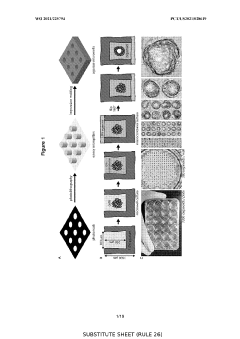
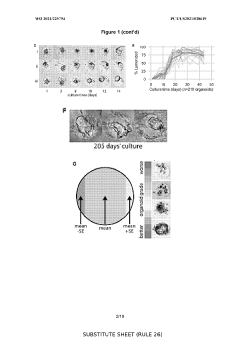
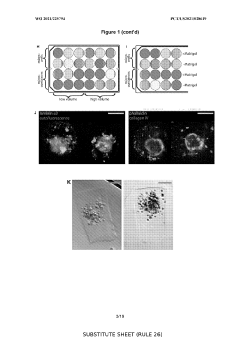
How Do Organoid Culture Systems Address Environmental Needs?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering. These three-dimensional cellular structures self-organize to mimic the architecture and functionality of native organs, providing unprecedented opportunities for studying human development, disease modeling, drug screening, and personalized medicine. The evolution of organoid technology can be traced back to the early 2000s, with significant breakthroughs occurring in 2009 when Sato and colleagues developed the first intestinal organoids from Lgr5+ stem cells.
The trajectory of organoid technology has been marked by continuous refinement of culture protocols and expansion into diverse organ systems. From initial successes with intestinal organoids, the field has rapidly expanded to include brain, liver, kidney, pancreas, lung, and many other organ-specific organoids. This progression reflects the growing understanding of stem cell biology and the microenvironmental factors that guide organ development and maintenance.
Environmental considerations have become increasingly central to organoid research, as researchers recognize that recreating physiologically relevant conditions is essential for developing organoids that accurately represent in vivo tissues. Traditional culture systems often fail to recapitulate the complex environmental cues present in native tissues, limiting the translational potential of organoid models.
The primary objective of modern organoid culture systems is to address these environmental needs by creating biomimetic conditions that support organoid development, maturation, and functionality. This includes optimizing physical parameters such as oxygen tension, mechanical forces, and spatial organization, as well as biochemical factors including growth factor gradients, extracellular matrix composition, and metabolite availability.
Recent technological innovations aim to overcome environmental limitations through the development of advanced bioreactors, microfluidic systems, and bioprinting approaches that enable precise control over the organoid microenvironment. These technologies facilitate the creation of more complex and physiologically relevant organoid models with enhanced functionality and stability.
The ultimate goal of organoid technology extends beyond basic research applications to include regenerative medicine, where organoids could potentially serve as transplantable tissue sources for treating organ dysfunction or damage. Achieving this ambitious objective requires addressing critical environmental challenges related to scalability, reproducibility, and long-term viability of organoid cultures.
As the field continues to evolve, researchers are increasingly focusing on integrating multiple organ systems into "body-on-a-chip" platforms that recapitulate inter-organ interactions and systemic responses. These advanced systems represent the frontier of organoid technology, promising to revolutionize drug development, toxicity testing, and personalized medicine approaches by providing more holistic models of human physiology.
The trajectory of organoid technology has been marked by continuous refinement of culture protocols and expansion into diverse organ systems. From initial successes with intestinal organoids, the field has rapidly expanded to include brain, liver, kidney, pancreas, lung, and many other organ-specific organoids. This progression reflects the growing understanding of stem cell biology and the microenvironmental factors that guide organ development and maintenance.
Environmental considerations have become increasingly central to organoid research, as researchers recognize that recreating physiologically relevant conditions is essential for developing organoids that accurately represent in vivo tissues. Traditional culture systems often fail to recapitulate the complex environmental cues present in native tissues, limiting the translational potential of organoid models.
The primary objective of modern organoid culture systems is to address these environmental needs by creating biomimetic conditions that support organoid development, maturation, and functionality. This includes optimizing physical parameters such as oxygen tension, mechanical forces, and spatial organization, as well as biochemical factors including growth factor gradients, extracellular matrix composition, and metabolite availability.
Recent technological innovations aim to overcome environmental limitations through the development of advanced bioreactors, microfluidic systems, and bioprinting approaches that enable precise control over the organoid microenvironment. These technologies facilitate the creation of more complex and physiologically relevant organoid models with enhanced functionality and stability.
