Organoid Culture Systems' Role in Genetic Research
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering. Since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers' laboratory, this field has experienced exponential growth, transforming our approach to modeling human development and disease. The evolution of organoid technology has progressed through several distinct phases, beginning with basic two-dimensional cell culture systems to the current sophisticated three-dimensional structures that recapitulate complex organ functionality.
The initial breakthrough in organoid development came with the identification of adult stem cell markers and the establishment of culture conditions that maintain stemness while allowing differentiation. This was followed by the development of protocols for generating organoids from pluripotent stem cells, which opened new avenues for studying early developmental processes. Recent advances have focused on enhancing organoid complexity through co-culture systems, bioengineering approaches, and integration with microfluidic platforms to better mimic in vivo conditions.
In the context of genetic research, organoids have emerged as invaluable tools that bridge the gap between traditional cell culture models and animal studies. They provide physiologically relevant systems for studying gene function, genetic disorders, and gene-environment interactions in a human-specific context. The ability to generate patient-derived organoids has particularly revolutionized personalized medicine approaches, allowing researchers to study disease mechanisms in patient-specific genetic backgrounds.
The primary objectives of organoid research in genetics include developing more accurate models of genetic diseases, establishing platforms for high-throughput genetic screening, and creating systems for testing gene therapy approaches. Researchers aim to enhance organoid complexity to better recapitulate the cellular heterogeneity and tissue architecture of native organs, thereby improving their predictive value for genetic studies. Additionally, there is a growing focus on standardizing organoid protocols to ensure reproducibility across laboratories and facilitate comparative genetic analyses.
Future directions in organoid technology for genetic research include the integration of advanced genome editing techniques like CRISPR-Cas9, the development of multi-organ systems to study systemic genetic effects, and the incorporation of immune components to investigate gene-immune interactions. Researchers are also working toward scaling up organoid production and reducing costs to make these technologies more accessible for widespread genetic research applications.
The convergence of organoid technology with single-cell sequencing, spatial transcriptomics, and computational modeling is expected to provide unprecedented insights into gene regulatory networks and developmental processes, potentially revolutionizing our understanding of genetic contributions to human development and disease.
The initial breakthrough in organoid development came with the identification of adult stem cell markers and the establishment of culture conditions that maintain stemness while allowing differentiation. This was followed by the development of protocols for generating organoids from pluripotent stem cells, which opened new avenues for studying early developmental processes. Recent advances have focused on enhancing organoid complexity through co-culture systems, bioengineering approaches, and integration with microfluidic platforms to better mimic in vivo conditions.
In the context of genetic research, organoids have emerged as invaluable tools that bridge the gap between traditional cell culture models and animal studies. They provide physiologically relevant systems for studying gene function, genetic disorders, and gene-environment interactions in a human-specific context. The ability to generate patient-derived organoids has particularly revolutionized personalized medicine approaches, allowing researchers to study disease mechanisms in patient-specific genetic backgrounds.
The primary objectives of organoid research in genetics include developing more accurate models of genetic diseases, establishing platforms for high-throughput genetic screening, and creating systems for testing gene therapy approaches. Researchers aim to enhance organoid complexity to better recapitulate the cellular heterogeneity and tissue architecture of native organs, thereby improving their predictive value for genetic studies. Additionally, there is a growing focus on standardizing organoid protocols to ensure reproducibility across laboratories and facilitate comparative genetic analyses.
Future directions in organoid technology for genetic research include the integration of advanced genome editing techniques like CRISPR-Cas9, the development of multi-organ systems to study systemic genetic effects, and the incorporation of immune components to investigate gene-immune interactions. Researchers are also working toward scaling up organoid production and reducing costs to make these technologies more accessible for widespread genetic research applications.
The convergence of organoid technology with single-cell sequencing, spatial transcriptomics, and computational modeling is expected to provide unprecedented insights into gene regulatory networks and developmental processes, potentially revolutionizing our understanding of genetic contributions to human development and disease.
