How Do Organoid Culture Systems Optimize Pharmaceutical Applications?
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Objectives
Organoid technology has evolved significantly since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers and colleagues. This breakthrough demonstrated that adult stem cells could self-organize into three-dimensional structures resembling their tissue of origin. The evolution of organoid technology represents a convergence of stem cell biology, developmental biology, and tissue engineering principles that has revolutionized our understanding of human development and disease.
Prior to organoids, pharmaceutical research relied heavily on traditional two-dimensional cell cultures and animal models, both with significant limitations in recapitulating human physiology. Two-dimensional cultures lack the complex architecture and cellular interactions of native tissues, while animal models often fail to predict human-specific responses due to species differences. These limitations have contributed to high failure rates in drug development pipelines, with approximately 90% of drug candidates failing in clinical trials despite promising preclinical results.
The technological evolution of organoid systems has been marked by several key advancements. The identification and characterization of tissue-specific stem cells provided the cellular foundation. Concurrently, the development of specialized extracellular matrices and defined culture media containing tissue-specific growth factors enabled the creation of microenvironments supporting organoid formation. More recently, bioengineering approaches incorporating microfluidics and bioprinting have enhanced the complexity and functionality of organoid systems.
Today's organoid technology encompasses diverse tissue types including intestinal, liver, pancreatic, brain, kidney, and lung organoids, each with specific applications in pharmaceutical research. These systems aim to provide physiologically relevant models for drug screening, toxicity testing, and personalized medicine approaches that better predict human responses than conventional methods.
The primary objectives of organoid culture systems in pharmaceutical applications include establishing reproducible and scalable platforms for high-throughput drug screening, developing disease-specific models that accurately recapitulate pathophysiology, and creating patient-derived organoids for personalized medicine approaches. Additionally, researchers aim to integrate organoids with other technologies such as organ-on-chip platforms to create more complex multi-organ systems.
Looking forward, the field is working toward standardization of protocols to enhance reproducibility across laboratories, automation of organoid generation and maintenance to increase throughput, and integration of functional readouts to better assess drug responses. The ultimate goal is to establish organoid technology as a mainstream tool in pharmaceutical development that significantly improves prediction of drug efficacy and safety in humans, thereby reducing attrition rates in clinical trials and accelerating the delivery of effective therapies to patients.
Prior to organoids, pharmaceutical research relied heavily on traditional two-dimensional cell cultures and animal models, both with significant limitations in recapitulating human physiology. Two-dimensional cultures lack the complex architecture and cellular interactions of native tissues, while animal models often fail to predict human-specific responses due to species differences. These limitations have contributed to high failure rates in drug development pipelines, with approximately 90% of drug candidates failing in clinical trials despite promising preclinical results.
The technological evolution of organoid systems has been marked by several key advancements. The identification and characterization of tissue-specific stem cells provided the cellular foundation. Concurrently, the development of specialized extracellular matrices and defined culture media containing tissue-specific growth factors enabled the creation of microenvironments supporting organoid formation. More recently, bioengineering approaches incorporating microfluidics and bioprinting have enhanced the complexity and functionality of organoid systems.
Today's organoid technology encompasses diverse tissue types including intestinal, liver, pancreatic, brain, kidney, and lung organoids, each with specific applications in pharmaceutical research. These systems aim to provide physiologically relevant models for drug screening, toxicity testing, and personalized medicine approaches that better predict human responses than conventional methods.
The primary objectives of organoid culture systems in pharmaceutical applications include establishing reproducible and scalable platforms for high-throughput drug screening, developing disease-specific models that accurately recapitulate pathophysiology, and creating patient-derived organoids for personalized medicine approaches. Additionally, researchers aim to integrate organoids with other technologies such as organ-on-chip platforms to create more complex multi-organ systems.
Looking forward, the field is working toward standardization of protocols to enhance reproducibility across laboratories, automation of organoid generation and maintenance to increase throughput, and integration of functional readouts to better assess drug responses. The ultimate goal is to establish organoid technology as a mainstream tool in pharmaceutical development that significantly improves prediction of drug efficacy and safety in humans, thereby reducing attrition rates in clinical trials and accelerating the delivery of effective therapies to patients.
Pharmaceutical Industry Demand for Advanced Drug Testing Models
The pharmaceutical industry is experiencing a paradigm shift in drug development methodologies, driven by escalating costs and high failure rates in traditional testing approaches. Current estimates indicate that bringing a single drug to market costs approximately $2.6 billion, with development timelines extending beyond 10 years. More concerning is the 90% failure rate of drug candidates in clinical trials, predominantly due to lack of efficacy or unexpected toxicity that wasn't detected in preclinical models.