The ultimate goal of organoid technology extends beyond basic research applications to include regenerative medicine, where organoids could potentially serve as transplantable tissue sources for treating organ dysfunction or damage. Achieving this ambitious objective requires addressing critical environmental challenges related to scalability, reproducibility, and long-term viability of organoid cultures.
As the field continues to evolve, researchers are increasingly focusing on integrating multiple organ systems into "body-on-a-chip" platforms that recapitulate inter-organ interactions and systemic responses. These advanced systems represent the frontier of organoid technology, promising to revolutionize drug development, toxicity testing, and personalized medicine approaches by providing more holistic models of human physiology.
Market Analysis for Organoid Culture Applications
The global organoid culture systems market is experiencing robust growth, driven primarily by increasing applications in drug discovery, personalized medicine, and disease modeling. Current market valuations place this sector at approximately 1.5 billion USD in 2023, with projections indicating a compound annual growth rate of 22-25% over the next five years. This accelerated growth trajectory reflects the expanding utility of organoid technologies across multiple biomedical research domains.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These entities leverage organoid culture systems extensively for drug screening and toxicity testing, significantly reducing development costs and time-to-market for new therapeutic compounds. Academic and research institutions constitute the second-largest segment at approximately 30%, focusing primarily on fundamental research and disease modeling applications.
Regionally, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the highest growth rate due to increasing research funding and expanding biotechnology sectors. This regional distribution highlights the global recognition of organoid technologies as essential tools in modern biomedical research.
The market for environmentally sustainable organoid culture systems is emerging as a significant niche, with estimated growth rates exceeding the overall market average by 3-5 percentage points. This sub-segment addresses the environmental impact concerns associated with traditional culture methods, including resource consumption, waste generation, and energy usage. Companies offering solutions with reduced environmental footprints are gaining competitive advantages, particularly among environmentally conscious research institutions and pharmaceutical companies with strong sustainability commitments.
Key market drivers include the rising prevalence of chronic diseases necessitating better in vitro models, increasing R&D investments in regenerative medicine, and growing adoption of personalized medicine approaches. Additionally, technological advancements enabling more physiologically relevant organoid systems are expanding potential applications across oncology, neurology, gastroenterology, and regenerative medicine.
Market challenges primarily revolve around high costs associated with specialized media and growth factors, technical complexities in maintaining consistent organoid cultures, and regulatory uncertainties regarding clinical applications. These factors currently limit broader market penetration, particularly in smaller research institutions and developing regions with constrained research budgets.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of the total market share. These entities leverage organoid culture systems extensively for drug screening and toxicity testing, significantly reducing development costs and time-to-market for new therapeutic compounds. Academic and research institutions constitute the second-largest segment at approximately 30%, focusing primarily on fundamental research and disease modeling applications.
Regionally, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the highest growth rate due to increasing research funding and expanding biotechnology sectors. This regional distribution highlights the global recognition of organoid technologies as essential tools in modern biomedical research.
The market for environmentally sustainable organoid culture systems is emerging as a significant niche, with estimated growth rates exceeding the overall market average by 3-5 percentage points. This sub-segment addresses the environmental impact concerns associated with traditional culture methods, including resource consumption, waste generation, and energy usage. Companies offering solutions with reduced environmental footprints are gaining competitive advantages, particularly among environmentally conscious research institutions and pharmaceutical companies with strong sustainability commitments.
Key market drivers include the rising prevalence of chronic diseases necessitating better in vitro models, increasing R&D investments in regenerative medicine, and growing adoption of personalized medicine approaches. Additionally, technological advancements enabling more physiologically relevant organoid systems are expanding potential applications across oncology, neurology, gastroenterology, and regenerative medicine.
Market challenges primarily revolve around high costs associated with specialized media and growth factors, technical complexities in maintaining consistent organoid cultures, and regulatory uncertainties regarding clinical applications. These factors currently limit broader market penetration, particularly in smaller research institutions and developing regions with constrained research budgets.
Current Challenges in Organoid Environmental Control
Despite significant advancements in organoid technology, researchers continue to face substantial challenges in creating and maintaining optimal environmental conditions for organoid culture systems. The primary obstacle remains the accurate replication of the native tissue microenvironment, which involves complex interactions between cells, extracellular matrix components, and biochemical signaling molecules. Current culture systems struggle to fully recapitulate these intricate conditions, leading to variability in organoid development and functionality.
