What Makes Organoid Culture Systems Cutting-edge?
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Objectives
Organoid technology represents one of the most significant breakthroughs in biomedical research over the past decade. Originating from stem cell research advancements in the early 2000s, organoids emerged as three-dimensional cellular structures that self-organize to mimic the architecture and functionality of native organs. The evolution of this technology can be traced back to Hans Clevers' groundbreaking work in 2009, when his team generated the first intestinal organoids from Lgr5+ stem cells, demonstrating that adult stem cells could self-organize into organ-like structures when provided with appropriate growth factors.
The technological progression accelerated dramatically between 2010-2015, with researchers successfully developing protocols for creating organoids representing various organs including brain, liver, kidney, pancreas, and retina. This period marked the transition from two-dimensional cell culture systems to more physiologically relevant three-dimensional models that better recapitulate in vivo cellular organization, differentiation patterns, and tissue-specific functions.
A critical milestone in organoid technology development was the integration of extracellular matrix components, particularly Matrigel, which provided the necessary physical scaffolding and biochemical cues for proper organoid formation. Subsequently, advances in bioengineering approaches have enabled more precise control over organoid development through customized hydrogels, microfluidic systems, and bioprinting technologies.
The primary objective of organoid technology is to establish physiologically relevant models that bridge the gap between traditional cell culture systems and animal models. These "mini-organs" aim to recapitulate key aspects of organ development, structure, and function at a level of complexity not achievable with conventional two-dimensional cultures, while avoiding the ethical concerns and species-specific differences associated with animal testing.
Current research objectives focus on enhancing organoid complexity through vascularization, incorporation of immune components, and development of multi-organ systems. These advancements seek to address limitations in nutrient diffusion, immune interactions, and organ-organ crosstalk that are essential for modeling complex physiological and pathological processes.
The technology aims to revolutionize personalized medicine by enabling patient-specific disease modeling, drug screening, and regenerative medicine applications. By generating organoids from patient-derived cells, researchers can study disease mechanisms, test therapeutic responses, and potentially develop personalized treatment strategies. Furthermore, organoids hold promise for toxicology testing, reducing reliance on animal models while providing human-relevant data for safety assessment of pharmaceuticals and chemicals.
Looking forward, the field is moving toward standardization of protocols, scaling up production, and integration with other cutting-edge technologies such as CRISPR gene editing, single-cell sequencing, and artificial intelligence to enhance reproducibility, throughput, and predictive capacity of organoid systems.
The technological progression accelerated dramatically between 2010-2015, with researchers successfully developing protocols for creating organoids representing various organs including brain, liver, kidney, pancreas, and retina. This period marked the transition from two-dimensional cell culture systems to more physiologically relevant three-dimensional models that better recapitulate in vivo cellular organization, differentiation patterns, and tissue-specific functions.
A critical milestone in organoid technology development was the integration of extracellular matrix components, particularly Matrigel, which provided the necessary physical scaffolding and biochemical cues for proper organoid formation. Subsequently, advances in bioengineering approaches have enabled more precise control over organoid development through customized hydrogels, microfluidic systems, and bioprinting technologies.
The primary objective of organoid technology is to establish physiologically relevant models that bridge the gap between traditional cell culture systems and animal models. These "mini-organs" aim to recapitulate key aspects of organ development, structure, and function at a level of complexity not achievable with conventional two-dimensional cultures, while avoiding the ethical concerns and species-specific differences associated with animal testing.
Current research objectives focus on enhancing organoid complexity through vascularization, incorporation of immune components, and development of multi-organ systems. These advancements seek to address limitations in nutrient diffusion, immune interactions, and organ-organ crosstalk that are essential for modeling complex physiological and pathological processes.
The technology aims to revolutionize personalized medicine by enabling patient-specific disease modeling, drug screening, and regenerative medicine applications. By generating organoids from patient-derived cells, researchers can study disease mechanisms, test therapeutic responses, and potentially develop personalized treatment strategies. Furthermore, organoids hold promise for toxicology testing, reducing reliance on animal models while providing human-relevant data for safety assessment of pharmaceuticals and chemicals.