Market Analysis of Organoid Applications in Genetics
The organoid market within genetic research has experienced remarkable growth, with the global organoid market valued at approximately $1.3 billion in 2022 and projected to reach $3.4 billion by 2027, representing a compound annual growth rate (CAGR) of 21.2%. This substantial growth is primarily driven by increasing applications in genetic disease modeling, personalized medicine, and drug discovery processes that require accurate genetic representation.
Demand for organoid technologies in genetic research is particularly strong in oncology, where patient-derived organoids enable precise genetic profiling of tumors and testing of targeted therapies. The cancer segment currently dominates the market, accounting for roughly 35% of organoid applications, followed by stem cell research (25%) and regenerative medicine (20%). Pharmaceutical companies represent the largest end-user segment, comprising approximately 40% of the market share.
Regionally, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate of 25% annually due to increasing investments in biotechnology research infrastructure and favorable regulatory environments for genetic research.
Key demand drivers include the rising prevalence of genetic disorders, growing interest in precision medicine approaches, and limitations of traditional 2D cell cultures in accurately representing genetic complexity. The shift toward reducing animal testing in pharmaceutical development has further accelerated adoption of genetically relevant organoid models, with over 60% of major pharmaceutical companies now incorporating organoid platforms into their genetic research workflows.
Market challenges include high production costs, with specialized organoid culture systems for genetic applications typically costing 3-5 times more than conventional cell culture systems. Technical barriers to standardization and scalability also persist, particularly for complex genetic manipulation procedures within organoid systems.
Emerging market opportunities include integration with CRISPR gene editing technologies, which has grown by approximately 35% annually since 2019. The development of biobanks containing genetically diverse organoid collections represents another high-growth segment, with several commercial biobanks reporting 40-50% annual increases in organoid acquisition and distribution volumes for genetic research applications.
Demand for organoid technologies in genetic research is particularly strong in oncology, where patient-derived organoids enable precise genetic profiling of tumors and testing of targeted therapies. The cancer segment currently dominates the market, accounting for roughly 35% of organoid applications, followed by stem cell research (25%) and regenerative medicine (20%). Pharmaceutical companies represent the largest end-user segment, comprising approximately 40% of the market share.
Regionally, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate of 25% annually due to increasing investments in biotechnology research infrastructure and favorable regulatory environments for genetic research.
Key demand drivers include the rising prevalence of genetic disorders, growing interest in precision medicine approaches, and limitations of traditional 2D cell cultures in accurately representing genetic complexity. The shift toward reducing animal testing in pharmaceutical development has further accelerated adoption of genetically relevant organoid models, with over 60% of major pharmaceutical companies now incorporating organoid platforms into their genetic research workflows.
Market challenges include high production costs, with specialized organoid culture systems for genetic applications typically costing 3-5 times more than conventional cell culture systems. Technical barriers to standardization and scalability also persist, particularly for complex genetic manipulation procedures within organoid systems.
Emerging market opportunities include integration with CRISPR gene editing technologies, which has grown by approximately 35% annually since 2019. The development of biobanks containing genetically diverse organoid collections represents another high-growth segment, with several commercial biobanks reporting 40-50% annual increases in organoid acquisition and distribution volumes for genetic research applications.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid culture systems, several critical challenges continue to impede their full potential in genetic research applications. The primary obstacle remains reproducibility and standardization. Current protocols exhibit considerable variability between laboratories, resulting in organoids with inconsistent cellular composition, architecture, and functionality. This variability significantly hampers comparative studies and limits the reliability of genetic manipulation experiments across different research settings.
Matrix composition represents another substantial challenge. The extracellular matrix (ECM) components used in organoid culture, particularly Matrigel, are often derived from animal sources with batch-to-batch variations. These inconsistencies affect organoid development and introduce unwanted variables in genetic studies. Furthermore, the undefined nature of these matrices complicates the interpretation of genetic manipulation outcomes, as interactions between genetic factors and matrix components remain poorly characterized.
Vascularization deficiency presents a significant limitation for long-term organoid culture and maturation. Without proper blood vessel formation, organoids frequently develop necrotic cores as they grow beyond certain dimensions, restricting their size and longevity. This limitation particularly affects genetic studies requiring extended observation periods or those investigating genes involved in tissue-level interactions and systemic responses.
