What Are the Prospects of Organoid Culture Systems in 2024?
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Objectives
Organoid technology has evolved significantly since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers and colleagues. This breakthrough demonstrated that adult stem cells could self-organize into three-dimensional structures resembling their tissue of origin. The evolution of organoid technology represents a convergence of advances in stem cell biology, developmental biology, and tissue engineering that has revolutionized our understanding of human development and disease.
The historical trajectory shows a rapid expansion from simple epithelial organoids to increasingly complex multi-cellular structures. Between 2011 and 2015, researchers successfully developed organoids representing various organs including liver, pancreas, brain, kidney, and lung. By 2018-2020, the field witnessed significant improvements in culture protocols, enabling longer-term maintenance and enhanced physiological relevance of these miniature organ models.
A pivotal advancement came with the integration of bioengineering approaches, including the use of hydrogels with tunable mechanical properties and the incorporation of microfluidic systems to better mimic the native tissue microenvironment. The development of chemically defined matrices has further standardized organoid culture, addressing reproducibility challenges that initially limited widespread adoption.
The primary technical objectives for organoid systems in 2024 focus on several key areas. First, enhancing organoid complexity through co-culture systems that incorporate multiple cell types, including immune cells, stromal components, and vascular elements. Second, improving maturation protocols to better recapitulate adult tissue functionality, as many current organoids remain developmentally immature. Third, increasing scalability and reproducibility to facilitate industrial applications in drug discovery and toxicology testing.
Another critical objective is the development of personalized medicine applications, where patient-derived organoids serve as "avatars" for individualized drug screening and therapeutic decision-making. This approach has shown particular promise in oncology and rare genetic disorders, with clinical implementation beginning to emerge in specialized centers.
The integration of advanced imaging technologies and multi-omics analytical approaches represents another frontier, enabling deeper phenotypic characterization and mechanistic insights. Researchers are also working toward standardization of protocols and quality control metrics to ensure consistency across laboratories and facilitate regulatory acceptance of organoid-based assays.
Looking forward, the field aims to develop more complex organ systems with functional vasculature and innervation, potentially leading to organoid-based organ replacement therapies. The convergence of organoid technology with bioprinting and organ-on-chip platforms presents exciting opportunities for creating increasingly sophisticated human tissue models that could significantly reduce reliance on animal testing while accelerating therapeutic development.
The historical trajectory shows a rapid expansion from simple epithelial organoids to increasingly complex multi-cellular structures. Between 2011 and 2015, researchers successfully developed organoids representing various organs including liver, pancreas, brain, kidney, and lung. By 2018-2020, the field witnessed significant improvements in culture protocols, enabling longer-term maintenance and enhanced physiological relevance of these miniature organ models.
A pivotal advancement came with the integration of bioengineering approaches, including the use of hydrogels with tunable mechanical properties and the incorporation of microfluidic systems to better mimic the native tissue microenvironment. The development of chemically defined matrices has further standardized organoid culture, addressing reproducibility challenges that initially limited widespread adoption.
The primary technical objectives for organoid systems in 2024 focus on several key areas. First, enhancing organoid complexity through co-culture systems that incorporate multiple cell types, including immune cells, stromal components, and vascular elements. Second, improving maturation protocols to better recapitulate adult tissue functionality, as many current organoids remain developmentally immature. Third, increasing scalability and reproducibility to facilitate industrial applications in drug discovery and toxicology testing.
Another critical objective is the development of personalized medicine applications, where patient-derived organoids serve as "avatars" for individualized drug screening and therapeutic decision-making. This approach has shown particular promise in oncology and rare genetic disorders, with clinical implementation beginning to emerge in specialized centers.
The integration of advanced imaging technologies and multi-omics analytical approaches represents another frontier, enabling deeper phenotypic characterization and mechanistic insights. Researchers are also working toward standardization of protocols and quality control metrics to ensure consistency across laboratories and facilitate regulatory acceptance of organoid-based assays.
Looking forward, the field aims to develop more complex organ systems with functional vasculature and innervation, potentially leading to organoid-based organ replacement therapies. The convergence of organoid technology with bioprinting and organ-on-chip platforms presents exciting opportunities for creating increasingly sophisticated human tissue models that could significantly reduce reliance on animal testing while accelerating therapeutic development.
