Organoid Culture Systems vs. Scaffold-Based Methods
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology has evolved significantly over the past decade, transforming from rudimentary cell culture techniques to sophisticated three-dimensional models that recapitulate key aspects of organ development and function. The journey began in the early 2000s with the identification of adult stem cells capable of self-renewal and differentiation into organ-specific cell types. A pivotal breakthrough occurred in 2009 when Sato and colleagues developed the first intestinal organoids from Lgr5+ stem cells, establishing fundamental principles for organoid culture.
The evolution continued with the development of diverse culture systems, transitioning from simple suspension cultures to more complex matrix-embedded approaches. Initially, researchers relied heavily on Matrigel, a basement membrane extract derived from mouse sarcoma cells, which provided essential extracellular matrix components and growth factors. Subsequently, synthetic hydrogels emerged as alternatives, offering better reproducibility and customization capabilities.
Parallel to organoid culture systems, scaffold-based methods have developed along a distinct trajectory. These approaches utilize pre-fabricated structures to guide cell organization and tissue formation. Early scaffold technologies employed simple porous materials, while contemporary systems incorporate bioprinting, microfluidics, and nanofabrication to create precisely engineered microenvironments with controlled physical and biochemical properties.
The convergence of these two methodological streams has created a rich technological landscape with complementary strengths and limitations. Organoid culture systems excel in recapitulating developmental processes and maintaining cellular heterogeneity, while scaffold-based methods offer superior control over spatial organization and mechanical properties. This technological diversity has expanded the application scope from basic developmental biology to personalized medicine, drug screening, and regenerative therapy.
Current research objectives focus on addressing several key challenges in both approaches. For organoid systems, researchers aim to enhance reproducibility, scalability, and vascularization. For scaffold-based methods, the integration of dynamic mechanical cues and improved biomaterial functionality represent primary goals. Both fields seek to develop standardized protocols that ensure consistent results across laboratories and applications.
The ultimate objective of this technological evolution is to create physiologically relevant models that faithfully represent human organ function and disease pathology. This includes developing organoid systems with integrated immune components, vascular networks, and neural innervation. Additionally, researchers are working toward high-throughput platforms compatible with automated screening and analysis, facilitating broader adoption in pharmaceutical development and precision medicine applications.
The evolution continued with the development of diverse culture systems, transitioning from simple suspension cultures to more complex matrix-embedded approaches. Initially, researchers relied heavily on Matrigel, a basement membrane extract derived from mouse sarcoma cells, which provided essential extracellular matrix components and growth factors. Subsequently, synthetic hydrogels emerged as alternatives, offering better reproducibility and customization capabilities.
Parallel to organoid culture systems, scaffold-based methods have developed along a distinct trajectory. These approaches utilize pre-fabricated structures to guide cell organization and tissue formation. Early scaffold technologies employed simple porous materials, while contemporary systems incorporate bioprinting, microfluidics, and nanofabrication to create precisely engineered microenvironments with controlled physical and biochemical properties.
The convergence of these two methodological streams has created a rich technological landscape with complementary strengths and limitations. Organoid culture systems excel in recapitulating developmental processes and maintaining cellular heterogeneity, while scaffold-based methods offer superior control over spatial organization and mechanical properties. This technological diversity has expanded the application scope from basic developmental biology to personalized medicine, drug screening, and regenerative therapy.
Current research objectives focus on addressing several key challenges in both approaches. For organoid systems, researchers aim to enhance reproducibility, scalability, and vascularization. For scaffold-based methods, the integration of dynamic mechanical cues and improved biomaterial functionality represent primary goals. Both fields seek to develop standardized protocols that ensure consistent results across laboratories and applications.
The ultimate objective of this technological evolution is to create physiologically relevant models that faithfully represent human organ function and disease pathology. This includes developing organoid systems with integrated immune components, vascular networks, and neural innervation. Additionally, researchers are working toward high-throughput platforms compatible with automated screening and analysis, facilitating broader adoption in pharmaceutical development and precision medicine applications.
Market Analysis for 3D Cell Culture Technologies
The 3D cell culture technologies market has experienced substantial growth in recent years, reaching approximately $1.7 billion in 2022 and projected to expand at a CAGR of 14.8% through 2030. This growth is primarily driven by increasing applications in drug discovery, cancer research, regenerative medicine, and personalized healthcare solutions.