Looking forward, the field is moving toward standardization of protocols, scaling up production, and integration with other cutting-edge technologies such as CRISPR gene editing, single-cell sequencing, and artificial intelligence to enhance reproducibility, throughput, and predictive capacity of organoid systems.
Market Analysis for 3D Cell Culture Applications
The 3D cell culture market has experienced significant growth in recent years, with a global market value reaching $1.7 billion in 2022 and projected to expand at a CAGR of 14.8% through 2030. This robust growth is primarily driven by increasing applications in drug discovery, cancer research, regenerative medicine, and personalized healthcare solutions. Organoid culture systems represent one of the fastest-growing segments within this market, accounting for approximately 25% of the total 3D cell culture market share.
The pharmaceutical and biotechnology sectors currently dominate the demand landscape, collectively representing over 60% of the total market consumption. These industries leverage organoid technologies primarily for drug screening, toxicity testing, and disease modeling applications. Academic and research institutions constitute another significant market segment, contributing approximately 20% to the overall market demand, with their focus primarily on basic research and technology development.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the highest growth rate in the coming years due to increasing R&D investments and government initiatives supporting biotechnology advancement.
The market for organoid-specific reagents and media has emerged as a particularly lucrative segment, growing at nearly 18% annually. Specialized extracellular matrices, growth factors, and defined media formulations tailored for specific organoid types command premium pricing, with profit margins exceeding 60% for some proprietary formulations.
Customer segmentation reveals distinct needs across different user groups. Pharmaceutical companies prioritize scalability and reproducibility for high-throughput screening applications. Academic researchers focus more on physiological relevance and model complexity. Clinical laboratories increasingly seek standardized protocols and validated systems for diagnostic applications.
Market barriers include high costs associated with specialized equipment and consumables, technical complexity requiring specialized expertise, and challenges in achieving consistent results across different batches. The average cost per organoid culture experiment ranges from $500 to $2,000, significantly higher than traditional 2D culture methods.
Emerging market opportunities include integration with complementary technologies such as microfluidics, AI-driven analysis platforms, and bioprinting. The patient-derived organoid market segment is experiencing particularly rapid growth, with increasing applications in precision medicine and companion diagnostics.
The pharmaceutical and biotechnology sectors currently dominate the demand landscape, collectively representing over 60% of the total market consumption. These industries leverage organoid technologies primarily for drug screening, toxicity testing, and disease modeling applications. Academic and research institutions constitute another significant market segment, contributing approximately 20% to the overall market demand, with their focus primarily on basic research and technology development.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the highest growth rate in the coming years due to increasing R&D investments and government initiatives supporting biotechnology advancement.
The market for organoid-specific reagents and media has emerged as a particularly lucrative segment, growing at nearly 18% annually. Specialized extracellular matrices, growth factors, and defined media formulations tailored for specific organoid types command premium pricing, with profit margins exceeding 60% for some proprietary formulations.
Customer segmentation reveals distinct needs across different user groups. Pharmaceutical companies prioritize scalability and reproducibility for high-throughput screening applications. Academic researchers focus more on physiological relevance and model complexity. Clinical laboratories increasingly seek standardized protocols and validated systems for diagnostic applications.
Market barriers include high costs associated with specialized equipment and consumables, technical complexity requiring specialized expertise, and challenges in achieving consistent results across different batches. The average cost per organoid culture experiment ranges from $500 to $2,000, significantly higher than traditional 2D culture methods.
Emerging market opportunities include integration with complementary technologies such as microfluidics, AI-driven analysis platforms, and bioprinting. The patient-derived organoid market segment is experiencing particularly rapid growth, with increasing applications in precision medicine and companion diagnostics.
Current Organoid Systems and Technical Barriers
Organoid culture systems have evolved significantly over the past decade, with various platforms now available for different tissue types. Brain organoids represent one of the most advanced systems, utilizing pluripotent stem cells to generate three-dimensional structures that recapitulate aspects of human brain development. These systems have enabled unprecedented insights into neurodevelopmental disorders and brain evolution. Similarly, intestinal organoids derived from adult stem cells have become valuable tools for studying gut physiology, host-microbe interactions, and intestinal diseases.
