What Are the Regulatory Hurdles for Organoid Culture Systems?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Regulatory Landscape and Research Objectives
Organoid technology has evolved significantly over the past decade, transforming from a laboratory curiosity to a promising platform for drug development, personalized medicine, and disease modeling. The regulatory landscape surrounding organoid culture systems, however, remains complex and fragmented, presenting significant challenges for researchers and commercial entities seeking to advance this technology into clinical applications.
The historical development of organoid regulation has been characterized by a patchwork approach, with different countries and regions establishing varying frameworks. Initially, organoids were primarily governed by existing regulations for cell culture and tissue engineering. As their potential for clinical applications became apparent, regulatory bodies began considering more specific guidelines, though comprehensive frameworks remain elusive in most jurisdictions.
Current regulatory considerations for organoid systems span multiple domains, including cell sourcing ethics, quality control standards, and clinical translation pathways. The use of human-derived materials, particularly stem cells, introduces ethical and legal complexities that vary significantly across global regions. Additionally, the three-dimensional nature of organoids and their ability to self-organize present novel regulatory challenges not adequately addressed by existing frameworks for traditional cell cultures.
The primary technical objectives for advancing organoid regulatory frameworks include establishing standardized protocols for derivation, culture, and characterization. These standards must balance the need for reproducibility with the inherent biological variability that makes organoids valuable as models. Furthermore, developing validated quality metrics that correlate with organoid functionality represents a critical goal for enabling regulatory approval pathways.
From a market perspective, the regulatory landscape directly impacts commercialization strategies for organoid-based products and services. Current ambiguities create barriers to investment and slow the translation of research findings into clinical applications. Clarifying regulatory pathways would likely accelerate market development and expand access to organoid technologies across healthcare sectors.
Looking forward, the evolution of organoid regulation will likely follow a trajectory toward harmonization of international standards, development of organoid-specific guidelines, and integration with existing frameworks for advanced therapy medicinal products. This evolution will require collaborative efforts between researchers, industry stakeholders, and regulatory authorities to establish science-based approaches that ensure safety while enabling innovation.
The ultimate goal of regulatory research in this field is to create frameworks that protect patient safety while facilitating the development of organoid technologies that can address unmet medical needs. This balance requires ongoing dialogue between scientific and regulatory communities to ensure that governance evolves in parallel with technological capabilities.
The historical development of organoid regulation has been characterized by a patchwork approach, with different countries and regions establishing varying frameworks. Initially, organoids were primarily governed by existing regulations for cell culture and tissue engineering. As their potential for clinical applications became apparent, regulatory bodies began considering more specific guidelines, though comprehensive frameworks remain elusive in most jurisdictions.
Current regulatory considerations for organoid systems span multiple domains, including cell sourcing ethics, quality control standards, and clinical translation pathways. The use of human-derived materials, particularly stem cells, introduces ethical and legal complexities that vary significantly across global regions. Additionally, the three-dimensional nature of organoids and their ability to self-organize present novel regulatory challenges not adequately addressed by existing frameworks for traditional cell cultures.
The primary technical objectives for advancing organoid regulatory frameworks include establishing standardized protocols for derivation, culture, and characterization. These standards must balance the need for reproducibility with the inherent biological variability that makes organoids valuable as models. Furthermore, developing validated quality metrics that correlate with organoid functionality represents a critical goal for enabling regulatory approval pathways.
From a market perspective, the regulatory landscape directly impacts commercialization strategies for organoid-based products and services. Current ambiguities create barriers to investment and slow the translation of research findings into clinical applications. Clarifying regulatory pathways would likely accelerate market development and expand access to organoid technologies across healthcare sectors.
Looking forward, the evolution of organoid regulation will likely follow a trajectory toward harmonization of international standards, development of organoid-specific guidelines, and integration with existing frameworks for advanced therapy medicinal products. This evolution will require collaborative efforts between researchers, industry stakeholders, and regulatory authorities to establish science-based approaches that ensure safety while enabling innovation.
The ultimate goal of regulatory research in this field is to create frameworks that protect patient safety while facilitating the development of organoid technologies that can address unmet medical needs. This balance requires ongoing dialogue between scientific and regulatory communities to ensure that governance evolves in parallel with technological capabilities.