Within this expanding market, organoid culture systems and scaffold-based methods represent two dominant technological approaches with distinct market positioning. Organoid systems currently hold approximately 35% of the market share, valued at around $595 million, while scaffold-based methods account for approximately 42%, representing roughly $714 million. The remaining market share is distributed among other technologies including spheroid cultures, microfluidic systems, and bioprinting platforms.
Pharmaceutical and biotechnology companies constitute the largest end-user segment, accounting for approximately 48% of the total market. Academic and research institutions follow at 32%, with healthcare providers and contract research organizations making up the remainder. This distribution reflects the significant investment in drug development applications, where both organoid and scaffold-based systems offer valuable screening platforms.
Regional analysis reveals North America as the dominant market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China, South Korea, and Japan, demonstrates the highest growth rate at approximately 16.5% annually, driven by increasing research funding and expanding biotechnology sectors.
Key market drivers include rising demand for physiologically relevant in vitro models, growing emphasis on reducing animal testing, and increasing prevalence of chronic diseases necessitating novel therapeutic approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid drug screening and vaccine development platforms.
Market challenges include high implementation costs, technical complexity, and standardization issues. The average cost of establishing a comprehensive 3D cell culture laboratory ranges from $200,000 to $500,000, creating significant barriers to entry for smaller research institutions and companies.
Future market trends indicate increasing integration of artificial intelligence and machine learning with 3D culture technologies, growing demand for patient-derived models, and development of high-throughput screening platforms compatible with both organoid and scaffold-based approaches. The market is also witnessing a shift toward hybrid systems that combine the advantages of both methodologies, potentially creating a new high-growth segment estimated to reach $300 million by 2028.
Within this expanding market, organoid culture systems and scaffold-based methods represent two dominant technological approaches with distinct market positioning. Organoid systems currently hold approximately 35% of the market share, valued at around $595 million, while scaffold-based methods account for approximately 42%, representing roughly $714 million. The remaining market share is distributed among other technologies including spheroid cultures, microfluidic systems, and bioprinting platforms.
Pharmaceutical and biotechnology companies constitute the largest end-user segment, accounting for approximately 48% of the total market. Academic and research institutions follow at 32%, with healthcare providers and contract research organizations making up the remainder. This distribution reflects the significant investment in drug development applications, where both organoid and scaffold-based systems offer valuable screening platforms.
Regional analysis reveals North America as the dominant market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China, South Korea, and Japan, demonstrates the highest growth rate at approximately 16.5% annually, driven by increasing research funding and expanding biotechnology sectors.
Key market drivers include rising demand for physiologically relevant in vitro models, growing emphasis on reducing animal testing, and increasing prevalence of chronic diseases necessitating novel therapeutic approaches. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid drug screening and vaccine development platforms.
Market challenges include high implementation costs, technical complexity, and standardization issues. The average cost of establishing a comprehensive 3D cell culture laboratory ranges from $200,000 to $500,000, creating significant barriers to entry for smaller research institutions and companies.
Future market trends indicate increasing integration of artificial intelligence and machine learning with 3D culture technologies, growing demand for patient-derived models, and development of high-throughput screening platforms compatible with both organoid and scaffold-based approaches. The market is also witnessing a shift toward hybrid systems that combine the advantages of both methodologies, potentially creating a new high-growth segment estimated to reach $300 million by 2028.
Current Challenges in Organoid and Scaffold Technologies
Despite significant advancements in both organoid culture systems and scaffold-based methods, several critical challenges persist that limit their widespread application in research and clinical settings. Organoid culture systems face reproducibility issues, with batch-to-batch variations affecting experimental outcomes. The self-organizing nature of organoids, while advantageous for mimicking in vivo development, introduces unpredictability in structural formation and cellular composition, complicating standardization efforts across laboratories.
Maturation limitations represent another significant hurdle, as many organoid systems fail to achieve adult-like functionality or maintain long-term viability. This restricts their utility in modeling chronic diseases or age-related conditions. Additionally, current organoid systems often lack essential non-parenchymal components such as immune cells, vasculature, and neural innervation, limiting their physiological relevance.
Scaffold-based methods encounter different but equally challenging obstacles. Material selection remains problematic, as researchers must balance mechanical properties, biocompatibility, degradation rates, and cell-material interactions. Synthetic scaffolds offer better reproducibility but frequently lack the complex biochemical cues present in native extracellular matrices, while natural scaffolds provide better biological signals but suffer from batch variability and limited mechanical tunability.