Traditional drug testing platforms rely heavily on 2D cell cultures and animal models, both presenting significant limitations. Two-dimensional cell cultures fail to replicate the complex three-dimensional architecture and microenvironment of human tissues, resulting in poor predictive value for drug responses. Animal models, while providing systemic responses, often demonstrate poor translation to human outcomes due to fundamental species differences in physiology and metabolism.
These limitations have created urgent market demand for advanced drug testing models that better recapitulate human physiology. Pharmaceutical companies are actively seeking technologies that can provide more predictive preclinical data, thereby reducing late-stage clinical failures and associated financial losses. Industry surveys indicate that 78% of pharmaceutical R&D executives consider improving preclinical predictivity a top strategic priority.
Organoid culture systems have emerged as a promising solution to address these challenges. These three-dimensional cellular structures self-organize to mimic the architecture and functionality of native organs, offering significantly improved physiological relevance compared to conventional models. The global market for 3D cell culture technologies, including organoids, was valued at $1.1 billion in 2020 and is projected to grow at a CAGR of 14.8% through 2028.
Key market drivers include regulatory pressures to reduce animal testing, increasing focus on personalized medicine approaches, and the need for more efficient drug development pipelines. The FDA's Predictive Toxicology Roadmap specifically encourages the development and adoption of new technologies to better predict adverse effects in humans, creating regulatory momentum for organoid adoption.
Several pharmaceutical giants including Roche, AstraZeneca, and Merck have established dedicated programs exploring organoid applications in drug discovery and development. Industry partnerships with academic institutions and biotech companies specializing in organoid technologies have increased by 35% annually since 2018, highlighting the growing integration of these advanced models into pharmaceutical research workflows.
Traditional drug testing platforms rely heavily on 2D cell cultures and animal models, both presenting significant limitations. Two-dimensional cell cultures fail to replicate the complex three-dimensional architecture and microenvironment of human tissues, resulting in poor predictive value for drug responses. Animal models, while providing systemic responses, often demonstrate poor translation to human outcomes due to fundamental species differences in physiology and metabolism.
These limitations have created urgent market demand for advanced drug testing models that better recapitulate human physiology. Pharmaceutical companies are actively seeking technologies that can provide more predictive preclinical data, thereby reducing late-stage clinical failures and associated financial losses. Industry surveys indicate that 78% of pharmaceutical R&D executives consider improving preclinical predictivity a top strategic priority.
Organoid culture systems have emerged as a promising solution to address these challenges. These three-dimensional cellular structures self-organize to mimic the architecture and functionality of native organs, offering significantly improved physiological relevance compared to conventional models. The global market for 3D cell culture technologies, including organoids, was valued at $1.1 billion in 2020 and is projected to grow at a CAGR of 14.8% through 2028.
Key market drivers include regulatory pressures to reduce animal testing, increasing focus on personalized medicine approaches, and the need for more efficient drug development pipelines. The FDA's Predictive Toxicology Roadmap specifically encourages the development and adoption of new technologies to better predict adverse effects in humans, creating regulatory momentum for organoid adoption.
Several pharmaceutical giants including Roche, AstraZeneca, and Merck have established dedicated programs exploring organoid applications in drug discovery and development. Industry partnerships with academic institutions and biotech companies specializing in organoid technologies have increased by 35% annually since 2018, highlighting the growing integration of these advanced models into pharmaceutical research workflows.
Current Challenges in Organoid Culture Systems
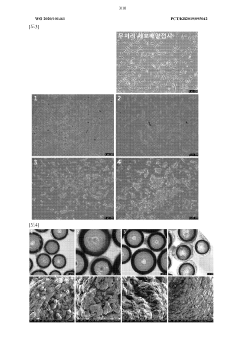
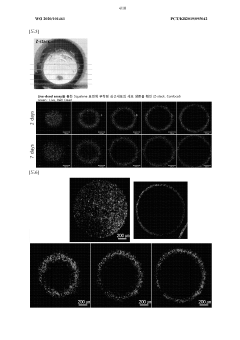
Despite significant advancements in organoid technology, several critical challenges persist in current organoid culture systems that limit their full potential in pharmaceutical applications. Reproducibility remains a primary concern, as variations in extracellular matrix components, growth factors, and culture conditions lead to inconsistent organoid formation and maturation. This variability creates obstacles for standardized drug screening protocols and reduces confidence in translating findings to clinical applications.