Liver and kidney organoids have also gained traction, though they face challenges in achieving full functional maturity. Current liver organoid systems can model basic hepatic functions but struggle to replicate complex detoxification processes. Kidney organoids, while promising for nephrotoxicity studies, still lack complete nephron structures and functional vasculature.
Despite these advances, significant technical barriers persist across organoid platforms. A primary challenge is the lack of standardization in culture protocols, leading to high variability between laboratories and even between batches within the same lab. This variability undermines reproducibility and complicates data interpretation, particularly for drug screening applications.
Vascularization remains another critical hurdle. Most organoid systems lack proper blood vessel networks, limiting nutrient and oxygen delivery to inner regions and restricting organoid size. This absence of vasculature also prevents modeling of systemic drug distribution and immune cell interactions, constraining the physiological relevance of these models.
Matrix dependency presents additional complications. Current systems rely heavily on animal-derived matrices like Matrigel, which suffer from batch-to-batch variability and undefined composition. These limitations impede scalability and regulatory approval for clinical applications. Efforts to develop synthetic alternatives have shown promise but have not yet achieved widespread adoption.
The absence of immune components represents another significant gap. Most organoid systems lack immune cells, limiting their utility for studying inflammatory responses and immune-mediated diseases. Co-culture approaches with immune cells have been developed but face challenges in maintaining long-term stability.
Maturation deficiencies also plague current systems. Many organoids remain developmentally immature, resembling fetal rather than adult tissues. This immaturity limits their applicability for modeling adult-onset diseases and age-related conditions. Approaches using extended culture periods or mechanical stimulation have shown some success in enhancing maturation but require further optimization.
Liver and kidney organoids have also gained traction, though they face challenges in achieving full functional maturity. Current liver organoid systems can model basic hepatic functions but struggle to replicate complex detoxification processes. Kidney organoids, while promising for nephrotoxicity studies, still lack complete nephron structures and functional vasculature.
Despite these advances, significant technical barriers persist across organoid platforms. A primary challenge is the lack of standardization in culture protocols, leading to high variability between laboratories and even between batches within the same lab. This variability undermines reproducibility and complicates data interpretation, particularly for drug screening applications.
Vascularization remains another critical hurdle. Most organoid systems lack proper blood vessel networks, limiting nutrient and oxygen delivery to inner regions and restricting organoid size. This absence of vasculature also prevents modeling of systemic drug distribution and immune cell interactions, constraining the physiological relevance of these models.
Matrix dependency presents additional complications. Current systems rely heavily on animal-derived matrices like Matrigel, which suffer from batch-to-batch variability and undefined composition. These limitations impede scalability and regulatory approval for clinical applications. Efforts to develop synthetic alternatives have shown promise but have not yet achieved widespread adoption.
The absence of immune components represents another significant gap. Most organoid systems lack immune cells, limiting their utility for studying inflammatory responses and immune-mediated diseases. Co-culture approaches with immune cells have been developed but face challenges in maintaining long-term stability.
Maturation deficiencies also plague current systems. Many organoids remain developmentally immature, resembling fetal rather than adult tissues. This immaturity limits their applicability for modeling adult-onset diseases and age-related conditions. Approaches using extended culture periods or mechanical stimulation have shown some success in enhancing maturation but require further optimization.
Mainstream Organoid Culture Methodologies
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems utilize specialized matrices, scaffolds, and growth factors to support the self-organization of stem cells into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and tissue architecture development, providing more physiologically relevant models than traditional 2D cultures for studying organ development, disease modeling, and drug testing.- 3D organoid culture methods and systems: Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The 3D environment allows cells to interact with each other and with extracellular matrix components in ways that better recapitulate in vivo development compared to traditional 2D cultures.
- Extracellular matrix components for organoid culture: Specific extracellular matrix components and scaffolds that support organoid growth and development. These include natural matrices like Matrigel, collagen, and laminin, as well as synthetic hydrogels with defined compositions. The physical and biochemical properties of these matrices can be tailored to support specific organoid types and developmental stages, influencing cell differentiation, proliferation, and organization.