Scalability presents a substantial challenge for both approaches. Organoids typically remain small due to diffusion limitations of nutrients and oxygen, while scaffold-based constructs struggle with uniform cell seeding and maintaining consistent cellular density throughout larger structures. This size limitation restricts applications requiring tissue-scale constructs for transplantation or industrial screening.
Cost and accessibility issues further impede widespread adoption. High-quality extracellular matrix components like Matrigel, essential for many organoid protocols, are expensive and exhibit batch variability. Similarly, advanced scaffold fabrication techniques such as 3D bioprinting require specialized equipment and expertise not readily available in all research settings.
Regulatory hurdles compound these technical challenges, particularly for clinical applications. The complex and variable nature of both technologies creates difficulties in establishing quality control parameters and standardized production protocols that meet regulatory requirements. Furthermore, the integration of these technologies with existing pharmaceutical screening platforms requires significant adaptation of established workflows and validation procedures.
Addressing these multifaceted challenges requires interdisciplinary collaboration between materials scientists, cell biologists, tissue engineers, and clinicians to develop next-generation solutions that combine the advantages of both approaches while mitigating their respective limitations.
Maturation limitations represent another significant hurdle, as many organoid systems fail to achieve adult-like functionality or maintain long-term viability. This restricts their utility in modeling chronic diseases or age-related conditions. Additionally, current organoid systems often lack essential non-parenchymal components such as immune cells, vasculature, and neural innervation, limiting their physiological relevance.
Scaffold-based methods encounter different but equally challenging obstacles. Material selection remains problematic, as researchers must balance mechanical properties, biocompatibility, degradation rates, and cell-material interactions. Synthetic scaffolds offer better reproducibility but frequently lack the complex biochemical cues present in native extracellular matrices, while natural scaffolds provide better biological signals but suffer from batch variability and limited mechanical tunability.
Scalability presents a substantial challenge for both approaches. Organoids typically remain small due to diffusion limitations of nutrients and oxygen, while scaffold-based constructs struggle with uniform cell seeding and maintaining consistent cellular density throughout larger structures. This size limitation restricts applications requiring tissue-scale constructs for transplantation or industrial screening.
Cost and accessibility issues further impede widespread adoption. High-quality extracellular matrix components like Matrigel, essential for many organoid protocols, are expensive and exhibit batch variability. Similarly, advanced scaffold fabrication techniques such as 3D bioprinting require specialized equipment and expertise not readily available in all research settings.
Regulatory hurdles compound these technical challenges, particularly for clinical applications. The complex and variable nature of both technologies creates difficulties in establishing quality control parameters and standardized production protocols that meet regulatory requirements. Furthermore, the integration of these technologies with existing pharmaceutical screening platforms requires significant adaptation of established workflows and validation procedures.
Addressing these multifaceted challenges requires interdisciplinary collaboration between materials scientists, cell biologists, tissue engineers, and clinicians to develop next-generation solutions that combine the advantages of both approaches while mitigating their respective limitations.
Comparative Analysis of Organoid vs Scaffold Methodologies
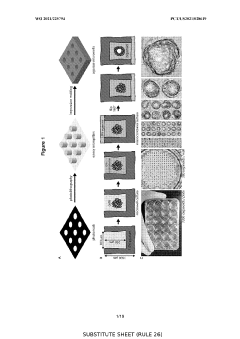
01 3D Scaffold-Based Organoid Culture Systems
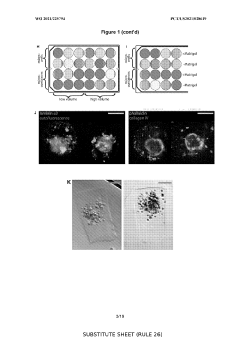
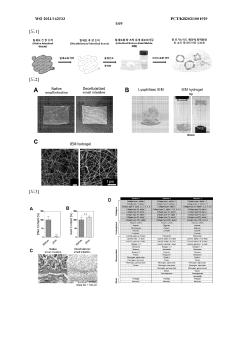
Three-dimensional scaffold-based systems provide structural support for organoid growth and development. These scaffolds mimic the extracellular matrix found in native tissues, allowing cells to organize into complex structures. Materials used include hydrogels, synthetic polymers, and decellularized tissues that can be tailored for specific tissue types. The scaffold architecture influences cell adhesion, migration, and differentiation, ultimately affecting organoid morphology and functionality.- 3D scaffold-based organoid culture systems: Three-dimensional scaffold-based methods provide structural support for organoid growth and development. These scaffolds mimic the extracellular matrix and provide physical cues that guide cell organization, differentiation, and function. Materials used for these scaffolds include natural polymers, synthetic hydrogels, and biocompatible materials that can be tailored to specific tissue types. The 3D environment allows for more physiologically relevant organoid formation compared to traditional 2D culture systems.