Scalability presents another significant hurdle, particularly for high-throughput pharmaceutical screening. Current methods often involve labor-intensive processes with low throughput, making large-scale drug testing economically unfeasible. The complex three-dimensional structure of organoids also complicates automated handling and imaging, further limiting industrial adoption.
Maturation limitations affect the physiological relevance of organoid models. Many organoid systems represent fetal or immature tissue states rather than adult functionality, potentially compromising their predictive value for drug efficacy and toxicity in adult patients. The absence of key mature cell types or functional properties in certain organoid systems creates blind spots in pharmaceutical testing.
Vascularization deficiency represents a critical physiological gap in current organoid systems. Without proper blood vessel networks, organoids face limitations in nutrient diffusion and waste removal, restricting their growth beyond certain dimensions and creating necrotic cores in larger structures. This absence of vasculature also prevents accurate modeling of drug distribution and metabolism, which depends heavily on circulatory systems.
Microenvironmental complexity remains inadequately recapitulated in most organoid platforms. The lack of immune components, stromal interactions, and mechanical forces that exist in native tissues limits the predictive capacity for immunomodulatory drugs and compounds affected by tissue-specific microenvironments.
Batch-to-batch variation in biological materials used for organoid culture introduces additional inconsistency. Matrigel and other animal-derived matrices exhibit compositional differences between lots, while patient-derived materials vary in quality and cellular composition, complicating standardization efforts essential for pharmaceutical validation.
Cost barriers further impede widespread adoption in drug discovery pipelines. Specialized media components, growth factors, and matrices required for organoid culture represent significant expenses that challenge cost-effectiveness compared to traditional cell culture methods, particularly during early-stage drug screening where large numbers of compounds need evaluation.
Scalability presents another significant hurdle, particularly for high-throughput pharmaceutical screening. Current methods often involve labor-intensive processes with low throughput, making large-scale drug testing economically unfeasible. The complex three-dimensional structure of organoids also complicates automated handling and imaging, further limiting industrial adoption.
Maturation limitations affect the physiological relevance of organoid models. Many organoid systems represent fetal or immature tissue states rather than adult functionality, potentially compromising their predictive value for drug efficacy and toxicity in adult patients. The absence of key mature cell types or functional properties in certain organoid systems creates blind spots in pharmaceutical testing.
Vascularization deficiency represents a critical physiological gap in current organoid systems. Without proper blood vessel networks, organoids face limitations in nutrient diffusion and waste removal, restricting their growth beyond certain dimensions and creating necrotic cores in larger structures. This absence of vasculature also prevents accurate modeling of drug distribution and metabolism, which depends heavily on circulatory systems.
Microenvironmental complexity remains inadequately recapitulated in most organoid platforms. The lack of immune components, stromal interactions, and mechanical forces that exist in native tissues limits the predictive capacity for immunomodulatory drugs and compounds affected by tissue-specific microenvironments.
Batch-to-batch variation in biological materials used for organoid culture introduces additional inconsistency. Matrigel and other animal-derived matrices exhibit compositional differences between lots, while patient-derived materials vary in quality and cellular composition, complicating standardization efforts essential for pharmaceutical validation.
Cost barriers further impede widespread adoption in drug discovery pipelines. Specialized media components, growth factors, and matrices required for organoid culture represent significant expenses that challenge cost-effectiveness compared to traditional cell culture methods, particularly during early-stage drug screening where large numbers of compounds need evaluation.
Established Organoid Culture Methodologies for Drug Testing
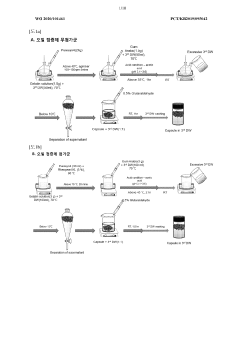
01 Matrix composition optimization for organoid culture
The composition of the extracellular matrix used in organoid culture systems significantly impacts organoid development and functionality. Optimizing the matrix components, such as using specific combinations of laminin, collagen, and other basement membrane proteins, can enhance organoid formation, structural organization, and cellular differentiation. Advanced matrix formulations may include growth factor-enriched hydrogels or synthetic matrices with tunable mechanical properties to better mimic the native tissue environment.- Matrix composition and scaffold design for organoid culture: The composition of extracellular matrix and scaffold design plays a crucial role in optimizing organoid culture systems. Specific matrix components can promote cell adhesion, proliferation, and differentiation, leading to more physiologically relevant organoid development. Advanced scaffold designs incorporating biocompatible materials can provide structural support while allowing for proper nutrient diffusion and cellular organization within the organoid structure.