Temperature and pH regulation present persistent challenges, as even minor fluctuations can significantly impact cellular behavior and organoid formation. Most conventional incubation systems provide only basic environmental control, lacking the precision required for sensitive organoid cultures. This limitation becomes particularly problematic during long-term culture experiments, where maintaining stable conditions over weeks or months is essential.
Oxygen tension control represents another critical challenge. Different tissues require specific oxygen concentrations that vary significantly from atmospheric levels. Current systems often fail to deliver precise oxygen gradients that mimic those found in native tissues, potentially affecting cellular differentiation pathways and metabolic functions within organoids. The inability to create these physiologically relevant oxygen microenvironments limits the biological accuracy of organoid models.
Nutrient delivery and waste removal mechanisms remain suboptimal in many culture systems. Unlike in vivo conditions where vasculature ensures continuous exchange, organoids typically rely on passive diffusion, which becomes increasingly inefficient as organoids grow larger. This limitation often results in necrotic cores in larger organoids and restricts their maximum achievable size, hampering their utility for certain applications.
Mechanical forces, including fluid shear stress and tissue compression, play crucial roles in organ development and function but are poorly represented in static culture systems. Current technologies struggle to incorporate these biomechanical cues in a physiologically relevant manner, potentially limiting the structural and functional maturation of organoids.
Standardization across different laboratory settings presents an additional challenge. The lack of universally accepted protocols and equipment specifications leads to significant variability in organoid cultures between research groups, complicating cross-laboratory validation and hindering clinical translation efforts. This inconsistency undermines the reproducibility of research findings and slows the broader adoption of organoid technologies.
Integration of real-time monitoring capabilities remains limited in current systems. Researchers often lack non-invasive methods to continuously assess organoid development, metabolic activity, and functional parameters without disrupting the culture environment. This deficiency makes it difficult to optimize culture conditions dynamically and to detect early signs of culture deterioration.
Temperature and pH regulation present persistent challenges, as even minor fluctuations can significantly impact cellular behavior and organoid formation. Most conventional incubation systems provide only basic environmental control, lacking the precision required for sensitive organoid cultures. This limitation becomes particularly problematic during long-term culture experiments, where maintaining stable conditions over weeks or months is essential.
Oxygen tension control represents another critical challenge. Different tissues require specific oxygen concentrations that vary significantly from atmospheric levels. Current systems often fail to deliver precise oxygen gradients that mimic those found in native tissues, potentially affecting cellular differentiation pathways and metabolic functions within organoids. The inability to create these physiologically relevant oxygen microenvironments limits the biological accuracy of organoid models.
Nutrient delivery and waste removal mechanisms remain suboptimal in many culture systems. Unlike in vivo conditions where vasculature ensures continuous exchange, organoids typically rely on passive diffusion, which becomes increasingly inefficient as organoids grow larger. This limitation often results in necrotic cores in larger organoids and restricts their maximum achievable size, hampering their utility for certain applications.
Mechanical forces, including fluid shear stress and tissue compression, play crucial roles in organ development and function but are poorly represented in static culture systems. Current technologies struggle to incorporate these biomechanical cues in a physiologically relevant manner, potentially limiting the structural and functional maturation of organoids.
Standardization across different laboratory settings presents an additional challenge. The lack of universally accepted protocols and equipment specifications leads to significant variability in organoid cultures between research groups, complicating cross-laboratory validation and hindering clinical translation efforts. This inconsistency undermines the reproducibility of research findings and slows the broader adoption of organoid technologies.
Integration of real-time monitoring capabilities remains limited in current systems. Researchers often lack non-invasive methods to continuously assess organoid development, metabolic activity, and functional parameters without disrupting the culture environment. This deficiency makes it difficult to optimize culture conditions dynamically and to detect early signs of culture deterioration.