Market Analysis for Organoid Culture Technologies
The global organoid culture technologies market is experiencing robust growth, valued at approximately $1.3 billion in 2023 with projections to reach $3.5 billion by 2028, representing a compound annual growth rate (CAGR) of 22.1%. This remarkable expansion is driven primarily by increasing applications in regenerative medicine, drug discovery, personalized medicine, and disease modeling.
The pharmaceutical and biotechnology sectors constitute the largest market segment, accounting for nearly 45% of the total market share. These industries leverage organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant testing platforms than traditional 2D cell cultures. The academic and research institutions segment follows closely, contributing approximately 30% to the market, while healthcare providers represent about 15%.
Geographically, North America dominates the market with approximately 40% share, attributed to substantial research funding, presence of major biotechnology companies, and favorable regulatory frameworks. Europe holds approximately 30% of the market, with countries like Germany, the UK, and the Netherlands leading in research output. The Asia-Pacific region represents the fastest-growing market at a CAGR of 25.3%, driven by increasing research investments in Japan, China, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating novel therapeutic approaches, growing demand for personalized medicine, and increasing concerns about animal testing ethics. Additionally, technological advancements in 3D bioprinting, microfluidics, and artificial intelligence integration are expanding organoid applications and market potential.
However, significant market restraints persist, particularly regulatory challenges. The lack of standardized regulatory frameworks specifically addressing organoid technologies creates uncertainty for commercial development. Regulatory agencies worldwide are struggling to classify these complex biological systems within existing frameworks, resulting in inconsistent approval processes across different jurisdictions.
Market analysis indicates that companies focusing on developing standardized, reproducible organoid culture systems with clear regulatory pathways are gaining competitive advantages. The market is witnessing strategic collaborations between biotechnology companies and regulatory consultants to navigate the complex regulatory landscape effectively.
Consumer trends show increasing demand for organoid technologies that can demonstrate regulatory compliance and standardization. End-users are willing to pay premium prices for systems with established regulatory acceptance, creating market opportunities for companies that can successfully address these regulatory hurdles while maintaining technological innovation.
The pharmaceutical and biotechnology sectors constitute the largest market segment, accounting for nearly 45% of the total market share. These industries leverage organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant testing platforms than traditional 2D cell cultures. The academic and research institutions segment follows closely, contributing approximately 30% to the market, while healthcare providers represent about 15%.
Geographically, North America dominates the market with approximately 40% share, attributed to substantial research funding, presence of major biotechnology companies, and favorable regulatory frameworks. Europe holds approximately 30% of the market, with countries like Germany, the UK, and the Netherlands leading in research output. The Asia-Pacific region represents the fastest-growing market at a CAGR of 25.3%, driven by increasing research investments in Japan, China, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating novel therapeutic approaches, growing demand for personalized medicine, and increasing concerns about animal testing ethics. Additionally, technological advancements in 3D bioprinting, microfluidics, and artificial intelligence integration are expanding organoid applications and market potential.
However, significant market restraints persist, particularly regulatory challenges. The lack of standardized regulatory frameworks specifically addressing organoid technologies creates uncertainty for commercial development. Regulatory agencies worldwide are struggling to classify these complex biological systems within existing frameworks, resulting in inconsistent approval processes across different jurisdictions.
Market analysis indicates that companies focusing on developing standardized, reproducible organoid culture systems with clear regulatory pathways are gaining competitive advantages. The market is witnessing strategic collaborations between biotechnology companies and regulatory consultants to navigate the complex regulatory landscape effectively.
Consumer trends show increasing demand for organoid technologies that can demonstrate regulatory compliance and standardization. End-users are willing to pay premium prices for systems with established regulatory acceptance, creating market opportunities for companies that can successfully address these regulatory hurdles while maintaining technological innovation.
Current Regulatory Challenges and Technical Limitations
Organoid culture systems face significant regulatory challenges globally due to their novel nature that blurs traditional boundaries between in vitro models and human tissues. Current regulatory frameworks were primarily designed for conventional cell cultures or animal models, creating substantial gaps when applied to organoids. The FDA, EMA, and other regulatory bodies have yet to establish specific guidelines for organoid development, validation, and commercialization, resulting in case-by-case evaluations that create uncertainty and delays for researchers and companies.