- Growth factor and signaling pathway modulation: Optimization of growth factor combinations and concentrations is essential for directing organoid development. By modulating specific signaling pathways through timed introduction of growth factors, researchers can control stem cell differentiation and organoid maturation. This approach allows for the creation of more complex and functionally mature organoids that better recapitulate in vivo tissue architecture and function.
- Automated monitoring and culture condition control systems: Implementation of automated systems for continuous monitoring and adjustment of culture conditions enhances organoid development consistency. These systems can regulate parameters such as temperature, pH, oxygen levels, and nutrient availability in real-time, ensuring optimal growth conditions. Machine learning algorithms can analyze growth patterns and automatically adjust culture parameters to maximize organoid quality and reproducibility.
- Co-culture techniques and vascularization strategies: Advanced co-culture techniques incorporating multiple cell types can enhance organoid complexity and functionality. Integration of endothelial cells and supporting stromal components enables the development of vascularized organoids with improved nutrient delivery and waste removal. These approaches result in larger, more mature organoids that better mimic the cellular heterogeneity and interactions found in native tissues.
- Bioreactor design and scale-up technologies: Specialized bioreactor systems designed specifically for organoid culture facilitate scaling up production while maintaining quality. These systems incorporate optimized mixing, perfusion, and mechanical stimulation to enhance nutrient distribution and waste removal. Advanced bioreactors can support long-term culture of larger organoids and enable high-throughput applications for drug screening and personalized medicine approaches.
02 Growth factor and signaling molecule optimization
Precise combinations and concentrations of growth factors and signaling molecules are critical for successful organoid culture. Optimization involves determining the ideal temporal sequence of factor addition, concentration gradients, and synergistic combinations that promote specific developmental pathways. These factors can be tailored to enhance proliferation, differentiation, or maturation phases of organoid development, resulting in more physiologically relevant in vitro models.Expand Specific Solutions03 Culture medium formulation and feeding strategies
Optimizing culture medium formulations involves balancing nutrients, hormones, vitamins, and trace elements to support organoid growth while preventing differentiation into unwanted cell types. Advanced feeding strategies include perfusion systems, timed-release of factors, and metabolite monitoring to maintain optimal conditions. Customized media formulations for different organoid types or developmental stages can significantly improve organoid viability, longevity, and functionality.Expand Specific Solutions04 Physical and mechanical parameter optimization
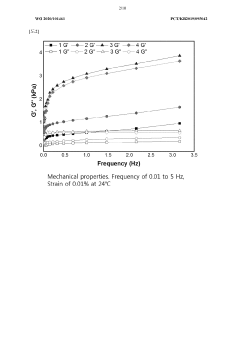
The physical and mechanical parameters of organoid culture systems, including oxygen tension, temperature gradients, mechanical stimulation, and spatial organization, significantly impact organoid development. Optimization may involve using bioreactors with controlled mechanical forces, oxygen tension regulators, or microfluidic devices that create physiologically relevant microenvironments. These parameters can be adjusted to promote specific tissue architectures or functional characteristics in the developing organoids.Expand Specific Solutions05 Computational modeling and AI-based optimization
Advanced computational approaches and artificial intelligence can accelerate the optimization of organoid culture systems. Machine learning algorithms can analyze large datasets of culture conditions and outcomes to identify optimal parameter combinations. Predictive modeling helps design experiments more efficiently by simulating organoid development under various conditions. These computational tools enable systematic optimization of multiple parameters simultaneously, reducing the time and resources required for empirical testing.Expand Specific Solutions
Leading Organizations in Organoid Research and Development
Organoid culture systems are currently in a growth phase within pharmaceutical applications, with the market expanding rapidly due to increasing adoption in drug discovery and personalized medicine. The global market size is estimated to reach several billion dollars by 2025, driven by the technology's ability to better predict drug responses compared to traditional 2D cell cultures. Technical maturity varies across applications, with companies demonstrating different specialization levels. Leading players like STEMCELL Technologies and Cellesce have developed standardized production platforms, while Xilis and Tempus AI integrate AI for precision medicine applications. Academic institutions (Duke University, University of Washington) collaborate with industry partners like Cell Microsystems to advance microfluidic technologies, creating a dynamic ecosystem that bridges research innovation with commercial pharmaceutical applications.