- Growth factors and signaling molecules for organoid development: Specific growth factors, morphogens, and signaling molecules that regulate organoid formation and maturation. These bioactive compounds are added to culture media in precise combinations and sequences to recapitulate developmental signaling pathways. They direct stem cell differentiation, pattern formation, and functional maturation of organoids, allowing researchers to model organ development and disease in vitro.
- Bioreactor systems for organoid culture: Specialized bioreactor systems designed for the large-scale production and maintenance of organoids. These systems provide controlled environments with optimized conditions for oxygen tension, nutrient delivery, waste removal, and mechanical stimulation. Advanced bioreactors may incorporate perfusion systems, mechanical stimulation, or electrical stimulation to enhance organoid development and functionality, enabling applications in drug screening and regenerative medicine.
- Disease modeling and drug screening using organoid systems: Applications of organoid culture systems for modeling human diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, enabling personalized medicine approaches. These systems allow for high-throughput drug screening in more physiologically relevant models compared to traditional cell cultures, potentially improving the translation of preclinical findings to clinical outcomes and reducing reliance on animal testing.
02 Stem Cell-Derived Organoid Systems
Methods for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve specific differentiation protocols that guide stem cells through developmental pathways to form organ-specific structures. The resulting organoids can recapitulate key aspects of organ development and function, making them valuable tools for studying human biology and disease mechanisms.Expand Specific Solutions03 Specialized Media and Growth Factors for Organoid Culture
Formulations of culture media and growth factors specifically designed to support organoid growth and development. These specialized media contain combinations of signaling molecules, nutrients, and supplements that promote stem cell maintenance, differentiation, and self-organization into organoids. The precise composition of these media is critical for directing the formation of specific organ types and maintaining long-term organoid cultures.Expand Specific Solutions04 Disease Modeling with Organoid Systems
Applications of organoid culture systems for modeling human diseases, including cancer, genetic disorders, and infectious diseases. Patient-derived organoids can recapitulate disease phenotypes and serve as personalized models for studying disease mechanisms and testing therapeutic interventions. These systems enable researchers to investigate disease progression in a controlled environment and develop targeted treatments based on individual patient characteristics.Expand Specific Solutions05 Bioengineering Approaches for Organoid Culture
Advanced bioengineering techniques to enhance organoid culture systems, including microfluidic devices, bioprinting, and biomaterial scaffolds. These approaches provide better control over the organoid microenvironment, improve nutrient delivery, and enable the creation of more complex multi-organ systems. Engineered organoid platforms can incorporate features such as vasculature, immune components, and mechanical stimuli to better mimic in vivo conditions and improve the physiological relevance of organoid models.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
Organoid culture systems represent a cutting-edge frontier in biomedical research, currently in an early growth phase with rapidly expanding applications. The global market for organoid technologies is projected to reach significant scale as these 3D cellular models increasingly replace traditional 2D cultures and animal testing. Technologically, the field shows varying maturity levels across different applications, with companies like STEMCELL Technologies, Cell Microsystems, and Molecular Devices leading instrumentation development, while Mimetas, Organoidsciences, and CYTOO focus on specialized culture platforms. Academic institutions including Johns Hopkins University, University of Washington, and Friedrich Miescher Institute collaborate extensively with industry partners to advance fundamental research. The integration of organoid systems with complementary technologies like microfluidics and high-content imaging, pioneered by companies such as SABIC Global Technologies and Texas Instruments, is accelerating the field's development toward standardized, reproducible applications in drug discovery and personalized medicine.
Cell Microsystems, Inc.