A major regulatory hurdle involves the classification of organoids, as they don't fit neatly into existing categories like medical devices, biological products, or cell therapies. This ambiguity affects everything from research approval processes to commercialization pathways. Additionally, the use of human-derived materials in organoid systems raises complex ethical and legal questions regarding informed consent, particularly for biobanking and long-term storage of patient-derived organoids.
Quality control represents another significant challenge, as regulatory agencies require standardized protocols for reproducibility and safety. However, the inherent biological variability in organoids makes establishing universal quality metrics exceptionally difficult. Current regulations demand batch-to-batch consistency that organoid systems struggle to achieve due to their complex developmental processes and cellular heterogeneity.
From a technical perspective, organoid culture systems face several limitations that compound regulatory challenges. Scalability remains problematic, with difficulties in maintaining consistent growth conditions and cellular architecture in large-scale production settings. This creates barriers for both clinical applications and drug screening platforms that require high-throughput capabilities to meet regulatory standards for reliability.
The lack of standardized culture protocols across laboratories further complicates regulatory compliance. Variations in extracellular matrix components, growth factors, and culture conditions lead to significant differences in organoid phenotypes, making cross-validation between research centers challenging and hindering the establishment of reference standards required by regulatory bodies.
Vascularization represents another critical technical limitation, as most current organoid systems lack proper blood vessel networks. This absence affects nutrient delivery to inner cell layers, limiting organoid size and longevity while raising questions about their physiological relevance as disease models. Regulatory agencies increasingly question whether non-vascularized organoids can accurately predict in vivo responses, particularly for drug toxicity and efficacy studies.
Finally, the integration of immune components remains technically challenging but is essential for modeling inflammatory responses and immune-mediated diseases. Without standardized methods to incorporate immune cells, organoids face regulatory scrutiny regarding their predictive value for immunological reactions to drugs and therapies.
A major regulatory hurdle involves the classification of organoids, as they don't fit neatly into existing categories like medical devices, biological products, or cell therapies. This ambiguity affects everything from research approval processes to commercialization pathways. Additionally, the use of human-derived materials in organoid systems raises complex ethical and legal questions regarding informed consent, particularly for biobanking and long-term storage of patient-derived organoids.
Quality control represents another significant challenge, as regulatory agencies require standardized protocols for reproducibility and safety. However, the inherent biological variability in organoids makes establishing universal quality metrics exceptionally difficult. Current regulations demand batch-to-batch consistency that organoid systems struggle to achieve due to their complex developmental processes and cellular heterogeneity.
From a technical perspective, organoid culture systems face several limitations that compound regulatory challenges. Scalability remains problematic, with difficulties in maintaining consistent growth conditions and cellular architecture in large-scale production settings. This creates barriers for both clinical applications and drug screening platforms that require high-throughput capabilities to meet regulatory standards for reliability.
The lack of standardized culture protocols across laboratories further complicates regulatory compliance. Variations in extracellular matrix components, growth factors, and culture conditions lead to significant differences in organoid phenotypes, making cross-validation between research centers challenging and hindering the establishment of reference standards required by regulatory bodies.
Vascularization represents another critical technical limitation, as most current organoid systems lack proper blood vessel networks. This absence affects nutrient delivery to inner cell layers, limiting organoid size and longevity while raising questions about their physiological relevance as disease models. Regulatory agencies increasingly question whether non-vascularized organoids can accurately predict in vivo responses, particularly for drug toxicity and efficacy studies.
Finally, the integration of immune components remains technically challenging but is essential for modeling inflammatory responses and immune-mediated diseases. Without standardized methods to incorporate immune cells, organoids face regulatory scrutiny regarding their predictive value for immunological reactions to drugs and therapies.
Current Compliance Strategies for Organoid Systems
01 Regulatory compliance for organoid culture systems
Organoid culture systems face significant regulatory hurdles due to their novel nature and biological complexity. These systems must comply with various regulatory frameworks that govern biological materials, cell cultures, and tissue engineering. Regulatory bodies require extensive documentation, validation, and standardization of organoid culture protocols to ensure safety and reproducibility. Compliance with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards is essential for organoids intended for clinical applications.- Regulatory challenges in organoid culture standardization: Organoid culture systems face significant regulatory hurdles related to standardization of protocols and materials. The lack of consistent manufacturing processes and quality control standards creates challenges for regulatory approval. Variations in culture conditions, growth factors, and matrix components can lead to inconsistent organoid development, making it difficult to establish regulatory frameworks that ensure reproducibility and safety for clinical applications.