Cellesce Ltd.
Technical Solution: Cellesce has developed a bioprocessing platform specifically designed for the scaled production of organoids for drug discovery and screening applications. Their proprietary technology uses a bioreactor-based expansion system that enables the mass production of standardized organoids while maintaining their complex 3D structure and genetic stability. The system employs controlled mechanical stimulation and optimized nutrient delivery to enhance organoid growth and uniformity. Cellesce's platform addresses a critical bottleneck in pharmaceutical applications by enabling the production of organoids at scale with reduced batch-to-batch variability. Their technology has been particularly successful with colorectal cancer organoids, providing pharmaceutical companies with consistent, high-quality patient-derived models for drug efficacy and toxicity testing. The company has also developed specialized protocols for cryopreservation and recovery that maintain organoid functionality.
Strengths: Scalable production capability addressing pharmaceutical industry volume requirements; enhanced standardization reducing experimental variability; automated processes reducing labor costs and human error; demonstrated success with colorectal cancer models. Weaknesses: Limited organ diversity compared to some competitors; significant initial investment required for implementation; technology may require adaptation for certain specialized organoid types; dependency on specific equipment systems.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has pioneered comprehensive organoid culture systems through their IntestiCult™ and other specialized media platforms designed to optimize pharmaceutical applications. Their technology provides defined, serum-free culture conditions that support long-term expansion of organoids while maintaining physiological relevance. The company has developed specialized matrices (like Matrigel® alternatives) and growth factor combinations that enhance organoid formation efficiency and reproducibility. Their systems support various organoid types including intestinal, hepatic, pancreatic, and cerebral organoids, making them versatile for drug screening applications. STEMCELL's platforms incorporate quality control measures to ensure batch-to-batch consistency critical for pharmaceutical validation studies, and they've developed cryopreservation protocols that maintain organoid viability and functionality after thawing.
Strengths: Comprehensive portfolio covering multiple organ systems; established quality control processes ensuring reproducibility; extensive technical support and validated protocols; compatible with high-throughput screening platforms. Weaknesses: Relatively high cost of specialized media components; some systems still require animal-derived components; optimization may be needed for specific pharmaceutical applications; learning curve for new users implementing the technology.
Critical Innovations in Organoid Matrix and Growth Factors
Organoid produced using carrier for cell culture, and method for evaluating drug toxicity using same
PatentWO2020101461A1
Innovation
- The development of organoids cultured using microcapsules containing gelatin, natural polymers, and oil thickener as a cell culture carrier, which allows for the creation of 3D organoids that mimic organ functions and respond to drug toxicity, enabling more accurate drug testing and personalized treatment approaches.
High-throughput automation of organoids for identifying therapeutic strategies
PatentActiveUS20210290632A1
Innovation
- A method involving the use of myosin II activators, such as thiadiazinone compounds, to prevent or shrink cysts in PKD, combined with an automated high-throughput screening platform using phenotypic organoid models derived from pluripotent stem cells to test therapeutic compound candidates.
Regulatory Framework for Organoid-Based Pharmaceutical Testing
The regulatory landscape for organoid-based pharmaceutical testing is evolving rapidly as these advanced 3D cellular models gain traction in drug development pipelines. Currently, the FDA, EMA, and other global regulatory bodies are working to establish standardized frameworks that accommodate these novel testing platforms while ensuring patient safety remains paramount.
Regulatory acceptance of organoid models requires validation against established testing methods. The FDA's Predictive Toxicology Roadmap (2017) specifically acknowledges the potential of advanced in vitro systems, including organoids, as alternatives to traditional animal testing. Similarly, the EMA has published guidance documents addressing the qualification process for novel methodologies in drug development that can be applied to organoid systems.
The ICH (International Council for Harmonisation) guidelines, particularly ICH S7A and ICH S7B for safety pharmacology studies, provide foundational frameworks that companies are adapting for organoid-based testing. These guidelines emphasize the importance of demonstrating correlation between in vitro results and clinical outcomes, a critical hurdle for organoid technology adoption.
Regulatory challenges specific to organoid systems include concerns about reproducibility, standardization, and validation. The heterogeneity inherent in organoids derived from different donors presents unique regulatory considerations regarding batch consistency and quality control. Regulatory bodies increasingly require robust protocols for organoid generation, characterization, and maintenance to ensure reliable pharmaceutical testing results.