The current inability to fully recapitulate native tissue microenvironments poses another critical challenge. Most organoid systems lack immune components, vasculature, and neural innervation present in vivo. This absence compromises studies of genetic factors involved in immune responses, inflammation, and neuroendocrine regulation. Additionally, the simplified microenvironment fails to represent the complex cell-cell interactions that influence gene expression and function in native tissues.
Scalability and cost-effectiveness remain significant barriers to widespread adoption of organoid technology in genetic research. Current protocols are labor-intensive, require specialized expertise, and involve expensive growth factors and matrices. These factors limit high-throughput genetic screening applications and accessibility to smaller research laboratories with limited resources.
Technical challenges in genetic manipulation of organoids further complicate research applications. While CRISPR-Cas9 and other gene editing technologies have been adapted for organoid systems, achieving consistent and efficient genetic modifications across all cells within an organoid remains difficult. This heterogeneity in genetic manipulation outcomes complicates the interpretation of phenotypic changes and functional analyses.
Matrix composition represents another substantial challenge. The extracellular matrix (ECM) components used in organoid culture, particularly Matrigel, are often derived from animal sources with batch-to-batch variations. These inconsistencies affect organoid development and introduce unwanted variables in genetic studies. Furthermore, the undefined nature of these matrices complicates the interpretation of genetic manipulation outcomes, as interactions between genetic factors and matrix components remain poorly characterized.
Vascularization deficiency presents a significant limitation for long-term organoid culture and maturation. Without proper blood vessel formation, organoids frequently develop necrotic cores as they grow beyond certain dimensions, restricting their size and longevity. This limitation particularly affects genetic studies requiring extended observation periods or those investigating genes involved in tissue-level interactions and systemic responses.
The current inability to fully recapitulate native tissue microenvironments poses another critical challenge. Most organoid systems lack immune components, vasculature, and neural innervation present in vivo. This absence compromises studies of genetic factors involved in immune responses, inflammation, and neuroendocrine regulation. Additionally, the simplified microenvironment fails to represent the complex cell-cell interactions that influence gene expression and function in native tissues.
Scalability and cost-effectiveness remain significant barriers to widespread adoption of organoid technology in genetic research. Current protocols are labor-intensive, require specialized expertise, and involve expensive growth factors and matrices. These factors limit high-throughput genetic screening applications and accessibility to smaller research laboratories with limited resources.
Technical challenges in genetic manipulation of organoids further complicate research applications. While CRISPR-Cas9 and other gene editing technologies have been adapted for organoid systems, achieving consistent and efficient genetic modifications across all cells within an organoid remains difficult. This heterogeneity in genetic manipulation outcomes complicates the interpretation of phenotypic changes and functional analyses.
Established Protocols for Genetic Studies Using Organoids
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems utilize specialized matrices, scaffolds, and growth factors to support the self-organization of stem cells into organ-like structures. The techniques enable long-term culture of organoids that maintain tissue-specific architecture and cellular diversity, providing valuable models for studying organ development, disease mechanisms, and drug responses.- 3D organoid culture methods and systems: Three-dimensional organoid culture systems that mimic in vivo tissue architecture and function. These systems typically involve specialized matrices, scaffolds, or suspension techniques that allow cells to self-organize into organ-like structures. The 3D environment promotes proper cell-cell interactions, differentiation, and physiological responses that are not achievable in traditional 2D cultures, making these systems valuable for studying organ development, disease modeling, and drug testing.
- Growth media formulations for organoid culture: Specialized growth media formulations designed to support the development and maintenance of organoids. These media typically contain specific combinations of growth factors, hormones, and supplements that promote stem cell proliferation, differentiation, and organoid formation. The composition of the media can be tailored to support different types of organoids, such as intestinal, liver, brain, or kidney organoids, by including tissue-specific signaling molecules and nutrients.