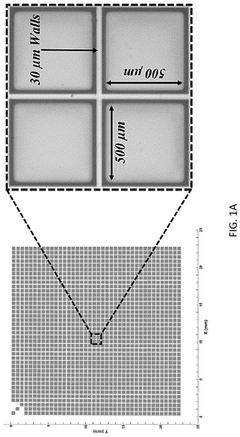
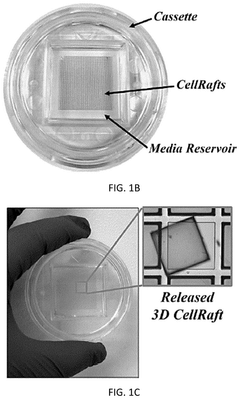
Technical Solution: Cell Microsystems has developed the CellRaft® Technology, an innovative platform for isolating, culturing, and analyzing single cells and organoids. Their system uses microwell arrays containing thousands of individual "rafts" on which organoids can be cultured and monitored over time. The CellRaft® AIR™ System enables researchers to identify and isolate specific organoids of interest based on morphological or fluorescent markers, allowing for selective retrieval without disturbing neighboring structures. This capability is particularly valuable for establishing clonal organoid lines or selecting organoids with specific characteristics. The company's technology supports time-lapse imaging to track organoid development from single cells through maturation, providing insights into morphogenesis processes. Their platform is compatible with downstream molecular analysis techniques, enabling genomic and transcriptomic profiling of selected organoids.
Strengths: Unparalleled capability for selecting and retrieving specific organoids of interest; minimal disruption during isolation preserves organoid integrity; compatible with downstream molecular analyses. Weaknesses: Limited throughput compared to some other screening platforms; requires specialized equipment not common in all laboratories; may require optimization for different organoid types.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive organoid culture systems through their IntestiCult™ and PancreaCult™ product lines. Their approach focuses on providing optimized media formulations that contain precisely balanced growth factors and small molecules to support the self-organization and differentiation of stem cells into functional organoids. Their IntestiCult™ Organoid Growth Medium (OGM) specifically supports the establishment and long-term culture of intestinal organoids from both human and mouse tissues, maintaining stem cell populations while allowing differentiation into specialized cell types. STEMCELL has also pioneered standardized protocols for organoid derivation, expansion, and cryopreservation, addressing key challenges in reproducibility. Their systems incorporate specialized extracellular matrix components that mimic the native stem cell niche, providing appropriate mechanical and biochemical cues for organoid development.
Strengths: Ready-to-use media formulations eliminate variability in preparation; comprehensive protocols enhance reproducibility across laboratories; extensive validation across multiple tissue types. Weaknesses: Relatively high cost for consumable media components in long-term studies; some applications may require customization beyond standard formulations; dependence on animal-derived matrix components in some systems.
Breakthrough Patents in Organoid Development
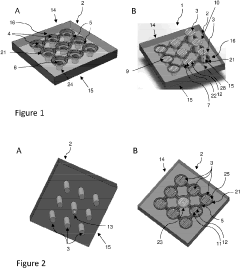
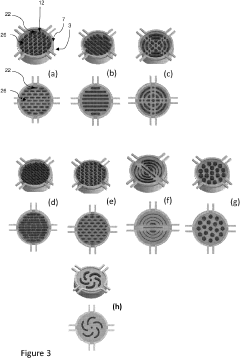
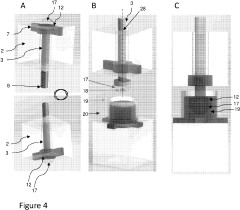
Micro-droplet dish
PatentPendingUS20230256449A1
Innovation
- A micro-droplet plate with a fixed element and moving elements that form, isolate, and seed gel droplets of specific volumes, utilizing hydrophilic surfaces with relief items to create and maintain microfluidic circuits for controlled dispensing and culture, enabling the formation of uniform gel droplets between 1 and 400 microliters.
Automated system for imaging, identification, and isolation of organoids
PatentPendingUS20240320992A1
Innovation
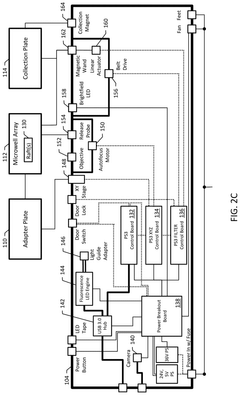
- An automated system utilizing a microarray with releasable cellrafts for culturing and imaging organoids, allowing for the polymerization of extracellular matrix within microwells, followed by automated imaging and release of selected organoids for transfer to a collection plate using a motorized needle and magnetic wand, enabling precise and efficient single-organoid imaging and isolation.
