Organoid Culture Systems and Bioengineering Strategies
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Research Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering over the past decade. These three-dimensional cellular structures self-organize to mimic the architecture and functionality of native organs, providing unprecedented opportunities for studying human development, disease modeling, drug screening, and regenerative medicine applications.
The evolution of organoid technology can be traced back to the groundbreaking work of Hans Clevers and colleagues in 2009, who established the first intestinal organoids from Lgr5+ stem cells. This milestone catalyzed rapid expansion across multiple organ systems, including brain, liver, kidney, pancreas, and lung organoids. The field has progressed from simple epithelial structures to increasingly complex multi-cellular systems that better recapitulate in vivo organ physiology.
Current organoid culture systems face significant limitations that constrain their research and clinical applications. These include inconsistent reproducibility, limited maturation, absence of vascular networks, and challenges in scalability. Additionally, most systems rely heavily on animal-derived matrices like Matrigel, which introduces batch-to-batch variability and regulatory concerns for clinical translation.
The primary objective of this research is to systematically evaluate existing organoid culture systems and identify innovative bioengineering strategies to overcome current limitations. Specifically, we aim to explore advanced biomaterials, microfluidic platforms, and bioprinting technologies that can enhance organoid development, functionality, and physiological relevance.
A key focus area involves developing synthetic hydrogel alternatives to Matrigel that offer tunable mechanical and biochemical properties while maintaining reproducibility. Additionally, we seek to investigate strategies for introducing vascularization and other supporting cell types to improve organoid maturation and functionality.
The research also aims to establish standardized protocols and quality control metrics for organoid generation, addressing the critical need for reproducibility in both research and potential clinical applications. This includes developing quantitative assessment methods for organoid morphology, gene expression profiles, and functional characteristics.
Long-term objectives include advancing organoid technology toward personalized medicine applications, such as patient-specific drug screening and toxicity testing. Furthermore, we aim to explore the potential of organoids in regenerative medicine, including their use as transplantable tissue constructs and as platforms for gene editing therapies.
The evolution of organoid technology can be traced back to the groundbreaking work of Hans Clevers and colleagues in 2009, who established the first intestinal organoids from Lgr5+ stem cells. This milestone catalyzed rapid expansion across multiple organ systems, including brain, liver, kidney, pancreas, and lung organoids. The field has progressed from simple epithelial structures to increasingly complex multi-cellular systems that better recapitulate in vivo organ physiology.
Current organoid culture systems face significant limitations that constrain their research and clinical applications. These include inconsistent reproducibility, limited maturation, absence of vascular networks, and challenges in scalability. Additionally, most systems rely heavily on animal-derived matrices like Matrigel, which introduces batch-to-batch variability and regulatory concerns for clinical translation.
The primary objective of this research is to systematically evaluate existing organoid culture systems and identify innovative bioengineering strategies to overcome current limitations. Specifically, we aim to explore advanced biomaterials, microfluidic platforms, and bioprinting technologies that can enhance organoid development, functionality, and physiological relevance.
A key focus area involves developing synthetic hydrogel alternatives to Matrigel that offer tunable mechanical and biochemical properties while maintaining reproducibility. Additionally, we seek to investigate strategies for introducing vascularization and other supporting cell types to improve organoid maturation and functionality.
The research also aims to establish standardized protocols and quality control metrics for organoid generation, addressing the critical need for reproducibility in both research and potential clinical applications. This includes developing quantitative assessment methods for organoid morphology, gene expression profiles, and functional characteristics.
Long-term objectives include advancing organoid technology toward personalized medicine applications, such as patient-specific drug screening and toxicity testing. Furthermore, we aim to explore the potential of organoids in regenerative medicine, including their use as transplantable tissue constructs and as platforms for gene editing therapies.