- Extracellular matrix components for organoid development: The use of specific extracellular matrix (ECM) components to support organoid growth and development. ECM proteins such as laminin, collagen, and Matrigel provide structural support and biochemical cues that guide cell organization, differentiation, and function within organoids. These components create a microenvironment that mimics the native tissue niche, enabling proper organoid formation and maintenance of tissue-specific characteristics.
- Stem cell-derived organoid technologies: Technologies focused on generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve specific protocols for stem cell isolation, expansion, and directed differentiation to create organoids representing different tissue types. The resulting organoids can be used for disease modeling, personalized medicine applications, regenerative therapy development, and toxicity screening.
- Bioreactor systems for organoid culture: Specialized bioreactor systems designed for the large-scale production, maintenance, and monitoring of organoids. These systems provide controlled environments with optimized conditions for oxygen tension, nutrient delivery, waste removal, and mechanical stimulation. Advanced bioreactors may incorporate perfusion systems, sensors for real-time monitoring, and automated feeding schedules to enhance organoid viability, reproducibility, and scalability for research and clinical applications.
02 Growth Media and Supplements for Organoid Culture
Specialized growth media formulations and supplements that support organoid development and maintenance. These include defined combinations of growth factors, hormones, small molecules, and extracellular matrix components tailored to specific organoid types. The media compositions promote stem cell proliferation, differentiation, and self-organization while maintaining cellular viability and functionality in long-term culture conditions.Expand Specific Solutions03 Disease Modeling with Organoid Systems
Applications of organoid culture systems for modeling human diseases and pathological conditions. Patient-derived organoids can recapitulate disease phenotypes, enabling studies of disease progression, genetic factors, and host-pathogen interactions. These models are particularly valuable for cancer research, infectious diseases, and genetic disorders, providing platforms for personalized medicine approaches and therapeutic development.Expand Specific Solutions04 Bioengineering Approaches for Organoid Culture
Advanced bioengineering technologies for enhancing organoid culture systems, including microfluidic devices, bioreactors, and bioprinting techniques. These approaches provide precise control over the organoid microenvironment, nutrient delivery, and mechanical stimuli. Engineered systems can incorporate vascularization, co-culture with supporting cells, and dynamic flow conditions to better mimic in vivo conditions and improve organoid maturation and functionality.Expand Specific Solutions05 Organoid-Based Screening and Therapeutic Applications
Utilization of organoid culture systems for drug screening, toxicity testing, and therapeutic development. Organoids provide physiologically relevant platforms for evaluating drug efficacy, safety profiles, and mechanisms of action. High-throughput screening approaches with organoids enable more efficient drug discovery and personalized medicine strategies. Additionally, organoids show potential for regenerative medicine applications, including tissue engineering and transplantation therapies.Expand Specific Solutions
Leading Institutions and Companies in Organoid Research
Organoid Culture Systems are currently in a growth phase within genetic research, with the market expanding rapidly due to increasing applications in disease modeling and personalized medicine. The global market size is projected to reach significant value as research institutions and biotechnology companies invest heavily in this technology. Leading players like STEMCELL Technologies, Cell Microsystems, and Organoidsciences are advancing the technical maturity of these systems, while research institutions such as Memorial Sloan Kettering Cancer Center, Johns Hopkins University, and University of Washington are driving innovation through fundamental research. Pharmaceutical companies are increasingly adopting organoid platforms for drug screening and development, with companies like Tempus AI integrating advanced analytics to enhance the utility of organoid data in genetic research applications.
Genome Research Ltd.
Technical Solution: Genome Research Ltd. has developed sophisticated organoid culture systems specifically optimized for genetic research applications. Their technology focuses on maintaining genomic stability in long-term organoid cultures, a critical factor for reliable genetic studies. The company's OrganoCulture™ platform incorporates defined media components that minimize genetic drift during extended culture periods, ensuring that genetic modifications introduced for research purposes remain stable. Their system includes specialized protocols for deriving organoids from genetically engineered mouse models and human tissues with specific genetic alterations. Genome Research has also pioneered methods for high-throughput genetic screening in organoid systems, including CRISPR library screening approaches that enable systematic functional genomics studies. Their technology has been particularly valuable for cancer genetics research, allowing the establishment of tumor organoids that preserve the genetic heterogeneity of original tumors.