Market Analysis for Organoid-Based Applications
The global organoid market is experiencing robust growth, with a valuation of approximately $1.5 billion in 2023 and projected to reach $3.9 billion by 2028, representing a compound annual growth rate (CAGR) of 21.2%. This significant expansion is driven by increasing applications across multiple sectors, with pharmaceutical research and development currently dominating the market share at roughly 40%.
Drug discovery and development represents the largest application segment, where organoids are revolutionizing preclinical testing by providing more physiologically relevant models than traditional 2D cell cultures. This application is expected to grow at a CAGR of 23.5% through 2028, fueled by the pharmaceutical industry's push to reduce the high failure rates in clinical trials and accelerate time-to-market for new therapeutics.
Personalized medicine applications are emerging as the fastest-growing segment, with a projected CAGR of 26.8%. Patient-derived organoids are increasingly being utilized for drug sensitivity testing and treatment selection, particularly in oncology where they can predict individual patient responses to specific therapies with reported accuracy rates of 80-85% in certain cancer types.
Regionally, North America currently leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate at 24.7% CAGR, driven by increasing research investments in countries like China, Japan, and South Korea, along with the establishment of new biobanks and research centers dedicated to organoid technology.
By disease area, cancer research dominates with approximately 35% market share, followed by gastrointestinal diseases (20%), neurological disorders (15%), and others. Notably, applications in infectious disease research, including viral pathogenesis studies, have gained significant traction following the COVID-19 pandemic, growing at 28.3% annually.
Commercial adoption of organoid technologies faces challenges including high costs, with average per-organoid development costs ranging from $1,000-$5,000, technical complexity requiring specialized expertise, and regulatory uncertainties. Despite these barriers, market penetration is accelerating as standardization improves and costs gradually decrease through technological advancements and economies of scale.
End-user analysis reveals pharmaceutical and biotechnology companies as the largest segment (50%), followed by academic and research institutions (30%), and contract research organizations (15%). Healthcare providers represent a small but rapidly growing segment as clinical applications begin to emerge.
Drug discovery and development represents the largest application segment, where organoids are revolutionizing preclinical testing by providing more physiologically relevant models than traditional 2D cell cultures. This application is expected to grow at a CAGR of 23.5% through 2028, fueled by the pharmaceutical industry's push to reduce the high failure rates in clinical trials and accelerate time-to-market for new therapeutics.
Personalized medicine applications are emerging as the fastest-growing segment, with a projected CAGR of 26.8%. Patient-derived organoids are increasingly being utilized for drug sensitivity testing and treatment selection, particularly in oncology where they can predict individual patient responses to specific therapies with reported accuracy rates of 80-85% in certain cancer types.
Regionally, North America currently leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate at 24.7% CAGR, driven by increasing research investments in countries like China, Japan, and South Korea, along with the establishment of new biobanks and research centers dedicated to organoid technology.
By disease area, cancer research dominates with approximately 35% market share, followed by gastrointestinal diseases (20%), neurological disorders (15%), and others. Notably, applications in infectious disease research, including viral pathogenesis studies, have gained significant traction following the COVID-19 pandemic, growing at 28.3% annually.
Commercial adoption of organoid technologies faces challenges including high costs, with average per-organoid development costs ranging from $1,000-$5,000, technical complexity requiring specialized expertise, and regulatory uncertainties. Despite these barriers, market penetration is accelerating as standardization improves and costs gradually decrease through technological advancements and economies of scale.
End-user analysis reveals pharmaceutical and biotechnology companies as the largest segment (50%), followed by academic and research institutions (30%), and contract research organizations (15%). Healthcare providers represent a small but rapidly growing segment as clinical applications begin to emerge.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid technology, several critical challenges continue to impede the full realization of organoid culture systems' potential. Reproducibility remains a primary concern, with researchers struggling to establish standardized protocols that yield consistent results across laboratories. This variability stems from differences in starting materials, culture conditions, and technical expertise, making cross-study comparisons and clinical translations difficult.
Scalability presents another significant hurdle, particularly for pharmaceutical applications and regenerative medicine. Current methods often produce limited quantities of organoids with variable quality, hampering high-throughput drug screening and therapeutic applications. The complex nature of organoid development requires substantial manual intervention, increasing costs and reducing throughput capacity.