Market Analysis of Organoid Applications in Biomedicine
The global organoid market has witnessed substantial growth in recent years, with a valuation of approximately $1.3 billion in 2022 and projections indicating a compound annual growth rate (CAGR) of 22.1% through 2030. This remarkable expansion is primarily driven by increasing applications in drug discovery, personalized medicine, and disease modeling, particularly in oncology and neurodegenerative disorders.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for over 40% of the total market share. These entities leverage organoid technology to reduce drug development costs and timelines by providing more physiologically relevant screening platforms compared to traditional 2D cell cultures. The cost-saving potential is significant, with estimates suggesting that incorporating organoid testing can reduce preclinical development expenses by up to 25%.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to increasing research investments and expanding biotechnology sectors in countries like China, Japan, and South Korea.
By application type, cancer research constitutes the largest segment (35%), followed by developmental biology (25%), regenerative medicine (20%), and toxicology studies (15%). The remaining 5% encompasses emerging applications such as infectious disease research, which gained significant attention during the COVID-19 pandemic.
Technological advancements in bioengineering strategies are creating new market opportunities. The integration of microfluidics with organoid systems has spawned the "organ-on-chip" subsegment, which is growing at a CAGR of 28%, outpacing the broader organoid market. Additionally, the development of bioprinting technologies compatible with organoid culture is opening new commercial avenues.
Challenges limiting market expansion include high production costs, with current estimates placing the cost of developing a single patient-derived organoid line between $10,000-$15,000. Standardization issues also persist, with reproducibility concerns affecting commercial adoption. Furthermore, regulatory frameworks for organoid-based products remain underdeveloped, creating uncertainty for commercial stakeholders.
Despite these challenges, venture capital investment in organoid technology startups has increased by 150% since 2018, reflecting strong market confidence. Strategic partnerships between academic institutions and industry players are becoming increasingly common, with over 60 such collaborations announced in the past three years, further indicating the commercial potential of organoid technologies in biomedicine.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for over 40% of the total market share. These entities leverage organoid technology to reduce drug development costs and timelines by providing more physiologically relevant screening platforms compared to traditional 2D cell cultures. The cost-saving potential is significant, with estimates suggesting that incorporating organoid testing can reduce preclinical development expenses by up to 25%.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to increasing research investments and expanding biotechnology sectors in countries like China, Japan, and South Korea.
By application type, cancer research constitutes the largest segment (35%), followed by developmental biology (25%), regenerative medicine (20%), and toxicology studies (15%). The remaining 5% encompasses emerging applications such as infectious disease research, which gained significant attention during the COVID-19 pandemic.
Technological advancements in bioengineering strategies are creating new market opportunities. The integration of microfluidics with organoid systems has spawned the "organ-on-chip" subsegment, which is growing at a CAGR of 28%, outpacing the broader organoid market. Additionally, the development of bioprinting technologies compatible with organoid culture is opening new commercial avenues.
Challenges limiting market expansion include high production costs, with current estimates placing the cost of developing a single patient-derived organoid line between $10,000-$15,000. Standardization issues also persist, with reproducibility concerns affecting commercial adoption. Furthermore, regulatory frameworks for organoid-based products remain underdeveloped, creating uncertainty for commercial stakeholders.
Despite these challenges, venture capital investment in organoid technology startups has increased by 150% since 2018, reflecting strong market confidence. Strategic partnerships between academic institutions and industry players are becoming increasingly common, with over 60 such collaborations announced in the past three years, further indicating the commercial potential of organoid technologies in biomedicine.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid technology, several critical challenges continue to impede the full realization of their potential in biomedical applications. The most persistent obstacle remains the lack of standardization across culture protocols, resulting in high variability between batches and laboratories. This inconsistency undermines reproducibility and hampers clinical translation, as organoids produced under seemingly identical conditions may exhibit different morphological and functional characteristics.
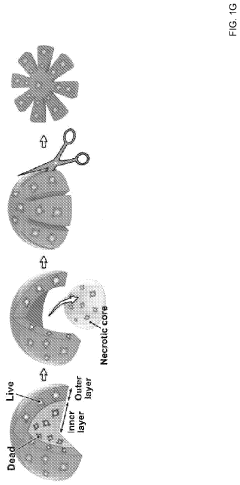
Vascularization represents another major hurdle in organoid development. Current culture systems typically fail to generate functional blood vessels, limiting nutrient and oxygen diffusion to approximately 200-400 μm. This constraint restricts organoid size and leads to necrotic cores in larger structures, preventing the accurate modeling of complex organ architectures and functions that depend on proper vascularization.