Strengths: Exceptional focus on genomic stability in long-term cultures; established protocols for genetic screening applications; strong integration with next-generation sequencing workflows. Weaknesses: More specialized focus may limit applications in some tissue types; systems require significant technical expertise to implement effectively.
Cell Microsystems, Inc.
Technical Solution: Cell Microsystems has developed the CellRaft™ Technology, an innovative platform that enhances organoid culture for genetic research applications. Their system enables single-cell isolation and clonal organoid formation, which is particularly valuable for studying genetic heterogeneity within tissues. The technology incorporates microwell arrays with releasable cell rafts that allow researchers to select and isolate individual organoids based on morphological or fluorescent genetic markers. This capability is crucial for establishing genetically homogeneous organoid lines or isolating rare genetic variants. Cell Microsystems has optimized their platform for downstream genetic analysis, including whole-genome sequencing and transcriptomic profiling of isolated organoids. Their AIR™ System automates the isolation process, improving reproducibility for genetic studies that require precise selection of organoid populations with specific genetic characteristics.
Strengths: Unparalleled capability for single-cell derived organoid isolation; excellent integration with downstream genetic analysis workflows; automated systems that enhance reproducibility. Weaknesses: Higher initial investment compared to traditional culture methods; specialized equipment requirements may limit accessibility for some research groups.
Key Innovations in Organoid-Based Genetic Modeling
Automated cell passaging
PatentWO2024196827A1
Innovation
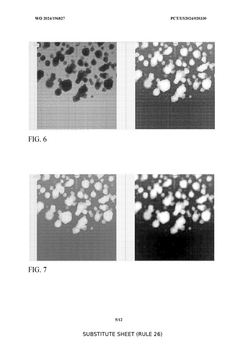
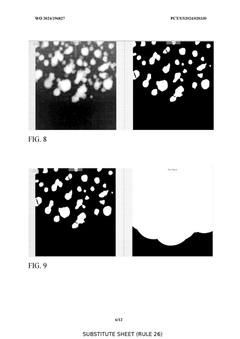
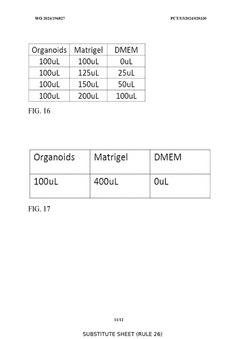
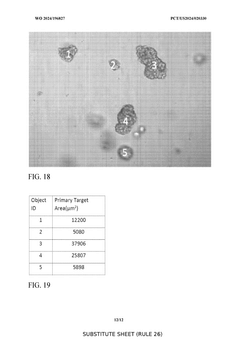
- A substantially automated process for identifying and disrupting extracellular matrix structures using a liquid handler with pipette tips, involving image processing to determine x,y coordinates and patterns for disrupting the ECM, allowing for precise removal and fragmentation of cells, and subsequent seeding in new culture plates.
Extracellular Matrix Gels, and Organoid Cultures Comprising the Same
PatentPendingUS20220389375A1
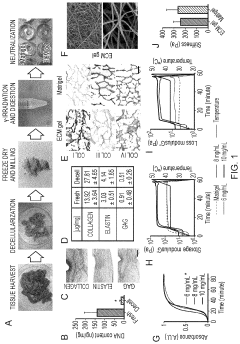
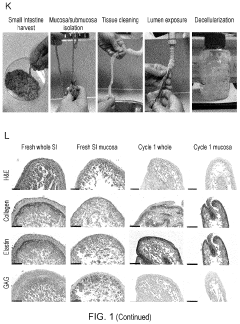
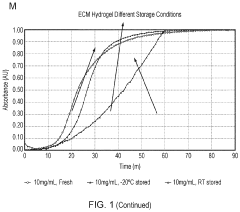
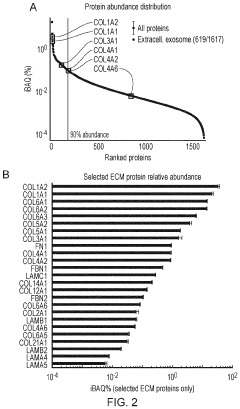
Innovation
- Development of ECM gels derived from decellularized tissues, specifically porcine small intestinal mucosa/submucosa, which provide a physiological and mechanical environment comparable to commercial gels, enriched with key ECM proteins relevant to organoid formation, and capable of supporting intestinal and other endodermal tissue-derived organoids, including angiogenesis in vivo.