- Hydrogel-based matrices for organoid development: Hydrogel-based matrices provide a supportive environment for organoid growth by mimicking the natural extracellular matrix. These matrices can be composed of natural materials like Matrigel, collagen, or synthetic alternatives that offer tunable mechanical and biochemical properties. Hydrogels enable the encapsulation of cells in a three-dimensional space, allowing for cell-cell interactions and self-organization into complex structures. The porosity and degradability of these matrices can be adjusted to facilitate nutrient diffusion and organoid expansion.
- Bioprinting technologies for organoid fabrication: Bioprinting technologies enable the precise deposition of cells and supporting materials to create organoids with defined architectures. These methods utilize various printing techniques, including extrusion-based, inkjet, and laser-assisted bioprinting, to position cells and bioinks in specific patterns. Bioprinted organoids can incorporate multiple cell types in predetermined spatial arrangements, allowing for the recreation of complex tissue structures. This approach offers high reproducibility and scalability for organoid production and can be integrated with microfluidic systems for enhanced functionality.
- Microfluidic systems for dynamic organoid culture: Microfluidic platforms provide dynamic culture environments for organoids by enabling controlled fluid flow, nutrient delivery, and waste removal. These systems can incorporate channels, chambers, and barriers that mimic physiological conditions and facilitate the formation of tissue-specific microenvironments. Microfluidic organoid cultures allow for real-time monitoring, precise manipulation of biochemical gradients, and integration with sensing technologies. The dynamic nature of these systems supports long-term culture maintenance and enhances organoid maturation and functionality.
- Biomaterial functionalization for enhanced organoid development: Functionalization of biomaterials with bioactive molecules enhances organoid development by providing specific biochemical cues. These modifications can include the incorporation of growth factors, adhesion molecules, and tissue-specific proteins that guide cell behavior and tissue formation. Functionalized scaffolds can promote cell attachment, proliferation, differentiation, and organization into complex structures. This approach allows for the creation of specialized microenvironments that support the development of specific organoid types and can be tailored to mimic various disease states or developmental stages.
02 Biomaterial Composition for Organoid Development
The composition of biomaterials used in organoid culture significantly impacts cell behavior and tissue formation. Natural materials like Matrigel, collagen, and fibrin provide bioactive cues that support cell growth and differentiation. Synthetic materials offer greater control over physical properties and can be modified with bioactive molecules to enhance cell attachment and function. Hybrid approaches combining natural and synthetic materials leverage the advantages of both to create optimal microenvironments for specific organoid types.Expand Specific Solutions03 Dynamic Culture Systems for Organoid Maturation
Dynamic culture systems incorporate mechanical stimulation and fluid flow to enhance organoid development and maturation. Bioreactors provide controlled environments where parameters such as oxygen tension, nutrient delivery, and waste removal can be precisely regulated. These systems often include perfusion capabilities that improve mass transfer throughout larger organoid structures. Mechanical forces applied through these systems can stimulate mechanosensitive pathways that promote tissue-specific differentiation and organization, resulting in more physiologically relevant organoid models.Expand Specific Solutions04 Growth Factor Delivery Systems in Scaffold-Based Methods
Controlled delivery of growth factors and morphogens is crucial for directing organoid development in scaffold-based culture systems. Various techniques have been developed to incorporate bioactive molecules into scaffolds, including encapsulation in degradable microspheres, covalent binding to scaffold materials, and affinity-based sequestration. These approaches enable spatial and temporal control over growth factor presentation, mimicking the complex signaling environments found during natural organogenesis and tissue development.Expand Specific Solutions05 Vascularization Strategies for Complex Organoids
Developing vascularized organoids remains a significant challenge in the field. Various approaches have been implemented to promote blood vessel formation within organoid structures, including co-culture with endothelial cells, incorporation of angiogenic factors, and the use of microfluidic devices. Scaffold designs with pre-patterned channels or sacrificial materials can create vessel-like networks within organoids. These vascularization strategies are essential for supporting the growth of larger, more complex organoid systems by facilitating nutrient and oxygen delivery to core regions.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The organoid culture systems and scaffold-based methods market is currently in a growth phase, with increasing adoption across biomedical research and drug development sectors. The global market size is estimated to reach $3.5-4 billion by 2027, driven by applications in disease modeling, regenerative medicine, and personalized therapeutics. Technologically, organoid systems are advancing rapidly with companies like STEMCELL Technologies, Molecular Devices, and Cell Microsystems leading innovation in self-organizing 3D cultures. Meanwhile, scaffold-based approaches are being refined by institutions such as École Polytechnique Fédérale de Lausanne and Finnadvance, focusing on structural support systems. Academic-industry partnerships are accelerating, with research powerhouses like Wuhan University, Sun Yat-Sen University, and Rutgers collaborating with commercial entities to bridge fundamental research and clinical applications, suggesting a maturing but still evolving technological landscape.