The extracellular matrix (ECM) components used in organoid culture present additional challenges. Matrigel, the most commonly used matrix, is derived from mouse sarcoma cells, introducing batch-to-batch variability and potential xenogeneic contamination that complicates clinical applications. Furthermore, its undefined composition makes it difficult to determine which specific ECM components influence organoid development.
Current organoid systems also struggle to recapitulate the complex cellular heterogeneity found in native tissues. Many protocols favor the expansion of epithelial components while underrepresenting stromal, immune, and neural elements that are crucial for proper organ function and disease modeling. This limitation is particularly problematic when studying conditions involving multiple cell types, such as inflammatory disorders or cancer.
The absence of mechanical and physiological stimuli in static culture conditions represents another significant challenge. In vivo, tissues experience various forces including fluid flow, mechanical stretching, and compression that influence cellular behavior and tissue development. Conventional organoid cultures lack these dynamic cues, resulting in models that may not accurately reflect in vivo tissue architecture and function.
Long-term maintenance of organoids remains problematic, with most systems showing deterioration in structure and function over extended culture periods. This limitation restricts the study of chronic diseases, aging processes, and long-term drug effects. Additionally, current methods for cryopreservation often result in reduced viability and altered functionality upon thawing, complicating biobanking efforts and consistent experimental planning.
Vascularization represents another major hurdle in organoid development. Current culture systems typically fail to generate functional blood vessels, limiting nutrient and oxygen diffusion to approximately 200-400 μm. This constraint restricts organoid size and leads to necrotic cores in larger structures, preventing the accurate modeling of complex organ architectures and functions that depend on proper vascularization.
The extracellular matrix (ECM) components used in organoid culture present additional challenges. Matrigel, the most commonly used matrix, is derived from mouse sarcoma cells, introducing batch-to-batch variability and potential xenogeneic contamination that complicates clinical applications. Furthermore, its undefined composition makes it difficult to determine which specific ECM components influence organoid development.
Current organoid systems also struggle to recapitulate the complex cellular heterogeneity found in native tissues. Many protocols favor the expansion of epithelial components while underrepresenting stromal, immune, and neural elements that are crucial for proper organ function and disease modeling. This limitation is particularly problematic when studying conditions involving multiple cell types, such as inflammatory disorders or cancer.
The absence of mechanical and physiological stimuli in static culture conditions represents another significant challenge. In vivo, tissues experience various forces including fluid flow, mechanical stretching, and compression that influence cellular behavior and tissue development. Conventional organoid cultures lack these dynamic cues, resulting in models that may not accurately reflect in vivo tissue architecture and function.
Long-term maintenance of organoids remains problematic, with most systems showing deterioration in structure and function over extended culture periods. This limitation restricts the study of chronic diseases, aging processes, and long-term drug effects. Additionally, current methods for cryopreservation often result in reduced viability and altered functionality upon thawing, complicating biobanking efforts and consistent experimental planning.
State-of-the-Art Organoid Culture Methodologies
01 3D Organoid Culture Systems
Three-dimensional organoid culture systems that mimic the natural microenvironment of organs. These systems provide a more physiologically relevant platform for studying organ development, disease modeling, and drug testing. The 3D structure allows cells to organize themselves in a manner similar to in vivo conditions, enabling better recapitulation of tissue architecture and function.- 3D Organoid Culture Systems: Three-dimensional organoid culture systems that mimic the natural microenvironment of organs. These systems utilize specialized scaffolds, matrices, and bioreactors to support the growth and differentiation of organoids. The 3D culture environment allows cells to self-organize into structures that resemble the architecture and functionality of native tissues, providing more physiologically relevant models for research and drug testing.
- Bioreactor Technologies for Organoid Culture: Advanced bioreactor systems designed specifically for organoid cultivation that provide controlled environments for long-term culture. These technologies incorporate features such as continuous perfusion, mechanical stimulation, and precise control of oxygen and nutrient delivery. Specialized bioreactors enable the scaling up of organoid production while maintaining consistent quality and functionality, which is essential for applications in regenerative medicine and drug discovery.