Current Environmental Control Solutions for Organoids
01 Temperature and humidity control in organoid culture systems
Maintaining optimal temperature and humidity levels is crucial for successful organoid culture. Environmental control systems that provide stable temperature (typically 37°C) and appropriate humidity (95-100%) create conditions that mimic the in vivo environment. These systems often include incubators with precise regulation capabilities and monitoring technologies to ensure consistent conditions throughout the culture period, which is essential for organoid development and function.- Temperature and humidity control in organoid culture systems: Maintaining optimal temperature and humidity levels is crucial for successful organoid culture. Environmental control systems that provide stable temperature (typically 37°C) and appropriate humidity (95-100%) create conditions that mimic the in vivo environment. These systems often include incubators with precise regulation capabilities and monitoring tools to ensure consistent environmental parameters, which are essential for organoid growth, differentiation, and long-term viability.
- Gas exchange and oxygen concentration requirements: Organoid cultures require specific gas compositions to thrive, particularly controlled oxygen and carbon dioxide levels. Most systems maintain CO2 at 5% to stabilize pH in culture media, while oxygen requirements can vary depending on the organoid type. Some tissues benefit from hypoxic conditions (1-5% O2) that better replicate their native environment, while others require normoxic conditions. Advanced culture systems incorporate gas permeable materials and automated regulation systems to maintain optimal gas exchange and prevent hypoxic or hyperoxic stress.
- Matrix and scaffold requirements for 3D organization: The extracellular matrix environment is critical for proper organoid development and structural organization. Culture systems typically incorporate natural hydrogels like Matrigel or synthetic alternatives that provide mechanical support and biochemical cues. These matrices must offer appropriate stiffness, porosity, and bioactive components to facilitate cell adhesion, migration, and self-organization. Advanced scaffold designs may include gradients of stiffness or bioactive molecules to better mimic tissue-specific niches and support complex organoid architecture.
- Nutrient delivery and waste removal systems: Efficient nutrient delivery and waste removal are essential for organoid viability, particularly as they grow in size and complexity. Culture systems must provide consistent access to growth factors, vitamins, amino acids, and other nutrients while removing metabolic waste products that can become toxic. Microfluidic platforms, perfusion systems, and bioreactors are increasingly used to create dynamic culture environments that improve nutrient diffusion and waste clearance compared to static culture methods, supporting larger and more physiologically relevant organoid models.
- Monitoring and automation technologies for organoid culture: Advanced monitoring and automation technologies are being integrated into organoid culture systems to maintain optimal environmental conditions and reduce variability. These include real-time sensors for pH, oxygen, temperature, and metabolite levels, combined with automated feedback systems that adjust conditions as needed. Imaging technologies allow non-invasive monitoring of organoid development, while machine learning algorithms can analyze growth patterns and predict optimal culture parameters. These technologies enable more reproducible organoid culture with reduced human intervention and improved standardization.
02 Gas exchange and oxygen concentration regulation
Organoid cultures require specific oxygen concentrations that often differ from atmospheric levels. Culture systems must provide controlled gas exchange mechanisms to maintain appropriate oxygen and carbon dioxide levels. Some organoids benefit from hypoxic conditions that mimic their native tissue environment, while others require higher oxygen levels. Advanced systems incorporate sensors and regulators to dynamically adjust gas concentrations based on the specific requirements of different organoid types.Expand Specific Solutions03 Matrix support and mechanical stimulation
The physical environment plays a critical role in organoid development. Culture systems must provide appropriate extracellular matrix components or synthetic alternatives that support three-dimensional growth and cellular organization. Some advanced systems incorporate mechanical stimulation features that mimic physiological forces such as fluid flow, stretching, or compression. These mechanical cues are essential for proper organoid maturation and functionality, particularly for organoids representing mechanosensitive tissues.Expand Specific Solutions04 Nutrient delivery and waste removal systems
Efficient nutrient delivery and waste removal are essential for organoid viability and growth. Culture systems must ensure adequate diffusion of nutrients to all cells within the three-dimensional structure while removing metabolic waste products. This often involves specialized perfusion systems, microfluidic platforms, or bioreactors that create a dynamic culture environment. These systems help prevent necrosis in the organoid core and support long-term culture maintenance.Expand Specific Solutions05 Monitoring and automation technologies