Maturation limitations constitute a persistent technical barrier. Many organoids fail to achieve the full functional and structural complexity of their corresponding adult organs, instead resembling fetal or embryonic tissues. This developmental arrest restricts their utility as accurate disease models, especially for adult-onset conditions. The absence of certain cell types, incomplete cellular differentiation, and inadequate tissue architecture all contribute to this limitation.
Vascularization deficiency represents perhaps the most significant physiological challenge. Without proper blood vessel networks, organoids typically develop necrotic cores as they grow beyond 200-300 micrometers in diameter. This size constraint severely limits their ability to model whole-organ physiology and restricts their growth potential. Current approaches using microfluidics and bioprinting show promise but remain technically demanding.
The extracellular matrix (ECM) components used in organoid culture, particularly Matrigel, introduce variability and complicate clinical applications. These animal-derived matrices have batch-to-batch inconsistencies and raise regulatory concerns for therapeutic applications. Developing synthetic alternatives that recapitulate the complex biochemical and mechanical properties of natural ECM remains challenging.
Integration of immune components represents an emerging challenge as researchers seek to model immune-mediated diseases and immunotherapy responses. Current organoid systems typically lack immune cells and vasculature necessary for modeling immune responses, limiting their utility in immunology research and personalized medicine applications.
Cost barriers persist throughout organoid research, with specialized media components, growth factors, and ECM materials driving expenses that restrict accessibility. These financial constraints particularly affect clinical implementations and widespread adoption in resource-limited settings, creating inequities in research capabilities globally.
Scalability presents another significant hurdle, particularly for pharmaceutical applications and regenerative medicine. Current methods often produce limited quantities of organoids with variable quality, hampering high-throughput drug screening and therapeutic applications. The complex nature of organoid development requires substantial manual intervention, increasing costs and reducing throughput capacity.
Maturation limitations constitute a persistent technical barrier. Many organoids fail to achieve the full functional and structural complexity of their corresponding adult organs, instead resembling fetal or embryonic tissues. This developmental arrest restricts their utility as accurate disease models, especially for adult-onset conditions. The absence of certain cell types, incomplete cellular differentiation, and inadequate tissue architecture all contribute to this limitation.
Vascularization deficiency represents perhaps the most significant physiological challenge. Without proper blood vessel networks, organoids typically develop necrotic cores as they grow beyond 200-300 micrometers in diameter. This size constraint severely limits their ability to model whole-organ physiology and restricts their growth potential. Current approaches using microfluidics and bioprinting show promise but remain technically demanding.
The extracellular matrix (ECM) components used in organoid culture, particularly Matrigel, introduce variability and complicate clinical applications. These animal-derived matrices have batch-to-batch inconsistencies and raise regulatory concerns for therapeutic applications. Developing synthetic alternatives that recapitulate the complex biochemical and mechanical properties of natural ECM remains challenging.
Integration of immune components represents an emerging challenge as researchers seek to model immune-mediated diseases and immunotherapy responses. Current organoid systems typically lack immune cells and vasculature necessary for modeling immune responses, limiting their utility in immunology research and personalized medicine applications.
Cost barriers persist throughout organoid research, with specialized media components, growth factors, and ECM materials driving expenses that restrict accessibility. These financial constraints particularly affect clinical implementations and widespread adoption in resource-limited settings, creating inequities in research capabilities globally.
State-of-the-Art Organoid Culture Methodologies
01 3D organoid culture systems and methods
Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems involve culturing stem cells or progenitor cells in specific conditions that allow them to self-organize into organ-like structures. The methods include providing appropriate extracellular matrix components, growth factors, and other signaling molecules to support organoid development. These 3D culture systems are valuable for studying organ development, disease modeling, and drug screening.- 3D Organoid Culture Methods: Three-dimensional organoid culture systems mimic the in vivo environment more accurately than traditional 2D cultures. These systems typically involve embedding stem cells or tissue-derived cells in extracellular matrix components like Matrigel, allowing cells to self-organize into structures that resemble native organs. The 3D environment promotes proper cell-cell interactions, differentiation, and functional development of organoids, making them valuable models for studying organ development, disease mechanisms, and drug responses.