Cell Microsystems, Inc.
Technical Solution: Cell Microsystems has developed the CellRaft® Technology, a unique approach that bridges organoid culture and scaffold-based methods. Their platform utilizes arrays of thousands of microwells (CellRafts®) on which cells can be cultured individually or in small groups. For organoid applications, their system allows for the isolation and culture of single stem cells or small organoid fragments, enabling clonal organoid development with precise tracking of lineage. The company's AIR™ System provides automated imaging and retrieval of selected organoids based on morphological criteria or growth characteristics. This technology addresses a critical challenge in organoid research by enabling the selection of organoids with specific desired properties. Cell Microsystems has demonstrated applications in patient-derived tumor organoid culture, where their platform enables the isolation of organoids with specific drug responses or genetic characteristics. Their approach combines the self-organizing properties of organoids with the control and selectivity advantages of engineered culture systems.
Strengths: Precise single-cell control for clonal organoid development; automated selection and retrieval of organoids with specific characteristics; excellent traceability of organoid lineage; compatible with high-throughput screening. Weaknesses: Limited ability to create large, complex organoid structures; requires specialized equipment and training; higher cost per sample compared to traditional organoid culture methods; relatively new technology with more limited validation across tissue types.
Molecular Devices LLC
Technical Solution: Molecular Devices has developed the ImageXpress® Micro Confocal High-Content Imaging System combined with their 3D tissue analysis software, which represents a comprehensive approach to analyzing both organoid cultures and scaffold-based 3D models. Their technology focuses on advanced imaging and analysis rather than a specific culture method, providing tools to quantitatively assess the complex structures formed in 3D culture systems. The company's OrganoidProfiler™ software utilizes machine learning algorithms to automatically identify and characterize organoid structures, measuring parameters such as size, shape, and internal organization. For scaffold-based methods, their technology can analyze cell distribution, migration, and organization within various scaffold materials. Molecular Devices has also developed specialized microplates optimized for 3D culture imaging, with optical properties that enhance visualization of complex structures. Their system enables time-lapse imaging of developing organoids or scaffold-based cultures, providing insights into dynamic processes such as differentiation and morphogenesis.
Strengths: Comprehensive quantitative analysis of 3D structures; compatible with multiple culture methods and scaffold types; automated high-throughput imaging capabilities; sophisticated data analysis tools. Weaknesses: Focuses on analysis rather than providing a complete culture solution; requires significant investment in imaging infrastructure; complex software may require specialized training; primarily addresses the analytical rather than biological challenges of 3D culture.
Breakthrough Technologies in Extracellular Matrix Mimicry
Methods for organoids production
PatentWO2021225794A1
Innovation
- A method involving microcontainers with hydrogel walls and lids that allow cells to self-organize without exogenous extracellular matrix, using a culturing medium with biological colloids to achieve a higher density than the hydrogel lid, enabling the formation of organoids that exhibit contractility and physiological differentiation.
Scaffold derived from decellularized organ tissue, for organ organoid culture and transplantation, and production method therefor
PatentWO2021162533A1
Innovation
- Development of decellularized organ tissue-derived scaffolds that mimic the extracellular matrix of intestinal, gastric, and liver tissues, providing a tissue-specific microenvironment for organoid culture and transplantation, using a decellularization process to create hydrogel scaffolds from pig small intestine, stomach, and liver tissues.
Standardization and Reproducibility Considerations
Standardization and reproducibility represent critical challenges in both organoid culture systems and scaffold-based methods. The inherent complexity of three-dimensional cellular structures demands rigorous protocols to ensure consistent results across laboratories and experiments.