Advanced organoid culture systems incorporate monitoring technologies that track environmental parameters and organoid development in real-time. These include imaging systems for morphological assessment, sensors for measuring metabolic activity, and automated feedback mechanisms that adjust culture conditions as needed. Automation technologies help maintain consistent conditions over extended culture periods and reduce human intervention, which minimizes contamination risks and improves reproducibility in organoid culture experiments.Expand Specific Solutions
Leading Organizations in Organoid Technology Development
The organoid culture systems market is in a growth phase, characterized by increasing adoption across research and clinical applications. The market size is expanding rapidly due to rising demand for more physiologically relevant in vitro models. Technologically, the field is advancing from early-stage development toward standardization, with companies like STEMCELL Technologies, Molecular Devices, and Cell Microsystems leading innovation in automated culture platforms. Established players such as FUJIFILM and BOE Technology are investing in advanced environmental control systems, while specialized firms like Cellesce and Organoidsciences focus on bioprocessing technologies for scalable organoid production. Academic institutions including Johns Hopkins University and Baylor College of Medicine collaborate with industry partners to address key challenges in maintaining consistent environmental conditions for long-term organoid culture, driving the field toward greater reproducibility and clinical relevance.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has pioneered specialized extracellular matrix (ECM) formulations specifically designed for organoid culture systems. Their IntestiCult™ and CerebralCult™ product lines provide optimized growth media that recreate tissue-specific microenvironments through carefully balanced combinations of growth factors, cytokines, and metabolic supplements. The company has developed Corning® Matrigel® alternatives that offer improved batch-to-batch consistency while providing the necessary structural support for 3D organoid formation. Their PneumaCult™ technology addresses the unique gas exchange requirements of airway organoids by creating an air-liquid interface that mimics respiratory tract conditions[2]. STEMCELL's AggreWell™ plates enable controlled formation of uniformly-sized organoids, which is critical for standardizing experimental conditions and ensuring reproducible results. Recent innovations include their OrganoID™ system, which incorporates oxygen-permeable membranes that allow for more physiologically relevant oxygen gradients within developing organoids, addressing one of the key challenges in maintaining viable organoid cultures.
Strengths: Exceptional specialization in tissue-specific media formulations that precisely address the unique environmental requirements of different organoid types. Their products offer excellent standardization, reducing variability between experiments. Weaknesses: Their solutions often require complementary technologies from other providers for complete environmental control. The specialized nature of their products can make them relatively expensive for routine laboratory use.
Molecular Devices LLC
Technical Solution: Molecular Devices has developed advanced organoid culture platforms that integrate microfluidic technology with automated imaging systems to create dynamic microenvironments for 3D organoid growth. Their ImageXpress® Micro Confocal High-Content Imaging System combined with their 3D cell culture technology allows for real-time monitoring of organoid development while precisely controlling environmental parameters such as oxygen gradients, nutrient delivery, and mechanical forces. The company's CellReady® bioreactor systems incorporate sensors that continuously monitor pH, dissolved oxygen, and temperature, automatically adjusting conditions to maintain optimal growth parameters. Their platforms also feature programmable perfusion systems that mimic in vivo fluid dynamics, allowing for controlled delivery of growth factors and removal of waste products, which is critical for long-term organoid viability and functionality[1][3]. Recent innovations include integration of AI-based image analysis to automatically detect morphological changes in organoids in response to environmental modifications.
Strengths: Highly integrated systems combining imaging, environmental control, and automation enable precise monitoring and adjustment of multiple parameters simultaneously. Their platforms offer exceptional reproducibility through standardized protocols and automated workflows. Weaknesses: The comprehensive systems require significant initial investment and technical expertise to operate effectively. The closed-system nature of some platforms may limit flexibility for certain specialized research applications.
Key Innovations in Organoid Microenvironment Engineering
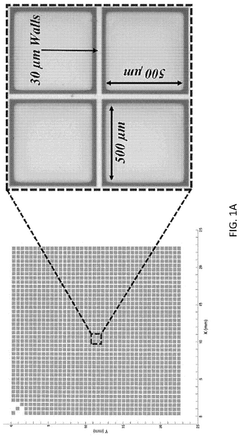
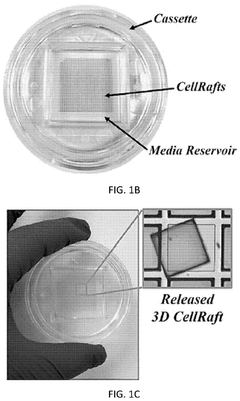
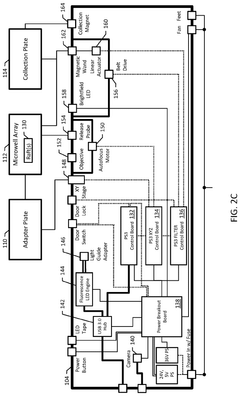
Automated system for imaging, identification, and isolation of organoids
PatentPendingUS20240360400A1
Innovation
- An automated system utilizing a microarray with releasable cellrafts for culturing and imaging organoids, allowing for the polymerization of extracellular matrix within microwells, followed by automated imaging and release of organoids for transfer to a collection plate, enabling precise imaging, phenotypic assessment, and isolation of individual organoids.