Ethical Implications of Organoid Use in Genetic Studies
The ethical landscape surrounding organoid use in genetic research presents complex challenges that require careful consideration. As organoids increasingly serve as sophisticated models for human development and disease, they raise profound questions about consent, privacy, and the moral status of these entities. The source of cells used to create organoids—often derived from human tissue samples—necessitates robust informed consent protocols that address not only immediate research applications but also potential future uses, data sharing, and commercialization possibilities.
Privacy concerns are particularly acute in genetic research using organoids, as these systems can reveal detailed genetic information about donors. With advances in single-cell sequencing and multi-omics approaches, organoids can generate comprehensive genetic profiles that may expose sensitive health information. This raises questions about data protection, anonymization practices, and the right of donors to control information derived from their biological materials.
The moral status of organoids represents perhaps the most philosophically challenging aspect of their use. As these structures become increasingly complex—particularly brain organoids that develop rudimentary neural activity—questions arise about consciousness, sentience, and the ethical boundaries of creating human-like tissues in laboratory settings. The scientific community continues to debate appropriate limitations on organoid complexity and functionality to prevent crossing ethical thresholds.
Cultural and religious perspectives further complicate the ethical framework, as different traditions hold varying views on the manipulation of human tissues and the creation of entities that mimic aspects of human development. These perspectives must be acknowledged in developing governance frameworks for organoid research.
Equity considerations also demand attention, as benefits from organoid-based genetic research may not be distributed fairly across populations. Historical biases in biomedical research could be perpetuated if organoid models primarily represent certain genetic backgrounds while underrepresenting others. Ensuring diversity in cell sources and addressing potential disparities in access to therapeutic applications derived from organoid research remains critical.
Regulatory frameworks for organoid research vary significantly across jurisdictions, creating challenges for international collaboration and standardization. While some regions have developed specific guidelines for organoid research, others rely on broader frameworks governing human tissue research or stem cell research, potentially leaving regulatory gaps that fail to address the unique ethical considerations of organoid systems in genetic studies.
Privacy concerns are particularly acute in genetic research using organoids, as these systems can reveal detailed genetic information about donors. With advances in single-cell sequencing and multi-omics approaches, organoids can generate comprehensive genetic profiles that may expose sensitive health information. This raises questions about data protection, anonymization practices, and the right of donors to control information derived from their biological materials.
The moral status of organoids represents perhaps the most philosophically challenging aspect of their use. As these structures become increasingly complex—particularly brain organoids that develop rudimentary neural activity—questions arise about consciousness, sentience, and the ethical boundaries of creating human-like tissues in laboratory settings. The scientific community continues to debate appropriate limitations on organoid complexity and functionality to prevent crossing ethical thresholds.
Cultural and religious perspectives further complicate the ethical framework, as different traditions hold varying views on the manipulation of human tissues and the creation of entities that mimic aspects of human development. These perspectives must be acknowledged in developing governance frameworks for organoid research.
Equity considerations also demand attention, as benefits from organoid-based genetic research may not be distributed fairly across populations. Historical biases in biomedical research could be perpetuated if organoid models primarily represent certain genetic backgrounds while underrepresenting others. Ensuring diversity in cell sources and addressing potential disparities in access to therapeutic applications derived from organoid research remains critical.
Regulatory frameworks for organoid research vary significantly across jurisdictions, creating challenges for international collaboration and standardization. While some regions have developed specific guidelines for organoid research, others rely on broader frameworks governing human tissue research or stem cell research, potentially leaving regulatory gaps that fail to address the unique ethical considerations of organoid systems in genetic studies.