- Growth Factors and Media Formulations: Specialized media formulations containing specific growth factors and signaling molecules are essential for successful organoid culture. These formulations typically include combinations of factors such as EGF, FGF, Wnt agonists, R-spondin, Noggin, and organ-specific factors that support stem cell maintenance and directed differentiation. The precise cocktail of growth factors determines the developmental trajectory and functional maturation of organoids, allowing researchers to generate specific organ types with appropriate cellular composition and architecture.
- Disease Modeling with Organoids: Organoid culture systems provide powerful platforms for modeling human diseases. Patient-derived organoids can recapitulate disease phenotypes, allowing for personalized medicine approaches. These systems are particularly valuable for studying genetic disorders, cancer, infectious diseases, and developmental abnormalities. By incorporating gene editing technologies like CRISPR-Cas9, researchers can create isogenic controls or introduce specific mutations to study disease mechanisms in a controlled environment, facilitating drug discovery and therapeutic development.
- Bioengineering Approaches for Organoid Culture: Advanced bioengineering approaches enhance organoid culture systems through the development of specialized scaffolds, microfluidic devices, and bioreactors. These technologies provide better control over the organoid microenvironment, including oxygen tension, nutrient delivery, and mechanical forces. Bioprinting and micropatterning techniques enable precise spatial organization of cells and matrix components, while perfusion systems improve nutrient exchange and waste removal, resulting in more physiologically relevant organoid models with improved longevity and functionality.
- Cryopreservation and Banking of Organoids: Methods for cryopreservation and banking of organoids are crucial for maintaining consistent experimental models and facilitating their widespread use. Specialized freezing protocols using cryoprotectants help preserve organoid viability and functionality during long-term storage. These techniques enable the establishment of biobanks containing diverse patient-derived organoids, which serve as valuable resources for drug screening, personalized medicine, and regenerative therapy development. Optimized thawing and recovery procedures ensure that organoids maintain their original characteristics after cryopreservation.
02 Stem cell-derived organoid technologies
Technologies for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve directing stem cell differentiation toward specific lineages and then supporting their self-organization into organoids. The technologies include specialized culture media formulations, growth factor combinations, and physical cues that guide stem cell fate and organization. These stem cell-derived organoids can represent various organ systems including gut, brain, liver, and kidney.Expand Specific Solutions03 Extracellular matrix components for organoid culture
Specific extracellular matrix (ECM) components and scaffolds that support organoid growth and development. These include natural matrices like Matrigel, collagen, and fibronectin, as well as synthetic hydrogels with defined compositions. The ECM provides structural support, facilitates cell-cell interactions, and presents biochemical cues that influence cell behavior and organoid formation. Different organoid types require specific ECM compositions to properly develop and maintain their structure and function.Expand Specific Solutions04 Microfluidic and bioreactor systems for organoid culture
Advanced culture platforms including microfluidic devices and bioreactor systems designed specifically for organoid culture. These systems provide controlled environments with precise regulation of nutrient delivery, waste removal, and mechanical stimulation. Microfluidic platforms can create gradients of growth factors and enable co-culture of different cell types. Bioreactors support scaling up organoid production and maintaining long-term cultures. These technologies enhance organoid maturation, functionality, and physiological relevance.Expand Specific Solutions05 Disease modeling and drug screening using organoid systems
Applications of organoid culture systems for modeling diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, enabling personalized medicine approaches. These systems are used to study cancer, genetic disorders, infectious diseases, and developmental abnormalities. Organoids provide physiologically relevant platforms for drug efficacy and toxicity testing, potentially reducing the need for animal testing. High-throughput screening methods have been developed to evaluate drug responses in organoid systems.Expand Specific Solutions
Leading Organizations in Organoid Research
The organoid culture systems market is experiencing rapid growth in 2024, transitioning from early development to commercial expansion phase. The market is projected to reach significant value due to increasing applications in drug discovery, personalized medicine, and disease modeling. Technologically, the field shows varying maturity levels across different players. Academic institutions like University of Washington, Peking University, and Johns Hopkins University are driving fundamental research, while specialized companies such as Xilis, Mimetas, and Organoidsciences are commercializing advanced platforms. STEMCELL Technologies and Cell Microsystems are establishing themselves as key suppliers of enabling technologies. The competitive landscape features a healthy mix of established research institutions, specialized biotechnology startups, and larger life science companies, indicating a maturing ecosystem with substantial growth potential.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has developed an advanced organoid culture platform that integrates microfluidic technology with bioprinting capabilities. Their system enables precise spatial organization of multiple cell types to create complex organoid structures with defined architecture. A*STAR's platform incorporates a gradient-generating system that can establish physiologically relevant chemical gradients across organoid cultures, mimicking developmental cues and organ-specific microenvironments. The technology utilizes a combination of synthetic and natural hydrogels with tunable mechanical properties, allowing researchers to match the stiffness of specific tissues being modeled. A*STAR has demonstrated successful application of their platform in creating brain organoids with distinct cortical layers, liver organoids with functional bile ducts, and intestinal organoids with crypt-villus architecture. Their system also incorporates non-invasive electrical impedance sensing for real-time monitoring of organoid development and function.
