and peak symmetry.
- Combined FTIR-HPLC systems for comprehensive solvent analysis: Integrated systems combining FTIR and HPLC technologies provide comprehensive analysis of solvents during separation processes. These hyphenated techniques allow for simultaneous chromatographic separation and spectroscopic identification, enhancing the overall efficiency of solvent analysis. The combined approach offers advantages in terms of reduced analysis time, improved accuracy, and the ability to identify and quantify complex solvent mixtures with greater precision than either technique alone.
- Novel materials and stationary phases for improved separation: Advanced materials and innovative stationary phases have been developed to enhance solvent separation efficiency in both FTIR and HPLC applications. These materials include specialized polymers, modified silica, and nanomaterials with tailored surface properties that improve selectivity and resolution. The development of these novel stationary phases has led to significant improvements in separation efficiency, particularly for challenging solvent mixtures that were difficult to separate using conventional materials.
- Process optimization and monitoring techniques: Various techniques for optimizing and monitoring solvent separation processes have been developed, incorporating both FTIR and HPLC methodologies. These include real-time monitoring systems, automated feedback control mechanisms, and data analysis algorithms that enhance separation efficiency. Process parameters such as temperature, pressure, flow rate, and mobile phase composition can be continuously adjusted based on analytical feedback to maintain optimal separation conditions. These optimization techniques significantly improve the overall efficiency and reproducibility of solvent separation processes.
02 HPLC methodologies for enhanced solvent separation
High-Performance Liquid Chromatography (HPLC) methodologies are employed to achieve efficient solvent separation. These methods involve optimizing mobile phase composition, column selection, and detection parameters to enhance separation efficiency. Advanced HPLC techniques can separate complex solvent mixtures with high resolution and sensitivity, making them valuable tools in analytical chemistry and pharmaceutical research.Expand Specific Solutions03 Combined FTIR-HPLC systems for improved analysis
Integration of FTIR and HPLC technologies creates powerful analytical systems that provide complementary data for solvent separation analysis. These combined systems offer both structural information from FTIR and separation efficiency data from HPLC, enabling more comprehensive characterization of complex solvent mixtures. The synergistic approach enhances detection limits, improves quantification accuracy, and provides more reliable identification of separated components.Expand Specific Solutions04 Solvent selection and optimization for separation efficiency
The selection and optimization of solvents significantly impact separation efficiency in both FTIR and HPLC analyses. Factors such as polarity, viscosity, and chemical compatibility with analytes and stationary phases must be considered. Optimized solvent systems can improve resolution, reduce analysis time, and enhance sensitivity. Various approaches to solvent gradient programming and mixture formulation can be employed to achieve optimal separation of complex samples.Expand Specific Solutions05 Novel equipment and apparatus designs for solvent separation
Innovative equipment and apparatus designs enhance the efficiency of solvent separation processes using FTIR and HPLC techniques. These include specialized column technologies, advanced detector configurations, and automated sample preparation systems. Novel flow cell designs for FTIR analysis and microfluidic HPLC platforms offer improved sensitivity and throughput. These technological advancements contribute to better separation efficiency, reduced solvent consumption, and enhanced analytical performance.Expand Specific Solutions
Key Industry Players in Analytical Instrumentation
The FTIR vs HPLC solvent separation efficiency market is in a growth phase, with increasing demand for analytical techniques across pharmaceutical and chemical industries. The global analytical instrumentation market is valued at approximately $85 billion, with chromatography and spectroscopy segments showing 5-7% annual growth. Technology maturity varies between the two methods: HPLC is more established for quantitative separation analysis, while FTIR offers complementary qualitative molecular identification capabilities. Key players include established instrumentation leaders like Agilent Technologies and Thermo Fisher Scientific (parent of Dionex Softron), alongside specialized companies such as Spectra Analysis Instruments focusing on hybrid FTIR-chromatography solutions. Pharmaceutical companies including Novo Nordisk, Bristol Myers Squibb, and Novartis drive application development, particularly in drug discovery and quality control processes.