- Safety and ethical considerations for clinical translation: The translation of organoid technologies to clinical settings faces regulatory hurdles related to safety assessment and ethical considerations. Regulatory bodies require comprehensive safety data addressing potential tumorigenicity, immunogenicity, and long-term stability of organoid-based therapies. Additionally, the use of human tissue-derived organoids raises ethical questions regarding informed consent, privacy, and ownership of biological materials, which must be addressed within regulatory frameworks.
- Manufacturing compliance and quality control systems: Establishing compliant manufacturing processes for organoid culture systems presents significant regulatory challenges. Good Manufacturing Practice (GMP) requirements necessitate validated equipment, controlled environments, and robust quality management systems. Regulatory agencies require documentation of process controls, batch consistency, and sterility assurance. The complex nature of organoid cultures demands specialized quality control metrics and validation methods that differ from traditional cell culture systems.
- Validation of organoid models for drug development: Regulatory acceptance of organoid models for drug development and toxicity testing requires validation against established methods. Demonstrating that organoid systems can reliably predict human responses presents regulatory challenges, as agencies require evidence of physiological relevance and predictive capability. Establishing standardized endpoints, reference compounds, and correlation with in vivo outcomes is necessary to gain regulatory acceptance of organoid-based testing platforms.
- Intellectual property and commercialization barriers: The commercialization of organoid culture systems faces regulatory hurdles related to intellectual property rights and market approval pathways. Patent protection for novel culture methods, media formulations, and applications creates a complex landscape for developers. Regulatory classification of organoid-based products (as devices, biologics, or combination products) affects approval pathways and requirements. International harmonization of regulations is lacking, creating additional barriers for global commercialization of organoid technologies.
02 Quality control and standardization challenges
A major regulatory hurdle for organoid culture systems involves establishing consistent quality control measures and standardization protocols. Variability in organoid development, structure, and function presents challenges for regulatory approval. Standardized methods for characterizing organoids, assessing their functionality, and ensuring batch-to-batch consistency are required. Developing reference standards and validated biomarkers for organoid quality assessment is crucial to overcome these regulatory challenges.Expand Specific Solutions03 Ethical and safety considerations
Organoid culture systems raise unique ethical and safety concerns that must be addressed to meet regulatory requirements. These include issues related to donor consent for cell sourcing, genetic modification of organoids, and potential tumorigenicity risks. Regulatory frameworks require comprehensive risk assessments and mitigation strategies for these concerns. Additionally, when organoids are derived from human tissues, especially for brain or reproductive organoids, specific ethical guidelines and oversight mechanisms are necessary to ensure responsible research and development.Expand Specific Solutions04 Validation for drug development and toxicity testing
Organoid culture systems used for drug development and toxicity testing face specific regulatory hurdles related to their validation as predictive models. Regulatory agencies require evidence demonstrating that organoid models accurately reflect in vivo human responses to drugs and toxins. This includes establishing correlations between organoid responses and clinical outcomes, determining the predictive value of organoid-based assays, and validating their reproducibility across different laboratories. Meeting these validation requirements is essential for the regulatory acceptance of organoid models as alternatives to traditional testing methods.Expand Specific Solutions05 Manufacturing and scale-up challenges
Scaling up organoid culture systems from research to clinical or commercial applications presents significant regulatory challenges. These include maintaining consistent quality during scale-up, establishing closed-system bioreactors that meet regulatory requirements, and developing appropriate storage and transportation methods. Regulatory frameworks demand robust process controls, environmental monitoring, and contamination prevention strategies. Additionally, the complex supply chains for materials used in organoid culture systems must comply with regulatory standards for sourcing, quality, and traceability.Expand Specific Solutions
Key Stakeholders in Organoid Research and Regulation
The organoid culture systems market is currently in a growth phase, characterized by increasing research activities and expanding applications in drug discovery and personalized medicine. The global market size is estimated to reach $3.5 billion by 2027, driven by rising investments in regenerative medicine and disease modeling. Technologically, the field is advancing rapidly but still faces significant regulatory challenges, particularly regarding standardization and ethical considerations. Leading players include STEMCELL Technologies, which has developed comprehensive culture media solutions, and Cellesce Ltd., specializing in bioprocessing technology for organoid expansion. Academic institutions like EPFL and Max Planck Society are pushing boundaries in fundamental research, while companies like Chuangxin International Biotechnology and Organoidsciences Ltd. are focusing on clinical applications and commercialization pathways through regulatory frameworks.