- Extracellular Matrix Components and Scaffolds: Innovative biomaterials and extracellular matrix formulations that support organoid development. These include natural and synthetic hydrogels, decellularized tissue matrices, and bioprinted scaffolds that provide structural support and biochemical cues for cell growth. The composition and mechanical properties of these materials can be tailored to specific tissue types, enhancing organoid formation, maturation, and functionality.
- Microfluidic Systems for Organoid Culture: Microfluidic platforms that enable precise control over the organoid microenvironment. These systems incorporate channels, chambers, and sensors to regulate fluid flow, nutrient exchange, and waste removal. Microfluidic technologies allow for high-throughput screening, real-time monitoring of organoid development, and the creation of organ-on-chip models that can simulate physiological conditions and organ interactions.
- Growth Factor Delivery and Signaling Modulation: Methods for controlled delivery of growth factors and modulation of signaling pathways in organoid cultures. These approaches include timed-release systems, gradient generators, and genetic modification techniques that regulate stem cell differentiation and tissue patterning. Precise manipulation of biochemical signals enables the development of more complex and functionally mature organoids that better recapitulate in vivo tissue characteristics.
02 Bioreactors and Perfusion Systems for Organoid Culture
Specialized bioreactors and perfusion systems designed for the cultivation and maintenance of organoids. These systems provide controlled environments with continuous nutrient supply and waste removal, mimicking physiological conditions. They enable long-term culture of organoids with improved viability, maturation, and functionality compared to static culture methods.Expand Specific Solutions03 Biomaterials and Scaffolds for Organoid Engineering
Advanced biomaterials and scaffolds that support organoid growth and development. These materials provide structural support and biochemical cues that guide cell organization and differentiation. Natural and synthetic hydrogels, extracellular matrix components, and bioprinted structures are used to create microenvironments that promote organoid formation with specific architectures and functions.Expand Specific Solutions04 Microfluidic Devices for Organoid Culture
Microfluidic platforms designed for precise control of the organoid microenvironment. These devices enable manipulation of fluid flow, gradients of nutrients and growth factors, and mechanical forces at microscale levels. They facilitate high-throughput screening, organ-on-chip applications, and studies of organoid responses to various stimuli under controlled conditions.Expand Specific Solutions05 Organoid Imaging and Analysis Technologies
Specialized technologies for imaging, monitoring, and analyzing organoids during development and experimentation. These include advanced microscopy techniques, biosensors, and analytical tools that enable real-time observation of organoid growth, morphology, and function. Such technologies provide insights into cellular behaviors, tissue organization, and responses to experimental conditions without disrupting the culture system.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in Organoid Field
The organoid culture systems and bioengineering strategies market is currently in a growth phase, with an estimated global market size of $1.5-2 billion and projected annual growth of 15-20%. The technology is transitioning from early development to commercial application, with varying degrees of maturity across different applications. Key players include established companies like Molecular Devices and Cell Microsystems, which focus on automation and single-cell isolation technologies, alongside emerging specialists such as Xilis and Cellesce that are developing scalable patient-derived organoid platforms. Academic institutions (Harvard, UPenn, KU Leuven) continue to drive fundamental research, while pharmaceutical companies increasingly adopt organoid technologies for drug discovery and personalized medicine applications. The competitive landscape reflects a blend of specialized biotech firms, research institutions, and larger life science tool providers working to address manufacturing scalability and standardization challenges.
Trustees of the University of Pennsylvania
Technical Solution: The University of Pennsylvania has developed advanced bioengineering approaches for organoid culture that focus on recreating the native tissue microenvironment with unprecedented precision. Their platform incorporates custom-designed hydrogel systems with tunable mechanical properties and degradation kinetics that can be matched to specific tissue types and developmental stages. Penn researchers have pioneered techniques for spatially patterned growth factor delivery within 3D matrices, creating directional cues that guide organoid development and cellular organization. Their system includes innovative microfluidic devices that enable the establishment of chemical gradients and controlled delivery of morphogens, mimicking developmental signaling patterns crucial for proper organogenesis. The Penn approach also features integration of primary tissue-specific stromal cells alongside stem cells, creating more complete niche environments that support organoid maturation and functionality. Their technology incorporates non-invasive monitoring capabilities through integrated biosensors that can track metabolic activity, pH changes, and secreted factors in real-time, providing valuable data on organoid health and function without disrupting the culture system.