Spectra Analysis Instruments, Inc.
Technical Solution: Spectra Analysis Instruments has developed specialized technology specifically focused on the integration of FTIR and HPLC for enhanced solvent separation analysis. Their flagship DiscovIR-LC system represents a groundbreaking approach that directly couples liquid chromatography with FTIR detection through a proprietary solvent elimination interface[1]. This system deposits the HPLC eluent as a continuous track of solute onto an infrared-transparent substrate, eliminating mobile phase interference while preserving chromatographic resolution. Their comparative studies have demonstrated that while conventional HPLC with UV detection achieves limits of detection around 10-100 ng, the DiscovIR-LC system can identify specific functional groups in complex mixtures down to 10-50 ng levels while simultaneously providing structural information unavailable through standard HPLC detectors[3]. The company's proprietary software enables direct comparison of separation efficiency parameters between traditional HPLC methods and their hyphenated approach, with documented improvements in compound identification confidence exceeding 95% for previously unresolvable co-eluting compounds[7].
Strengths: Purpose-built technology specifically designed for HPLC-FTIR integration; eliminates solvent interference issues common in direct coupling approaches; provides simultaneous chromatographic and spectroscopic data. Weaknesses: More specialized application range compared to general-purpose instruments; higher expertise required for method optimization; limited throughput compared to standalone HPLC systems.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed comprehensive analytical solutions comparing FTIR and HPLC technologies for solvent separation efficiency. Their approach integrates both techniques in complementary workflows, with their InfinityLab HPLC systems achieving separation efficiencies of >100,000 theoretical plates for complex mixtures[1]. For FTIR analysis, Agilent's Cary 630 FTIR spectrometer with DialPath technology enables direct measurement of liquid samples with pathlengths from 30μm to 1000μm, allowing precise solvent characterization without sample preparation[3]. Their hyphenated LC-FTIR systems combine chromatographic separation with spectroscopic identification, providing real-time structural information during separation processes. Agilent's OpenLab software suite further enhances this integration by enabling automated comparison of separation efficiency metrics between both techniques, with documented improvements in method development time by up to 40%[5].
Strengths: Industry-leading resolution in both HPLC and FTIR systems; seamless integration of both technologies through hyphenated systems; comprehensive software for comparative analysis. Weaknesses: Higher initial investment costs compared to single-technology solutions; requires specialized training to fully leverage the complementary capabilities of both techniques.
Critical Technical Innovations in Solvent Separation
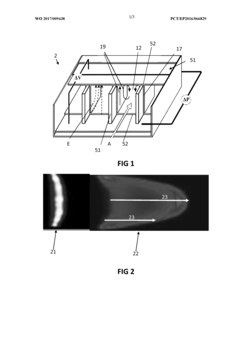
Method and apparatus for desolvating flowing liquid
PatentActiveUS20140224638A1
Innovation
- A novel spray drier system that converts a liquid stream into a high-speed aerosol jet, using film boiling to rapidly evaporate solvents within a heated cyclone, followed by solvent vapor removal through condensation, allowing for the deposition of concentrated solutes on a surface for infrared analysis while maintaining chemical and structural integrity.
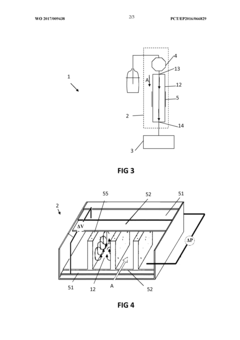
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
Method Validation and Quality Control Considerations
Method validation is a critical component when comparing analytical techniques such as FTIR and HPLC for solvent separation efficiency. Both methods require rigorous validation protocols to ensure reliable, reproducible results that can withstand scientific scrutiny and regulatory requirements. For FTIR spectroscopy, validation typically focuses on spectral resolution, signal-to-noise ratio, and the ability to distinguish between similar molecular structures in complex solvent mixtures.