Strengths: Integration of microfluidics with bioprinting for complex tissue architecture; tunable hydrogel system; gradient-generating capabilities; non-invasive monitoring systems; broad application across multiple organ types. Weaknesses: Complex technology requiring specialized expertise; higher initial setup costs; potential challenges in scaling for high-throughput applications; primarily research-focused with developing commercial applications.
Organoidsciences Ltd.
Technical Solution: Organoidsciences has developed a comprehensive organoid culture platform focused on patient-derived organoids (PDOs) for personalized medicine applications. Their proprietary matrix formulation enhances organoid formation efficiency by approximately 40% compared to conventional Matrigel-based systems. The company's technology incorporates defined growth factor combinations optimized for specific tissue types, enabling consistent organoid development across various organs including intestine, liver, pancreas, and brain tissues. Their automated culture system integrates robotic handling with real-time imaging capabilities, allowing for continuous monitoring of organoid growth and morphological changes. Organoidsciences has also pioneered cryopreservation protocols that maintain over 85% viability after thawing, addressing a critical challenge in organoid biobanking and distribution.
Strengths: Enhanced matrix formulation with superior organoid formation efficiency; tissue-specific growth factor optimization; integrated automation and imaging capabilities; advanced cryopreservation protocols. Weaknesses: Limited commercial scale compared to larger competitors; potentially higher cost structure; requires specialized equipment for full platform implementation; relatively new market entrant with developing clinical validation data.
Breakthrough Patents in Organoid Development
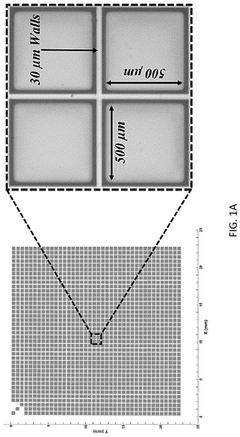
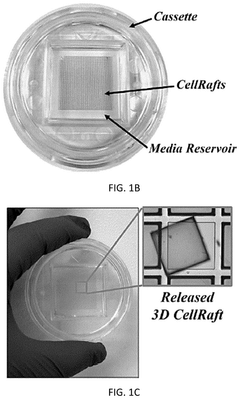
Automated system for imaging, identification, and isolation of organoids
PatentPendingUS20240320992A1
Innovation
- An automated system utilizing a microarray with releasable cellrafts for culturing and imaging organoids, allowing for the polymerization of extracellular matrix within microwells, followed by automated imaging and release of selected organoids for transfer to a collection plate using a motorized needle and magnetic wand, enabling precise and efficient single-organoid imaging and isolation.
Engineering of organoid culture for enhanced organogenesis in a dish
PatentPendingUS20240026261A1
Innovation
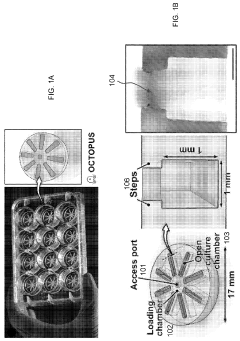
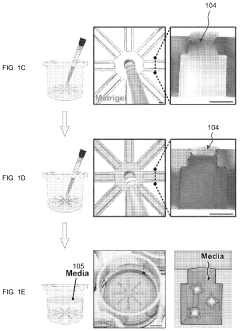
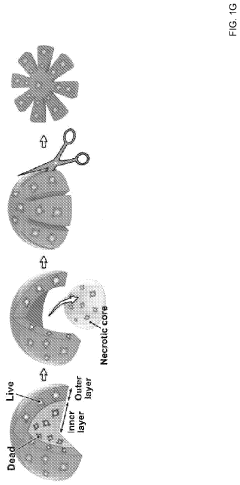
- The OCTOPUS device provides a 3D culture system with radially arranged culture chambers that reduce diffusion limitations by allowing unrestricted access to nutrients and oxygen, enabling continuous culture of organoids for extended periods without passaging, using a simple and scalable design compatible with standard cell culture plates.