Molecular Devices (Austria) GmbH
Technical Solution: Molecular Devices has developed an integrated hardware-software platform specifically designed to address regulatory challenges in organoid research and development. Their approach combines high-content imaging systems with automated culture maintenance technology that ensures standardization and reproducibility - key requirements for regulatory approval. The company's ImageXpress® system incorporates machine learning algorithms that quantitatively assess organoid morphology and function, providing the objective measurements required by regulatory agencies. Their technology includes comprehensive data management solutions that maintain complete records of organoid development, experimental conditions, and analytical results - creating the documentation trail necessary for regulatory compliance. Molecular Devices has also established validation protocols that demonstrate the reliability and reproducibility of their organoid analysis systems across multiple tissue types and experimental conditions, addressing a critical regulatory concern about consistency in complex biological models.
Strengths: Industry-leading integration of imaging and analysis technologies specifically validated for organoid applications; comprehensive data management systems that facilitate regulatory documentation; established presence in regulated markets with proven compliance track record. Weaknesses: High capital investment requirements for full platform implementation; complex systems require specialized training; primary focus on analytical rather than culture aspects of organoid technology.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive regulatory-compliant organoid culture systems through their STEMdiff™ and IntestiCult™ product lines. Their approach addresses regulatory hurdles by implementing standardized, animal component-free culture media that meets GMP requirements for clinical applications. The company has established a robust quality management system that ensures batch-to-batch consistency and traceability, critical for regulatory approval. Their technology includes defined extracellular matrices and growth factor supplements specifically designed to maintain genomic stability during long-term organoid culture - a key concern for regulators. STEMCELL has also developed validation protocols that demonstrate their culture systems' ability to produce organoids with consistent morphological and functional characteristics across multiple passages, addressing regulatory concerns about reproducibility and standardization.
Strengths: Industry-leading standardization protocols that facilitate regulatory compliance; extensive documentation systems that support IND applications; proprietary defined matrices that eliminate animal-derived components. Weaknesses: Higher cost compared to academic-developed systems; some applications still require customization beyond their standardized offerings; limited flexibility for novel organoid types not covered by their product portfolio.
Critical Regulatory Documents and Scientific Literature
Method for constructing human pluripotent stem cell-derived liver organoid having enhanced drug metabolic potential and liver organoid constructed by same method
PatentActiveUS20240141289A1
Innovation
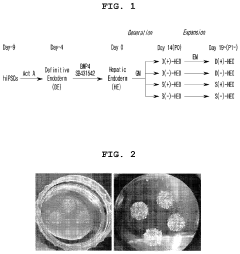
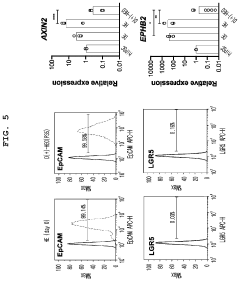
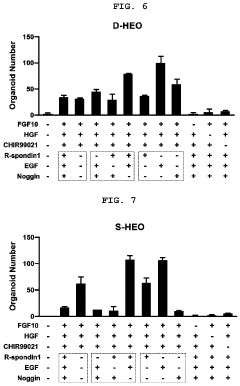
- A method for constructing human pluripotent stem cell-derived liver organoids by differentiating cells into definitive endoderm, hepatic endoderm, and finally liver organoids, using specific culture media without R-Spondin-1, Noggin, and EGF, which enhances drug metabolic capabilities and toxicity evaluation.