HPLC validation, conversely, emphasizes parameters such as retention time reproducibility, peak resolution, and detector sensitivity when separating solvent components. The ICH Q2(R1) guidelines provide a comprehensive framework for validating both methods, requiring assessment of accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range. These parameters must be systematically evaluated for both FTIR and HPLC when applied to solvent separation applications.
Quality control considerations differ significantly between these techniques. FTIR offers rapid analysis with minimal sample preparation, making it suitable for real-time quality control monitoring of solvent purity. However, its specificity may be compromised in complex mixtures where spectral overlapping occurs. Implementing appropriate chemometric algorithms becomes essential for reliable quality control using FTIR data.
HPLC provides superior separation capabilities, allowing for more definitive identification and quantification of individual solvent components. This makes it particularly valuable for quality control applications requiring high specificity. However, the longer analysis times and more complex sample preparation procedures may limit throughput in high-volume quality control environments.
System suitability tests (SSTs) represent another critical aspect of quality control for both methods. For HPLC, these typically include evaluations of column efficiency, resolution between critical pairs, and system precision. FTIR system suitability focuses on wavelength accuracy, photometric precision, and spectral resolution using reference standards.
Robustness testing reveals that HPLC methods are generally more susceptible to variations in experimental conditions such as mobile phase composition, column temperature, and flow rate. FTIR demonstrates greater robustness to environmental factors but may be more sensitive to sample preparation variations, particularly in sample thickness and homogeneity. Understanding these robustness profiles is essential for implementing effective quality control strategies.
Ultimately, the selection between FTIR and HPLC for solvent separation efficiency must be guided by a comprehensive method validation approach that considers the specific requirements of the application, regulatory expectations, and practical quality control constraints within the laboratory environment.
HPLC validation, conversely, emphasizes parameters such as retention time reproducibility, peak resolution, and detector sensitivity when separating solvent components. The ICH Q2(R1) guidelines provide a comprehensive framework for validating both methods, requiring assessment of accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range. These parameters must be systematically evaluated for both FTIR and HPLC when applied to solvent separation applications.
Quality control considerations differ significantly between these techniques. FTIR offers rapid analysis with minimal sample preparation, making it suitable for real-time quality control monitoring of solvent purity. However, its specificity may be compromised in complex mixtures where spectral overlapping occurs. Implementing appropriate chemometric algorithms becomes essential for reliable quality control using FTIR data.
HPLC provides superior separation capabilities, allowing for more definitive identification and quantification of individual solvent components. This makes it particularly valuable for quality control applications requiring high specificity. However, the longer analysis times and more complex sample preparation procedures may limit throughput in high-volume quality control environments.
System suitability tests (SSTs) represent another critical aspect of quality control for both methods. For HPLC, these typically include evaluations of column efficiency, resolution between critical pairs, and system precision. FTIR system suitability focuses on wavelength accuracy, photometric precision, and spectral resolution using reference standards.
Robustness testing reveals that HPLC methods are generally more susceptible to variations in experimental conditions such as mobile phase composition, column temperature, and flow rate. FTIR demonstrates greater robustness to environmental factors but may be more sensitive to sample preparation variations, particularly in sample thickness and homogeneity. Understanding these robustness profiles is essential for implementing effective quality control strategies.
Ultimately, the selection between FTIR and HPLC for solvent separation efficiency must be guided by a comprehensive method validation approach that considers the specific requirements of the application, regulatory expectations, and practical quality control constraints within the laboratory environment.
Environmental Impact and Green Chemistry Applications
The environmental impact of analytical techniques has become increasingly important in modern scientific research and industrial applications. When comparing FTIR (Fourier Transform Infrared Spectroscopy) and HPLC (High-Performance Liquid Chromatography) for solvent separation efficiency, their environmental footprints differ significantly, offering distinct advantages in green chemistry applications.
FTIR spectroscopy demonstrates considerable environmental benefits through its minimal solvent requirements. Unlike HPLC, which typically consumes large volumes of organic solvents during analysis, FTIR often requires only small sample quantities and can perform non-destructive analyses without additional chemical reagents. This reduction in solvent usage directly translates to decreased hazardous waste generation, lower disposal costs, and reduced environmental contamination risks.