Regulatory Framework for Organoid-Based Products
The regulatory landscape for organoid-based products is rapidly evolving as these technologies advance toward clinical applications. Currently, there exists significant regulatory ambiguity as organoids occupy a unique position between traditional cell cultures and complete organs, creating challenges for classification under existing frameworks. The FDA and EMA have begun developing specialized guidance documents for organoid technologies, though comprehensive regulatory pathways remain under development.
Key regulatory considerations include safety validation protocols, with particular emphasis on genetic stability, contamination risks, and tumorigenicity assessments. Regulatory bodies are increasingly requiring standardized characterization methods to ensure batch-to-batch consistency and functional equivalence to native tissues. This standardization represents a critical hurdle for commercial development of organoid-based products.
Ethical oversight frameworks are being established in parallel with technical regulations. These address consent procedures for donor tissue acquisition, data privacy concerns, and appropriate limitations on genetic modification of organoid systems. The International Society for Stem Cell Research updated its guidelines in 2023 to specifically address organoid research ethics, providing a foundation for national regulatory frameworks.
Manufacturing standards present another regulatory challenge, with current Good Manufacturing Practice (cGMP) requirements being adapted for organoid production. Regulatory agencies are developing specialized guidelines for scaling organoid technologies while maintaining quality control. The transition from research-grade to clinical-grade organoids necessitates stricter oversight of culture conditions, media components, and quality assurance protocols.
Market authorization pathways are gradually becoming clearer, with regulatory bodies implementing accelerated review processes for certain organoid applications, particularly those addressing rare diseases or unmet medical needs. However, reimbursement frameworks remain underdeveloped, creating uncertainty for commercial viability despite regulatory approval.
International harmonization efforts are underway through initiatives like the International Coalition of Medicines Regulatory Authorities, which established a working group on advanced therapy regulation inclusive of organoid technologies in late 2023. These efforts aim to reduce regulatory fragmentation across major markets and facilitate global development of organoid-based therapies.
Looking toward 2024-2025, we anticipate significant regulatory developments including finalized guidance documents from major agencies, establishment of reference standards for organoid characterization, and potentially the first conditional approvals for organoid-based diagnostic applications in oncology and rare disease settings.
Key regulatory considerations include safety validation protocols, with particular emphasis on genetic stability, contamination risks, and tumorigenicity assessments. Regulatory bodies are increasingly requiring standardized characterization methods to ensure batch-to-batch consistency and functional equivalence to native tissues. This standardization represents a critical hurdle for commercial development of organoid-based products.
Ethical oversight frameworks are being established in parallel with technical regulations. These address consent procedures for donor tissue acquisition, data privacy concerns, and appropriate limitations on genetic modification of organoid systems. The International Society for Stem Cell Research updated its guidelines in 2023 to specifically address organoid research ethics, providing a foundation for national regulatory frameworks.
Manufacturing standards present another regulatory challenge, with current Good Manufacturing Practice (cGMP) requirements being adapted for organoid production. Regulatory agencies are developing specialized guidelines for scaling organoid technologies while maintaining quality control. The transition from research-grade to clinical-grade organoids necessitates stricter oversight of culture conditions, media components, and quality assurance protocols.
Market authorization pathways are gradually becoming clearer, with regulatory bodies implementing accelerated review processes for certain organoid applications, particularly those addressing rare diseases or unmet medical needs. However, reimbursement frameworks remain underdeveloped, creating uncertainty for commercial viability despite regulatory approval.