Strengths: Exceptional control over mechanical and biochemical microenvironment parameters; sophisticated spatial patterning capabilities; integration of multiple cell types for more complete tissue representation; non-invasive monitoring capabilities. Weaknesses: Complex systems may require specialized expertise; higher technical barriers to implementation; potential challenges in scaling up these sophisticated approaches for high-throughput applications.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to organoid culture systems centers on their pioneering organ-on-chip technology that combines microfluidic devices with living human cells to create functional tissue models. Their platform features compartmentalized microchannels lined with living human cells that recreate tissue-tissue interfaces, physical microenvironments, and physiological functions of organs. Harvard researchers have developed specialized extracellular matrix (ECM) compositions that better mimic native tissue environments, enhancing organoid development and functionality. Their systems incorporate mechanical forces (such as breathing motions for lung models or peristaltic contractions for intestinal models) to recreate physiological stimuli essential for proper tissue function. Harvard has also developed advanced imaging techniques specifically optimized for 3D organoid visualization and analysis, allowing for real-time monitoring of cellular responses and developmental processes. Their bioengineering strategies include the integration of vascular networks within organoids to overcome size limitations caused by diffusion constraints, enabling the development of larger, more complex tissue structures with improved viability.
Strengths: Exceptional integration of mechanical forces that mimic physiological conditions; strong focus on tissue-tissue interfaces that better represent in vivo complexity; established protocols with extensive validation. Weaknesses: Systems can be technically complex to implement; higher cost and expertise requirements compared to simpler organoid culture methods; some models may still lack certain aspects of organ functionality.
Key Breakthroughs in Organoid Bioengineering
Engineering of organoid culture for enhanced organogenesis in a dish
PatentPendingUS20240026261A1
Innovation
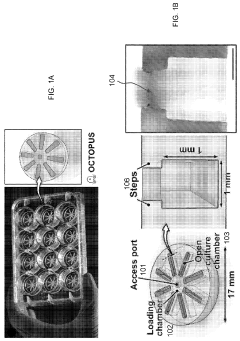
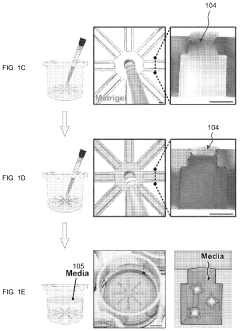
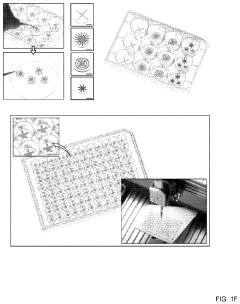
- The OCTOPUS device provides a 3D culture system with radially arranged culture chambers that reduce diffusion limitations by allowing unrestricted access to nutrients and oxygen, enabling continuous culture of organoids for extended periods without passaging, using a simple and scalable design compatible with standard cell culture plates.
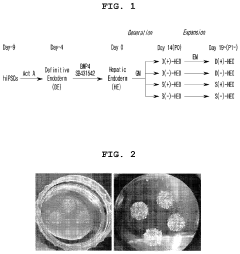
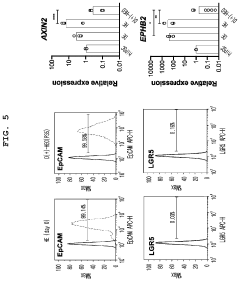
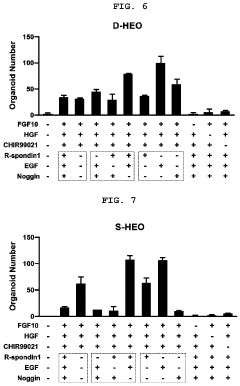
Method for constructing human pluripotent stem cell-derived liver organoid having enhanced drug metabolic potential and liver organoid constructed by same method
PatentActiveUS20240141289A1
Innovation
- A method for constructing human pluripotent stem cell-derived liver organoids by differentiating cells into definitive endoderm, hepatic endoderm, and finally liver organoids, using specific culture media without R-Spondin-1, Noggin, and EGF, which enhances drug metabolic capabilities and toxicity evaluation.