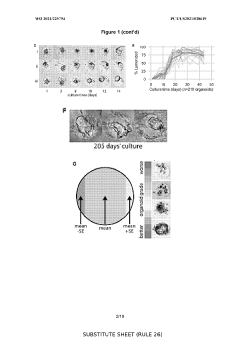
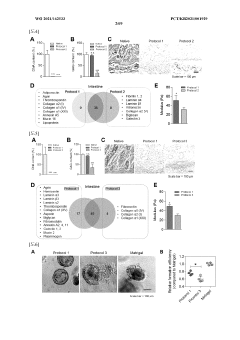
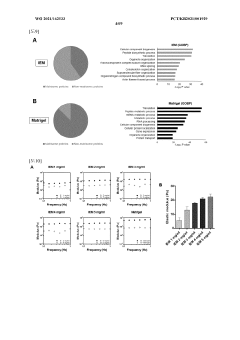
In organoid culture systems, batch-to-batch variability of extracellular matrix components, particularly Matrigel, introduces significant reproducibility concerns. Recent studies indicate that up to 35% of experimental variations can be attributed to matrix inconsistencies. This variability affects organoid formation efficiency, morphology, and functional characteristics, complicating cross-study comparisons and clinical applications.
Scaffold-based methods face different standardization challenges. The physical properties of scaffolds, including porosity, stiffness, and degradation rates, must be precisely controlled during manufacturing. Current production techniques show approximately 10-15% variation in critical parameters between batches, necessitating comprehensive quality control measures. Additionally, the interaction between cells and synthetic materials requires standardized characterization methods to ensure consistent cellular responses.
Protocol standardization remains underdeveloped in both approaches. A 2022 meta-analysis of 87 organoid studies revealed substantial methodological variations, with differences in media composition, growth factor concentrations, and culture durations. Similarly, scaffold-based methods employ diverse fabrication techniques and cell seeding protocols, further complicating reproducibility efforts.
Emerging technologies are addressing these challenges through automated culture systems and quality control measures. Microfluidic platforms for organoid culture have demonstrated a 40% reduction in variability compared to manual methods. For scaffold-based approaches, advanced manufacturing techniques like 3D bioprinting offer improved precision with parameter control within 5% variance.
International initiatives are working toward standardization, including the Human Cell Atlas Organoid Project and the International Society for Biofabrication's scaffold characterization guidelines. These efforts aim to establish minimum reporting requirements and standardized testing methods for both technologies.
The development of reference materials and quantitative assessment tools represents another promising direction. Digital image analysis algorithms now enable objective evaluation of organoid morphology and growth patterns, while mechanical testing protocols for scaffolds are becoming increasingly standardized, allowing meaningful comparisons between different research groups.
In organoid culture systems, batch-to-batch variability of extracellular matrix components, particularly Matrigel, introduces significant reproducibility concerns. Recent studies indicate that up to 35% of experimental variations can be attributed to matrix inconsistencies. This variability affects organoid formation efficiency, morphology, and functional characteristics, complicating cross-study comparisons and clinical applications.
Scaffold-based methods face different standardization challenges. The physical properties of scaffolds, including porosity, stiffness, and degradation rates, must be precisely controlled during manufacturing. Current production techniques show approximately 10-15% variation in critical parameters between batches, necessitating comprehensive quality control measures. Additionally, the interaction between cells and synthetic materials requires standardized characterization methods to ensure consistent cellular responses.
Protocol standardization remains underdeveloped in both approaches. A 2022 meta-analysis of 87 organoid studies revealed substantial methodological variations, with differences in media composition, growth factor concentrations, and culture durations. Similarly, scaffold-based methods employ diverse fabrication techniques and cell seeding protocols, further complicating reproducibility efforts.
Emerging technologies are addressing these challenges through automated culture systems and quality control measures. Microfluidic platforms for organoid culture have demonstrated a 40% reduction in variability compared to manual methods. For scaffold-based approaches, advanced manufacturing techniques like 3D bioprinting offer improved precision with parameter control within 5% variance.
International initiatives are working toward standardization, including the Human Cell Atlas Organoid Project and the International Society for Biofabrication's scaffold characterization guidelines. These efforts aim to establish minimum reporting requirements and standardized testing methods for both technologies.
The development of reference materials and quantitative assessment tools represents another promising direction. Digital image analysis algorithms now enable objective evaluation of organoid morphology and growth patterns, while mechanical testing protocols for scaffolds are becoming increasingly standardized, allowing meaningful comparisons between different research groups.