Regulatory Framework for Organoid-Based Research
The regulatory landscape for organoid-based research is evolving rapidly as these three-dimensional cellular structures gain prominence in biomedical applications. Currently, organoid research operates within a complex framework of existing regulations that were primarily designed for conventional cell cultures and animal models, creating significant regulatory gaps and uncertainties.
In the United States, the Food and Drug Administration (FDA) has begun developing specific guidance for organoid technologies, particularly focusing on their applications in drug development and toxicity testing. The FDA's framework emphasizes validation protocols and standardization requirements to ensure reproducibility of organoid-based assays. Meanwhile, the European Medicines Agency (EMA) has established working groups dedicated to evaluating the regulatory implications of advanced in vitro models, including organoids.
Ethical considerations form a critical component of the regulatory framework, especially for brain organoids and those derived from human embryonic stem cells. The International Society for Stem Cell Research (ISSCR) has published guidelines addressing ethical boundaries in organoid research, particularly concerning the development of cerebral organoids with potential neural activity and consciousness-like properties.
Data standardization represents another regulatory challenge, with initiatives like the Human Cell Atlas and the NIH's Standards Working Group developing protocols for data collection, analysis, and sharing across organoid research. These efforts aim to establish minimum reporting requirements and quality control metrics essential for regulatory compliance.
Intellectual property regulations surrounding organoid technologies present unique challenges, with patent landscapes becoming increasingly complex. Key patents cover fundamental organoid culture methods, specific tissue-type organoids, and commercial applications, creating potential barriers for research accessibility and commercialization.
Privacy regulations, including GDPR in Europe and HIPAA in the US, significantly impact organoid research using patient-derived materials. These frameworks mandate strict consent procedures and data protection measures when handling human biological samples for organoid generation.
Looking forward, regulatory bodies are moving toward adaptive frameworks that can accommodate the rapid technological advances in organoid systems while maintaining scientific rigor and ethical standards. International harmonization efforts are underway to develop consensus guidelines that balance innovation with appropriate oversight, recognizing organoids as a distinct category requiring specialized regulatory approaches.
In the United States, the Food and Drug Administration (FDA) has begun developing specific guidance for organoid technologies, particularly focusing on their applications in drug development and toxicity testing. The FDA's framework emphasizes validation protocols and standardization requirements to ensure reproducibility of organoid-based assays. Meanwhile, the European Medicines Agency (EMA) has established working groups dedicated to evaluating the regulatory implications of advanced in vitro models, including organoids.
Ethical considerations form a critical component of the regulatory framework, especially for brain organoids and those derived from human embryonic stem cells. The International Society for Stem Cell Research (ISSCR) has published guidelines addressing ethical boundaries in organoid research, particularly concerning the development of cerebral organoids with potential neural activity and consciousness-like properties.
Data standardization represents another regulatory challenge, with initiatives like the Human Cell Atlas and the NIH's Standards Working Group developing protocols for data collection, analysis, and sharing across organoid research. These efforts aim to establish minimum reporting requirements and quality control metrics essential for regulatory compliance.
Intellectual property regulations surrounding organoid technologies present unique challenges, with patent landscapes becoming increasingly complex. Key patents cover fundamental organoid culture methods, specific tissue-type organoids, and commercial applications, creating potential barriers for research accessibility and commercialization.
Privacy regulations, including GDPR in Europe and HIPAA in the US, significantly impact organoid research using patient-derived materials. These frameworks mandate strict consent procedures and data protection measures when handling human biological samples for organoid generation.
Looking forward, regulatory bodies are moving toward adaptive frameworks that can accommodate the rapid technological advances in organoid systems while maintaining scientific rigor and ethical standards. International harmonization efforts are underway to develop consensus guidelines that balance innovation with appropriate oversight, recognizing organoids as a distinct category requiring specialized regulatory approaches.