Ethical and Regulatory Framework for Organoid Research
The ethical and regulatory landscape surrounding organoid research presents a complex framework that continues to evolve alongside technological advancements. Current regulatory approaches vary significantly across jurisdictions, with some countries implementing specific guidelines for organoid research while others rely on broader frameworks governing stem cell research and human tissue use. The European Union has established comprehensive regulations through its Clinical Trials Regulation and Advanced Therapy Medicinal Products framework, which address aspects of organoid development and application.
In the United States, the FDA has begun developing specialized guidance for organoid technologies, particularly focusing on their potential use in drug screening and personalized medicine applications. Meanwhile, countries like Japan and Singapore have implemented progressive regulatory frameworks that aim to balance innovation with ethical considerations, often featuring expedited approval pathways for certain organoid applications.
Ethical considerations in organoid research center around several key domains. Consent issues are paramount, particularly regarding the sourcing of primary cells for organoid development and the potential future uses of patient-derived organoids. The concept of informed consent becomes especially challenging when considering unanticipated future research applications that may emerge as the technology advances.
Privacy and data protection represent another critical ethical dimension, as organoids derived from patient tissues contain complete genetic information that requires robust protection protocols. The potential commercialization of patient-derived organoids raises questions about ownership, benefit-sharing, and intellectual property rights that remain largely unresolved in many jurisdictions.
For brain organoids specifically, unique ethical questions arise regarding consciousness, sentience, and moral status. As these models become increasingly complex and functionally similar to human brain tissue, the scientific community must address fundamental questions about appropriate limitations and safeguards.
International harmonization efforts are emerging through organizations like the International Society for Stem Cell Research (ISSCR) and the World Health Organization, which have published guidelines addressing organoid research. These frameworks emphasize transparency, responsible innovation, and ongoing ethical oversight as essential components of research governance.
Moving forward, adaptive regulatory approaches that can evolve alongside technological advancements will be crucial. Stakeholder engagement involving scientists, ethicists, patient advocates, and regulatory bodies will be essential to developing frameworks that both protect ethical principles and enable scientific progress in this rapidly advancing field.
In the United States, the FDA has begun developing specialized guidance for organoid technologies, particularly focusing on their potential use in drug screening and personalized medicine applications. Meanwhile, countries like Japan and Singapore have implemented progressive regulatory frameworks that aim to balance innovation with ethical considerations, often featuring expedited approval pathways for certain organoid applications.
Ethical considerations in organoid research center around several key domains. Consent issues are paramount, particularly regarding the sourcing of primary cells for organoid development and the potential future uses of patient-derived organoids. The concept of informed consent becomes especially challenging when considering unanticipated future research applications that may emerge as the technology advances.
Privacy and data protection represent another critical ethical dimension, as organoids derived from patient tissues contain complete genetic information that requires robust protection protocols. The potential commercialization of patient-derived organoids raises questions about ownership, benefit-sharing, and intellectual property rights that remain largely unresolved in many jurisdictions.
For brain organoids specifically, unique ethical questions arise regarding consciousness, sentience, and moral status. As these models become increasingly complex and functionally similar to human brain tissue, the scientific community must address fundamental questions about appropriate limitations and safeguards.
International harmonization efforts are emerging through organizations like the International Society for Stem Cell Research (ISSCR) and the World Health Organization, which have published guidelines addressing organoid research. These frameworks emphasize transparency, responsible innovation, and ongoing ethical oversight as essential components of research governance.
Moving forward, adaptive regulatory approaches that can evolve alongside technological advancements will be crucial. Stakeholder engagement involving scientists, ethicists, patient advocates, and regulatory bodies will be essential to developing frameworks that both protect ethical principles and enable scientific progress in this rapidly advancing field.