The energy consumption profiles of these techniques also merit consideration from a sustainability perspective. FTIR instruments generally consume less electricity during operation compared to HPLC systems, which require high-pressure pumps, temperature-controlled columns, and sometimes specialized detectors. This reduced energy footprint makes FTIR a more environmentally friendly option for routine analyses where applicable.
In green chemistry applications, FTIR has found particular utility in real-time monitoring of environmentally friendly reaction processes. Its ability to provide rapid, non-invasive analysis supports the development of solvent-free reactions, bio-based solvents, and water-based reaction systems. Researchers can monitor reaction progress without interrupting processes or generating additional waste streams, aligning with several principles of green chemistry.
HPLC, despite its higher solvent consumption, has evolved to address environmental concerns through the development of green chromatography approaches. Modern HPLC methods increasingly incorporate biodegradable solvents, recycling systems, and miniaturized formats that reduce environmental impact. Techniques such as supercritical fluid chromatography (SFC) represent hybrid approaches that maintain HPLC's separation efficiency while dramatically reducing organic solvent requirements.
The life cycle assessment of both techniques reveals important considerations for sustainable laboratory practices. FTIR instruments typically have longer operational lifespans with fewer consumable parts, reducing electronic waste generation. Conversely, HPLC systems require regular replacement of columns, filters, and other components, contributing to laboratory waste streams.
For industries implementing green chemistry initiatives, the choice between these techniques often involves balancing environmental impact against analytical requirements. FTIR's advantages in waste reduction and energy efficiency make it preferable for screening applications and routine monitoring, while HPLC remains essential for complex separations despite its higher environmental cost. Hybrid approaches and complementary use of both techniques often represent the most sustainable analytical strategy.
FTIR spectroscopy demonstrates considerable environmental benefits through its minimal solvent requirements. Unlike HPLC, which typically consumes large volumes of organic solvents during analysis, FTIR often requires only small sample quantities and can perform non-destructive analyses without additional chemical reagents. This reduction in solvent usage directly translates to decreased hazardous waste generation, lower disposal costs, and reduced environmental contamination risks.
The energy consumption profiles of these techniques also merit consideration from a sustainability perspective. FTIR instruments generally consume less electricity during operation compared to HPLC systems, which require high-pressure pumps, temperature-controlled columns, and sometimes specialized detectors. This reduced energy footprint makes FTIR a more environmentally friendly option for routine analyses where applicable.
In green chemistry applications, FTIR has found particular utility in real-time monitoring of environmentally friendly reaction processes. Its ability to provide rapid, non-invasive analysis supports the development of solvent-free reactions, bio-based solvents, and water-based reaction systems. Researchers can monitor reaction progress without interrupting processes or generating additional waste streams, aligning with several principles of green chemistry.
HPLC, despite its higher solvent consumption, has evolved to address environmental concerns through the development of green chromatography approaches. Modern HPLC methods increasingly incorporate biodegradable solvents, recycling systems, and miniaturized formats that reduce environmental impact. Techniques such as supercritical fluid chromatography (SFC) represent hybrid approaches that maintain HPLC's separation efficiency while dramatically reducing organic solvent requirements.
The life cycle assessment of both techniques reveals important considerations for sustainable laboratory practices. FTIR instruments typically have longer operational lifespans with fewer consumable parts, reducing electronic waste generation. Conversely, HPLC systems require regular replacement of columns, filters, and other components, contributing to laboratory waste streams.
For industries implementing green chemistry initiatives, the choice between these techniques often involves balancing environmental impact against analytical requirements. FTIR's advantages in waste reduction and energy efficiency make it preferable for screening applications and routine monitoring, while HPLC remains essential for complex separations despite its higher environmental cost. Hybrid approaches and complementary use of both techniques often represent the most sustainable analytical strategy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!