Ethical Implications of Human Organoid Models
The development of human organoid models raises profound ethical questions that extend beyond technical considerations. As these miniaturized, three-dimensional tissue cultures increasingly mimic human organs' functionality, they challenge existing ethical frameworks and demand careful consideration from researchers, policymakers, and society at large.
Consent and ownership issues stand at the forefront of ethical concerns. When human cells are used to generate organoids, questions arise regarding donor consent scope, particularly for applications unforeseen at collection time. The commercialization of organoid lines derived from donated tissues further complicates matters, raising questions about who rightfully owns these biological entities and associated intellectual property.
Privacy considerations become increasingly relevant as organoids derived from specific individuals potentially reveal sensitive genetic information. This creates tension between advancing personalized medicine and protecting genetic privacy, especially when organoids are shared between research institutions or companies without robust anonymization protocols.
The moral status of organoids presents perhaps the most philosophically challenging dimension. As brain organoids develop increasingly complex neural networks, the scientific community must confront difficult questions about consciousness, sentience, and moral consideration. Current regulatory frameworks remain inadequate for addressing the unique ethical position of entities that blur traditional boundaries between cell cultures and developing organs.
Cultural and religious perspectives add another layer of complexity. Different belief systems hold varying views on the manipulation of human tissues and the creation of organ-like structures, potentially limiting global consensus on ethical guidelines. These perspectives must be respectfully incorporated into international research governance frameworks.
Looking forward, the field requires proactive ethical oversight that evolves alongside technical capabilities. Transparent governance structures involving diverse stakeholders—including ethicists, patient advocates, and religious representatives—are essential for developing guidelines that balance scientific progress with ethical responsibility.
The establishment of standardized informed consent protocols specifically addressing organoid research represents a critical step toward ethical practice. These protocols must clearly communicate potential commercial applications and long-term storage implications while respecting donor autonomy and cultural sensitivities.
As organoid technology advances toward greater complexity and functionality, the scientific community must remain vigilant about potential ethical boundaries, particularly regarding neural organoids that approach consciousness-like properties. This vigilance requires ongoing interdisciplinary dialogue that anticipates ethical challenges before they materialize in the laboratory.
Consent and ownership issues stand at the forefront of ethical concerns. When human cells are used to generate organoids, questions arise regarding donor consent scope, particularly for applications unforeseen at collection time. The commercialization of organoid lines derived from donated tissues further complicates matters, raising questions about who rightfully owns these biological entities and associated intellectual property.
Privacy considerations become increasingly relevant as organoids derived from specific individuals potentially reveal sensitive genetic information. This creates tension between advancing personalized medicine and protecting genetic privacy, especially when organoids are shared between research institutions or companies without robust anonymization protocols.
The moral status of organoids presents perhaps the most philosophically challenging dimension. As brain organoids develop increasingly complex neural networks, the scientific community must confront difficult questions about consciousness, sentience, and moral consideration. Current regulatory frameworks remain inadequate for addressing the unique ethical position of entities that blur traditional boundaries between cell cultures and developing organs.
Cultural and religious perspectives add another layer of complexity. Different belief systems hold varying views on the manipulation of human tissues and the creation of organ-like structures, potentially limiting global consensus on ethical guidelines. These perspectives must be respectfully incorporated into international research governance frameworks.
Looking forward, the field requires proactive ethical oversight that evolves alongside technical capabilities. Transparent governance structures involving diverse stakeholders—including ethicists, patient advocates, and religious representatives—are essential for developing guidelines that balance scientific progress with ethical responsibility.
The establishment of standardized informed consent protocols specifically addressing organoid research represents a critical step toward ethical practice. These protocols must clearly communicate potential commercial applications and long-term storage implications while respecting donor autonomy and cultural sensitivities.
As organoid technology advances toward greater complexity and functionality, the scientific community must remain vigilant about potential ethical boundaries, particularly regarding neural organoids that approach consciousness-like properties. This vigilance requires ongoing interdisciplinary dialogue that anticipates ethical challenges before they materialize in the laboratory.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!