Translational Applications in Drug Discovery and Personalized Medicine
Organoid culture systems and scaffold-based methods have revolutionized drug discovery and personalized medicine by providing more physiologically relevant models than traditional 2D cell cultures. These advanced 3D systems enable researchers to recapitulate complex tissue architectures and functions, offering unprecedented opportunities for translational applications.
In drug discovery, organoid cultures derived from patient tissues allow for high-throughput screening of drug candidates against disease-specific phenotypes. Pharmaceutical companies have increasingly adopted organoid platforms to evaluate drug efficacy, toxicity, and metabolism in pre-clinical stages. This approach significantly reduces the attrition rate of drug candidates in clinical trials by identifying potential failures earlier in the development pipeline, ultimately saving billions in R&D costs.
Scaffold-based methods complement organoid systems by providing structural support that mimics the extracellular matrix, enabling more controlled studies of cell-matrix interactions during drug testing. These methods have proven particularly valuable for testing compounds targeting diseases where the microenvironment plays a crucial role, such as fibrosis and cancer.
Patient-derived organoids (PDOs) have emerged as powerful tools for personalized medicine applications. By generating organoids from individual patients, clinicians can create "avatar" models to test multiple treatment options simultaneously, identifying the most effective therapeutic strategy for each patient. Several clinical studies have demonstrated strong correlations between drug responses in PDOs and actual patient outcomes, particularly in oncology.
The integration of these 3D culture technologies with advanced imaging and multi-omics approaches has further enhanced their translational value. Researchers can now monitor real-time drug responses while simultaneously analyzing molecular changes at the genomic, transcriptomic, and proteomic levels, providing comprehensive insights into drug mechanisms and resistance pathways.
Despite these advances, challenges remain in standardizing organoid and scaffold-based methods for clinical implementation. Variability in organoid generation, maturation time, and reproducibility presents obstacles for widespread adoption in clinical settings. However, recent developments in automated culture systems and quality control protocols are addressing these limitations.
The economic impact of these technologies on healthcare is substantial. By enabling precision medicine approaches, they potentially reduce ineffective treatments, minimize adverse effects, and improve patient outcomes. Market analyses project that organoid-based drug discovery and personalized medicine applications will grow at a CAGR of approximately 22% through 2028, reflecting their increasing adoption across pharmaceutical research and clinical practice.
In drug discovery, organoid cultures derived from patient tissues allow for high-throughput screening of drug candidates against disease-specific phenotypes. Pharmaceutical companies have increasingly adopted organoid platforms to evaluate drug efficacy, toxicity, and metabolism in pre-clinical stages. This approach significantly reduces the attrition rate of drug candidates in clinical trials by identifying potential failures earlier in the development pipeline, ultimately saving billions in R&D costs.
Scaffold-based methods complement organoid systems by providing structural support that mimics the extracellular matrix, enabling more controlled studies of cell-matrix interactions during drug testing. These methods have proven particularly valuable for testing compounds targeting diseases where the microenvironment plays a crucial role, such as fibrosis and cancer.
Patient-derived organoids (PDOs) have emerged as powerful tools for personalized medicine applications. By generating organoids from individual patients, clinicians can create "avatar" models to test multiple treatment options simultaneously, identifying the most effective therapeutic strategy for each patient. Several clinical studies have demonstrated strong correlations between drug responses in PDOs and actual patient outcomes, particularly in oncology.
The integration of these 3D culture technologies with advanced imaging and multi-omics approaches has further enhanced their translational value. Researchers can now monitor real-time drug responses while simultaneously analyzing molecular changes at the genomic, transcriptomic, and proteomic levels, providing comprehensive insights into drug mechanisms and resistance pathways.
Despite these advances, challenges remain in standardizing organoid and scaffold-based methods for clinical implementation. Variability in organoid generation, maturation time, and reproducibility presents obstacles for widespread adoption in clinical settings. However, recent developments in automated culture systems and quality control protocols are addressing these limitations.
The economic impact of these technologies on healthcare is substantial. By enabling precision medicine approaches, they potentially reduce ineffective treatments, minimize adverse effects, and improve patient outcomes. Market analyses project that organoid-based drug discovery and personalized medicine applications will grow at a CAGR of approximately 22% through 2028, reflecting their increasing adoption across pharmaceutical research and clinical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!