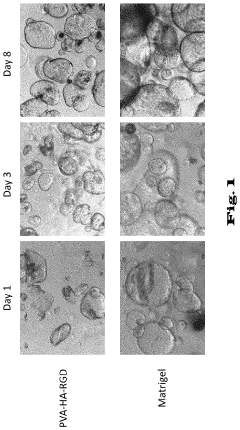
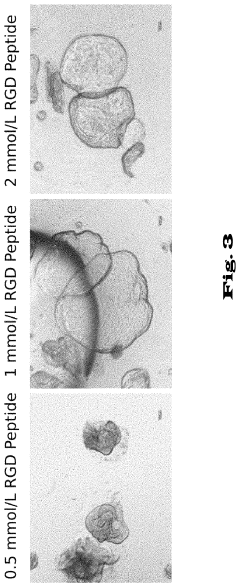
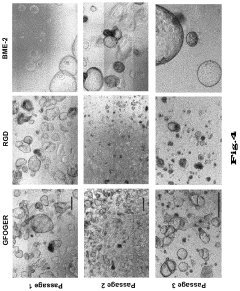
Hydrogels for cultivating pancreatic organoids
PatentInactiveUS20190367869A1
Innovation
- A chemically defined hydrogel composition with a reduced shear modulus, composed of functionalized polymer molecules and linker molecules, and low-molecular peptides with cell adhesion motifs, which allows for the cultivation and expansion of pancreatic cells into stable and functional 3D organoids without natural extracellular matrix proteins.
Ethical Considerations in Organoid Development
The ethical landscape surrounding organoid development presents complex challenges that require careful consideration as this technology advances. Organoids, as three-dimensional cellular structures that mimic organ functionality, raise profound questions about the moral status of these entities. While they lack consciousness and sentience, their increasing complexity and human-derived nature necessitate establishing clear ethical boundaries for their development and utilization.
Consent issues represent a primary ethical concern in organoid research. When human cells are harvested for organoid creation, comprehensive informed consent protocols must address not only immediate research applications but also potential future uses, commercial developments, and data sharing implications. The donor's right to withdraw consent becomes particularly complicated when their biological materials have been integrated into long-term research programs or commercialized products.
Privacy considerations emerge as organoids derived from specific individuals may contain their unique genetic information. This raises questions about genetic privacy protection, especially as organoid biobanks expand and research becomes increasingly collaborative across international boundaries with varying privacy regulations. The potential for re-identification of donors through their organoid-derived genetic data requires robust safeguards.
The development of cerebral organoids presents particularly nuanced ethical challenges. As these brain-like structures become more sophisticated, questions arise regarding the potential for consciousness or sentience. While current cerebral organoids remain far from achieving consciousness, establishing preemptive ethical frameworks becomes essential as technology advances toward greater neural complexity.
Commercialization and ownership questions introduce additional ethical dimensions. The patenting of organoid technologies, ownership of derived cell lines, and equitable benefit-sharing with tissue donors require careful consideration. The potential commodification of human-derived materials must be balanced against the need for sustainable research funding and development incentives.
Cultural and religious perspectives on organoid research vary significantly across societies. Some traditions may view certain applications of organoid technology as challenging fundamental beliefs about human dignity or the sanctity of life. Inclusive stakeholder engagement across diverse cultural contexts is essential for developing globally acceptable ethical frameworks that respect pluralistic values while advancing scientific progress.
Consent issues represent a primary ethical concern in organoid research. When human cells are harvested for organoid creation, comprehensive informed consent protocols must address not only immediate research applications but also potential future uses, commercial developments, and data sharing implications. The donor's right to withdraw consent becomes particularly complicated when their biological materials have been integrated into long-term research programs or commercialized products.
Privacy considerations emerge as organoids derived from specific individuals may contain their unique genetic information. This raises questions about genetic privacy protection, especially as organoid biobanks expand and research becomes increasingly collaborative across international boundaries with varying privacy regulations. The potential for re-identification of donors through their organoid-derived genetic data requires robust safeguards.
The development of cerebral organoids presents particularly nuanced ethical challenges. As these brain-like structures become more sophisticated, questions arise regarding the potential for consciousness or sentience. While current cerebral organoids remain far from achieving consciousness, establishing preemptive ethical frameworks becomes essential as technology advances toward greater neural complexity.
Commercialization and ownership questions introduce additional ethical dimensions. The patenting of organoid technologies, ownership of derived cell lines, and equitable benefit-sharing with tissue donors require careful consideration. The potential commodification of human-derived materials must be balanced against the need for sustainable research funding and development incentives.