International harmonization efforts are underway through initiatives like the International Coalition of Medicines Regulatory Authorities, which established a working group on advanced therapy regulation inclusive of organoid technologies in late 2023. These efforts aim to reduce regulatory fragmentation across major markets and facilitate global development of organoid-based therapies.
Looking toward 2024-2025, we anticipate significant regulatory developments including finalized guidance documents from major agencies, establishment of reference standards for organoid characterization, and potentially the first conditional approvals for organoid-based diagnostic applications in oncology and rare disease settings.
Ethical Implications of Organoid Research
As organoid research advances rapidly in 2024, the ethical landscape surrounding these miniaturized, simplified versions of organs becomes increasingly complex. The development of more sophisticated organoid culture systems raises profound questions about the moral status of these entities, particularly as they approach higher levels of complexity and functionality that mimic human organs.
The consent process for obtaining cells used in organoid research presents significant ethical challenges. While standard informed consent protocols exist for tissue donation, the unique nature of organoids—which can be maintained for extended periods and potentially develop advanced neural activity—necessitates more comprehensive consent frameworks that address long-term implications and potential applications not initially envisioned.
Privacy concerns emerge as organoids derived from specific individuals may contain their genetic information. This raises questions about data ownership, confidentiality, and the potential for identifying individuals through their organoid-derived data. The research community must establish robust protocols for anonymization and data protection to safeguard donor privacy while enabling scientific progress.
The development of cerebral organoids presents particularly challenging ethical dilemmas. As these brain-like structures become more sophisticated, questions arise about consciousness, sentience, and the potential for experiencing pain or distress. The scientific community continues to debate appropriate boundaries for neural organoid research and the need for specialized oversight mechanisms.
Equitable access to organoid technologies represents another critical ethical dimension. The high cost and technical expertise required for organoid culture systems may exacerbate existing healthcare disparities if benefits primarily flow to wealthy nations or populations. Ensuring fair distribution of the therapeutic and diagnostic advantages of organoid research requires deliberate policy interventions and international cooperation.
Regulatory frameworks for organoid research vary significantly across jurisdictions, creating potential for "ethics arbitrage" where researchers might conduct controversial work in regions with less stringent oversight. The development of harmonized international guidelines would help establish consistent ethical standards while still accommodating cultural differences in approaches to bioethics.
Looking forward, the ethical governance of organoid research will likely require adaptive frameworks that evolve alongside technological capabilities. Multi-stakeholder engagement—including scientists, ethicists, patient advocates, and the broader public—will be essential for developing guidelines that balance scientific progress with ethical considerations and societal values.
The consent process for obtaining cells used in organoid research presents significant ethical challenges. While standard informed consent protocols exist for tissue donation, the unique nature of organoids—which can be maintained for extended periods and potentially develop advanced neural activity—necessitates more comprehensive consent frameworks that address long-term implications and potential applications not initially envisioned.
Privacy concerns emerge as organoids derived from specific individuals may contain their genetic information. This raises questions about data ownership, confidentiality, and the potential for identifying individuals through their organoid-derived data. The research community must establish robust protocols for anonymization and data protection to safeguard donor privacy while enabling scientific progress.
The development of cerebral organoids presents particularly challenging ethical dilemmas. As these brain-like structures become more sophisticated, questions arise about consciousness, sentience, and the potential for experiencing pain or distress. The scientific community continues to debate appropriate boundaries for neural organoid research and the need for specialized oversight mechanisms.
Equitable access to organoid technologies represents another critical ethical dimension. The high cost and technical expertise required for organoid culture systems may exacerbate existing healthcare disparities if benefits primarily flow to wealthy nations or populations. Ensuring fair distribution of the therapeutic and diagnostic advantages of organoid research requires deliberate policy interventions and international cooperation.
Regulatory frameworks for organoid research vary significantly across jurisdictions, creating potential for "ethics arbitrage" where researchers might conduct controversial work in regions with less stringent oversight. The development of harmonized international guidelines would help establish consistent ethical standards while still accommodating cultural differences in approaches to bioethics.
Looking forward, the ethical governance of organoid research will likely require adaptive frameworks that evolve alongside technological capabilities. Multi-stakeholder engagement—including scientists, ethicists, patient advocates, and the broader public—will be essential for developing guidelines that balance scientific progress with ethical considerations and societal values.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!