Standardization and Quality Control in Organoid Systems
The standardization and quality control of organoid systems represent critical challenges in the advancement of organoid technology for genetic research applications. As these three-dimensional cellular structures gain prominence in modeling human development and disease, the scientific community faces significant hurdles in establishing consistent protocols that ensure reproducibility across laboratories and experiments.
Current standardization efforts focus on several key parameters including cell source selection, extracellular matrix composition, growth factor concentrations, and culture duration. The heterogeneity observed between organoid batches stems largely from variations in these factors, with studies showing that even minor protocol deviations can lead to substantial phenotypic differences. For instance, recent comparative analyses have demonstrated that organoids derived from the same tissue but cultured under slightly different conditions can exhibit up to 30% variation in gene expression profiles.
Quality control metrics are evolving rapidly, with researchers implementing multi-parameter assessment approaches. These typically include morphological evaluation through advanced imaging techniques, functional assays specific to the tissue being modeled, and comprehensive molecular characterization. Single-cell RNA sequencing has emerged as a particularly valuable tool, allowing researchers to verify cellular composition and maturation states within organoid cultures with unprecedented resolution.
International initiatives such as the Human Cell Atlas Organoid Project and the NIH-funded HuBMAP consortium are working to establish reference standards for organoid systems. These collaborative efforts aim to create benchmarks against which individual laboratories can validate their organoid cultures, potentially addressing the reproducibility crisis that has begun to emerge in organoid-based research publications.
Commercial entities have also entered this space, developing standardized organoid culture kits and quality control reagents. While these products offer convenience and potential consistency, their proprietary nature sometimes limits full protocol transparency, creating tensions between standardization and open science principles.
Regulatory considerations are increasingly important as organoid systems move toward clinical applications. The FDA and EMA have begun preliminary discussions on framework development for evaluating organoid-based models in drug screening and toxicity testing, with particular emphasis on quality control parameters that would ensure reliable prediction of human responses.
Machine learning approaches are being integrated into quality control workflows, with algorithms trained to identify optimal organoid phenotypes based on morphological features and growth characteristics. These automated systems promise to reduce subjective assessment and increase throughput in quality evaluation processes.
Current standardization efforts focus on several key parameters including cell source selection, extracellular matrix composition, growth factor concentrations, and culture duration. The heterogeneity observed between organoid batches stems largely from variations in these factors, with studies showing that even minor protocol deviations can lead to substantial phenotypic differences. For instance, recent comparative analyses have demonstrated that organoids derived from the same tissue but cultured under slightly different conditions can exhibit up to 30% variation in gene expression profiles.
Quality control metrics are evolving rapidly, with researchers implementing multi-parameter assessment approaches. These typically include morphological evaluation through advanced imaging techniques, functional assays specific to the tissue being modeled, and comprehensive molecular characterization. Single-cell RNA sequencing has emerged as a particularly valuable tool, allowing researchers to verify cellular composition and maturation states within organoid cultures with unprecedented resolution.
International initiatives such as the Human Cell Atlas Organoid Project and the NIH-funded HuBMAP consortium are working to establish reference standards for organoid systems. These collaborative efforts aim to create benchmarks against which individual laboratories can validate their organoid cultures, potentially addressing the reproducibility crisis that has begun to emerge in organoid-based research publications.
Commercial entities have also entered this space, developing standardized organoid culture kits and quality control reagents. While these products offer convenience and potential consistency, their proprietary nature sometimes limits full protocol transparency, creating tensions between standardization and open science principles.
Regulatory considerations are increasingly important as organoid systems move toward clinical applications. The FDA and EMA have begun preliminary discussions on framework development for evaluating organoid-based models in drug screening and toxicity testing, with particular emphasis on quality control parameters that would ensure reliable prediction of human responses.
Machine learning approaches are being integrated into quality control workflows, with algorithms trained to identify optimal organoid phenotypes based on morphological features and growth characteristics. These automated systems promise to reduce subjective assessment and increase throughput in quality evaluation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!