Methods for organoids production
PatentWO2021225794A1
Innovation
- A method involving microcontainers with hydrogel walls and lids that allow cells to self-organize without exogenous extracellular matrix, using a culturing medium with biological colloids to achieve a higher density than the hydrogel lid, enabling the formation of organoids that exhibit contractility and physiological differentiation.
Sustainability Aspects of Organoid Culture Technologies
The sustainability of organoid culture technologies represents a critical dimension in their development and widespread adoption. Current organoid systems, while revolutionary for biomedical research, present significant environmental challenges that must be addressed for responsible advancement of the field.
The resource intensity of organoid culture systems poses a primary sustainability concern. These systems typically require substantial quantities of specialized growth factors, culture media, and extracellular matrix components derived from animal sources. The production of these materials involves energy-intensive manufacturing processes and often generates considerable waste. Additionally, the single-use plasticware prevalent in organoid research contributes significantly to laboratory plastic waste streams, exacerbating environmental impact.
Energy consumption represents another environmental challenge. Organoid cultures require precise temperature control, typically maintained at 37°C for extended periods, sometimes weeks or months. The continuous operation of incubators, biosafety cabinets, and other laboratory equipment results in substantial energy expenditure, contributing to the carbon footprint of research facilities.
Water usage in organoid technologies also raises sustainability concerns. The preparation of culture media, washing procedures, and sterilization processes consume significant volumes of purified water. Furthermore, the disposal of potentially biohazardous waste from organoid cultures necessitates specialized treatment protocols that may involve additional environmental costs.
Recent innovations are beginning to address these sustainability challenges. The development of chemically defined, animal-free culture media reduces dependence on animal-derived components and improves reproducibility while decreasing environmental impact. Similarly, advances in microfluidic systems and miniaturized organoid platforms enable significant reductions in reagent consumption and waste generation.
Recycling initiatives specifically designed for laboratory plastics are emerging, with some manufacturers implementing take-back programs for culture vessels and other consumables. Additionally, energy-efficient laboratory equipment designed specifically for long-term cell culture is gaining traction, incorporating features such as improved insulation and smart power management systems.
The transition toward more sustainable organoid technologies will require coordinated efforts across the research ecosystem. This includes developing standardized sustainability metrics for organoid culture systems, incentivizing environmentally responsible practices through funding mechanisms, and fostering collaboration between researchers, manufacturers, and waste management specialists to create closed-loop systems for laboratory consumables.
The resource intensity of organoid culture systems poses a primary sustainability concern. These systems typically require substantial quantities of specialized growth factors, culture media, and extracellular matrix components derived from animal sources. The production of these materials involves energy-intensive manufacturing processes and often generates considerable waste. Additionally, the single-use plasticware prevalent in organoid research contributes significantly to laboratory plastic waste streams, exacerbating environmental impact.
Energy consumption represents another environmental challenge. Organoid cultures require precise temperature control, typically maintained at 37°C for extended periods, sometimes weeks or months. The continuous operation of incubators, biosafety cabinets, and other laboratory equipment results in substantial energy expenditure, contributing to the carbon footprint of research facilities.
Water usage in organoid technologies also raises sustainability concerns. The preparation of culture media, washing procedures, and sterilization processes consume significant volumes of purified water. Furthermore, the disposal of potentially biohazardous waste from organoid cultures necessitates specialized treatment protocols that may involve additional environmental costs.
Recent innovations are beginning to address these sustainability challenges. The development of chemically defined, animal-free culture media reduces dependence on animal-derived components and improves reproducibility while decreasing environmental impact. Similarly, advances in microfluidic systems and miniaturized organoid platforms enable significant reductions in reagent consumption and waste generation.