Translational Potential of Organoid Technologies
Organoid technologies represent a significant breakthrough in biomedical research, offering unprecedented potential for translational applications across multiple fields. The transition from laboratory research to clinical and commercial applications is accelerating, with organoids increasingly being utilized in drug discovery and development pipelines by pharmaceutical companies. These three-dimensional cellular structures provide more physiologically relevant models for drug screening, toxicity testing, and efficacy assessment compared to traditional two-dimensional cell cultures.
In personalized medicine, patient-derived organoids are emerging as powerful tools for tailoring treatment strategies. By generating organoids from individual patients' tissues, clinicians can test various therapeutic options to determine the most effective treatment approach before administering it to the patient. This application is particularly promising in oncology, where cancer organoids can predict patient-specific drug responses with high accuracy, potentially revolutionizing cancer treatment paradigms.
Regenerative medicine represents another frontier for organoid technology translation. Researchers are exploring the use of organoids as sources for transplantable tissues and organs, addressing the critical shortage of donor organs worldwide. While significant challenges remain, progress in bioengineering strategies is bringing this application closer to clinical reality, with early-stage clinical trials already underway for conditions such as inflammatory bowel disease and retinal disorders.
The diagnostic potential of organoids is also being realized, with applications in infectious disease research, genetic disorder modeling, and environmental toxin assessment. Organoid-based diagnostic platforms could enable more accurate disease detection and characterization, particularly for complex conditions with heterogeneous presentations.
Commercial interest in organoid technologies has surged, evidenced by increasing investment in organoid-focused biotechnology companies and partnerships between academic institutions and industry. The global market for organoid-based products and services is projected to grow substantially in the coming years, driven by applications in drug discovery, personalized medicine, and regenerative therapies.
Regulatory frameworks are evolving to accommodate these novel technologies, with agencies like the FDA and EMA developing guidelines for organoid-based assays and therapies. Standardization efforts are underway to ensure reproducibility and reliability of organoid models across different laboratories and applications, a critical step for broader clinical adoption.
Despite promising advances, several challenges must be addressed to fully realize the translational potential of organoid technologies, including scaling production, reducing costs, improving standardization, and resolving ethical considerations related to more complex neural and cerebral organoids.
In personalized medicine, patient-derived organoids are emerging as powerful tools for tailoring treatment strategies. By generating organoids from individual patients' tissues, clinicians can test various therapeutic options to determine the most effective treatment approach before administering it to the patient. This application is particularly promising in oncology, where cancer organoids can predict patient-specific drug responses with high accuracy, potentially revolutionizing cancer treatment paradigms.
Regenerative medicine represents another frontier for organoid technology translation. Researchers are exploring the use of organoids as sources for transplantable tissues and organs, addressing the critical shortage of donor organs worldwide. While significant challenges remain, progress in bioengineering strategies is bringing this application closer to clinical reality, with early-stage clinical trials already underway for conditions such as inflammatory bowel disease and retinal disorders.
The diagnostic potential of organoids is also being realized, with applications in infectious disease research, genetic disorder modeling, and environmental toxin assessment. Organoid-based diagnostic platforms could enable more accurate disease detection and characterization, particularly for complex conditions with heterogeneous presentations.
Commercial interest in organoid technologies has surged, evidenced by increasing investment in organoid-focused biotechnology companies and partnerships between academic institutions and industry. The global market for organoid-based products and services is projected to grow substantially in the coming years, driven by applications in drug discovery, personalized medicine, and regenerative therapies.
Regulatory frameworks are evolving to accommodate these novel technologies, with agencies like the FDA and EMA developing guidelines for organoid-based assays and therapies. Standardization efforts are underway to ensure reproducibility and reliability of organoid models across different laboratories and applications, a critical step for broader clinical adoption.
Despite promising advances, several challenges must be addressed to fully realize the translational potential of organoid technologies, including scaling production, reducing costs, improving standardization, and resolving ethical considerations related to more complex neural and cerebral organoids.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!