Cultural and religious perspectives on organoid research vary significantly across societies. Some traditions may view certain applications of organoid technology as challenging fundamental beliefs about human dignity or the sanctity of life. Inclusive stakeholder engagement across diverse cultural contexts is essential for developing globally acceptable ethical frameworks that respect pluralistic values while advancing scientific progress.
International Harmonization of Organoid Regulations
The global landscape of organoid research and application is currently fragmented by diverse regulatory frameworks across different countries and regions. This lack of harmonization creates significant barriers for international collaboration, technology transfer, and clinical translation of organoid technologies. Major regulatory bodies including the FDA in the United States, the EMA in Europe, and the PMDA in Japan have established varying requirements for organoid-based research, particularly concerning ethical considerations, quality control standards, and clinical application pathways.
Efforts toward international harmonization have begun through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which has started preliminary discussions on organoid technologies. The Organization for Economic Co-operation and Development (OECD) has also established working groups focused on developing consensus guidelines for advanced cellular models including organoids. These initiatives aim to create standardized frameworks that can be adopted across multiple jurisdictions.
Key areas requiring harmonization include consent procedures for human tissue sourcing, genetic modification protocols, quality assurance standards, and validation requirements for organoid models. The divergence in these areas currently creates significant compliance challenges for multinational research projects and commercial development programs. For instance, requirements for donor consent and privacy protection vary substantially between the EU's GDPR framework and regulations in countries like China, Japan, and the United States.
Regulatory convergence would particularly benefit the pharmaceutical industry, where organoid-based drug screening and toxicity testing face inconsistent acceptance criteria across different markets. A unified approach to validating organoid models as alternatives to animal testing would accelerate drug development timelines and reduce regulatory uncertainty. Several pharmaceutical consortia have begun advocating for such harmonization through position papers and stakeholder engagement with regulatory authorities.
Recent progress includes the establishment of the International Organoid Alliance in 2022, which brings together researchers, industry representatives, and regulatory experts from 27 countries to develop consensus standards. Their initial focus has been on creating standardized terminology, minimum reporting requirements, and quality control benchmarks that could form the foundation for harmonized regulations. The World Health Organization has also recognized this need and commissioned an expert committee to develop guidance on ethical and regulatory considerations for organoid research with potential global applicability.
The path toward full international harmonization will likely require a phased approach, beginning with agreement on fundamental principles and gradually addressing more complex regulatory issues. Successful models from other fields, such as the harmonization of medical device regulations through the International Medical Device Regulators Forum, provide potential templates for organoid regulation standardization.
Efforts toward international harmonization have begun through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which has started preliminary discussions on organoid technologies. The Organization for Economic Co-operation and Development (OECD) has also established working groups focused on developing consensus guidelines for advanced cellular models including organoids. These initiatives aim to create standardized frameworks that can be adopted across multiple jurisdictions.
Key areas requiring harmonization include consent procedures for human tissue sourcing, genetic modification protocols, quality assurance standards, and validation requirements for organoid models. The divergence in these areas currently creates significant compliance challenges for multinational research projects and commercial development programs. For instance, requirements for donor consent and privacy protection vary substantially between the EU's GDPR framework and regulations in countries like China, Japan, and the United States.
Regulatory convergence would particularly benefit the pharmaceutical industry, where organoid-based drug screening and toxicity testing face inconsistent acceptance criteria across different markets. A unified approach to validating organoid models as alternatives to animal testing would accelerate drug development timelines and reduce regulatory uncertainty. Several pharmaceutical consortia have begun advocating for such harmonization through position papers and stakeholder engagement with regulatory authorities.
Recent progress includes the establishment of the International Organoid Alliance in 2022, which brings together researchers, industry representatives, and regulatory experts from 27 countries to develop consensus standards. Their initial focus has been on creating standardized terminology, minimum reporting requirements, and quality control benchmarks that could form the foundation for harmonized regulations. The World Health Organization has also recognized this need and commissioned an expert committee to develop guidance on ethical and regulatory considerations for organoid research with potential global applicability.
The path toward full international harmonization will likely require a phased approach, beginning with agreement on fundamental principles and gradually addressing more complex regulatory issues. Successful models from other fields, such as the harmonization of medical device regulations through the International Medical Device Regulators Forum, provide potential templates for organoid regulation standardization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!