Recycling initiatives specifically designed for laboratory plastics are emerging, with some manufacturers implementing take-back programs for culture vessels and other consumables. Additionally, energy-efficient laboratory equipment designed specifically for long-term cell culture is gaining traction, incorporating features such as improved insulation and smart power management systems.
The transition toward more sustainable organoid technologies will require coordinated efforts across the research ecosystem. This includes developing standardized sustainability metrics for organoid culture systems, incentivizing environmentally responsible practices through funding mechanisms, and fostering collaboration between researchers, manufacturers, and waste management specialists to create closed-loop systems for laboratory consumables.
Regulatory Framework for Organoid-Based Research
The regulatory landscape governing organoid research is complex and evolving, reflecting the novel nature of these three-dimensional cellular structures that mimic organ functionality. Current regulatory frameworks primarily address organoids through existing regulations for stem cell research, tissue engineering, and biological materials. In the United States, the FDA has begun developing specific guidance for organoid technologies, particularly focusing on their applications in drug development and toxicity testing, where they serve as alternatives to animal models.
The European Medicines Agency (EMA) has established working groups dedicated to evaluating organoid technologies within their Advanced Therapy Medicinal Products (ATMP) framework. These regulations emphasize quality control parameters essential for environmental stability in organoid culture systems, including sterility standards, contamination prevention protocols, and validation requirements for environmental control equipment.
Ethical considerations form a significant component of the regulatory framework, particularly regarding the sourcing of cells for organoid development. Regulations typically mandate informed consent procedures, privacy protections for donor information, and ethical review board approvals. For brain organoids and other neurological models, additional ethical guidelines address concerns about consciousness and neural activity.
International harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standards for organoid-based testing methodologies. These standards aim to establish consistent environmental parameters across research institutions and commercial applications, facilitating reproducibility and reliability in organoid culture systems.
Regulatory gaps remain concerning the environmental sustainability of organoid culture systems. Current frameworks provide limited guidance on resource efficiency, waste management from culture media, or carbon footprint considerations. As organoid technologies scale toward industrial applications, regulatory bodies are beginning to incorporate sustainability metrics into their evaluation criteria.
Compliance documentation requirements typically include detailed records of environmental monitoring systems, calibration procedures, and validation protocols for equipment maintaining optimal culture conditions. These requirements ensure that temperature, humidity, gas composition, and other environmental factors remain within specified parameters throughout the organoid development process.
Looking forward, regulatory evolution will likely focus on standardizing environmental control systems for organoid cultures, establishing reference materials for quality control, and developing specialized frameworks for organoid biobanking that address long-term storage conditions and environmental stability requirements.
The European Medicines Agency (EMA) has established working groups dedicated to evaluating organoid technologies within their Advanced Therapy Medicinal Products (ATMP) framework. These regulations emphasize quality control parameters essential for environmental stability in organoid culture systems, including sterility standards, contamination prevention protocols, and validation requirements for environmental control equipment.
Ethical considerations form a significant component of the regulatory framework, particularly regarding the sourcing of cells for organoid development. Regulations typically mandate informed consent procedures, privacy protections for donor information, and ethical review board approvals. For brain organoids and other neurological models, additional ethical guidelines address concerns about consciousness and neural activity.
International harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standards for organoid-based testing methodologies. These standards aim to establish consistent environmental parameters across research institutions and commercial applications, facilitating reproducibility and reliability in organoid culture systems.
Regulatory gaps remain concerning the environmental sustainability of organoid culture systems. Current frameworks provide limited guidance on resource efficiency, waste management from culture media, or carbon footprint considerations. As organoid technologies scale toward industrial applications, regulatory bodies are beginning to incorporate sustainability metrics into their evaluation criteria.
Compliance documentation requirements typically include detailed records of environmental monitoring systems, calibration procedures, and validation protocols for equipment maintaining optimal culture conditions. These requirements ensure that temperature, humidity, gas composition, and other environmental factors remain within specified parameters throughout the organoid development process.
Looking forward, regulatory evolution will likely focus on standardizing environmental control systems for organoid cultures, establishing reference materials for quality control, and developing specialized frameworks for organoid biobanking that address long-term storage conditions and environmental stability requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!