The path toward regulatory acceptance is being facilitated through collaborative initiatives. The FDA's Organs-on-Chips program and the EMA's Innovation Task Force actively engage with researchers and industry to develop appropriate validation frameworks. Additionally, the OECD's Adverse Outcome Pathway framework provides a mechanistic foundation for linking organoid responses to human health outcomes, strengthening regulatory confidence.
Recent regulatory milestones include the FDA's acceptance of certain organoid data as supporting evidence in IND (Investigational New Drug) applications, though not yet as standalone alternatives to traditional testing. The EU's commitment to reducing animal testing through Directive 2010/63/EU has created regulatory incentives for developing and validating organoid-based alternatives, particularly for toxicity testing.
Looking forward, regulatory frameworks are expected to evolve toward a hybrid approach, where organoid testing complements traditional methods before potentially replacing some aspects of conventional testing paradigms. This transition will require continued dialogue between researchers, industry stakeholders, and regulatory authorities to establish consensus standards that balance innovation with patient safety.
Regulatory acceptance of organoid models requires validation against established testing methods. The FDA's Predictive Toxicology Roadmap (2017) specifically acknowledges the potential of advanced in vitro systems, including organoids, as alternatives to traditional animal testing. Similarly, the EMA has published guidance documents addressing the qualification process for novel methodologies in drug development that can be applied to organoid systems.
The ICH (International Council for Harmonisation) guidelines, particularly ICH S7A and ICH S7B for safety pharmacology studies, provide foundational frameworks that companies are adapting for organoid-based testing. These guidelines emphasize the importance of demonstrating correlation between in vitro results and clinical outcomes, a critical hurdle for organoid technology adoption.
Regulatory challenges specific to organoid systems include concerns about reproducibility, standardization, and validation. The heterogeneity inherent in organoids derived from different donors presents unique regulatory considerations regarding batch consistency and quality control. Regulatory bodies increasingly require robust protocols for organoid generation, characterization, and maintenance to ensure reliable pharmaceutical testing results.
The path toward regulatory acceptance is being facilitated through collaborative initiatives. The FDA's Organs-on-Chips program and the EMA's Innovation Task Force actively engage with researchers and industry to develop appropriate validation frameworks. Additionally, the OECD's Adverse Outcome Pathway framework provides a mechanistic foundation for linking organoid responses to human health outcomes, strengthening regulatory confidence.
Recent regulatory milestones include the FDA's acceptance of certain organoid data as supporting evidence in IND (Investigational New Drug) applications, though not yet as standalone alternatives to traditional testing. The EU's commitment to reducing animal testing through Directive 2010/63/EU has created regulatory incentives for developing and validating organoid-based alternatives, particularly for toxicity testing.
Looking forward, regulatory frameworks are expected to evolve toward a hybrid approach, where organoid testing complements traditional methods before potentially replacing some aspects of conventional testing paradigms. This transition will require continued dialogue between researchers, industry stakeholders, and regulatory authorities to establish consensus standards that balance innovation with patient safety.
Cost-Benefit Analysis of Organoid Systems vs Traditional Models
The economic evaluation of organoid culture systems compared to traditional pharmaceutical testing models reveals a complex cost-benefit landscape. Initially, organoid systems require significantly higher setup investments, with specialized equipment, advanced culture media, and skilled personnel representing substantial upfront costs. Estimates suggest that establishing a comprehensive organoid platform may cost 3-5 times more than conventional cell culture systems.
However, the long-term economic benefits become increasingly apparent when considering the pharmaceutical development pipeline. Traditional animal models and 2D cell cultures demonstrate a concerning 90% failure rate of drug candidates in clinical trials, with each failed drug costing pharmaceutical companies approximately $800 million to $1.4 billion. Organoid systems have demonstrated improved predictive validity, potentially reducing late-stage clinical failures by 25-30% according to recent industry analyses.
The resource efficiency of organoid systems further enhances their economic value proposition. These systems require smaller quantities of test compounds, reducing material costs by up to 60% compared to animal testing. Additionally, the accelerated testing timeline—approximately 2-3 weeks for organoid assays versus 2-3 months for animal studies—translates to significant time-cost savings in the development process.
Ethical considerations also factor into the cost-benefit equation. Regulatory pressures to reduce animal testing have financial implications, with organoid systems offering compliance advantages that may prevent costly regulatory delays. Several pharmaceutical companies report saving $2-5 million per drug development program through reduced animal testing requirements.
Scalability represents another economic dimension, with automated organoid platforms demonstrating potential for high-throughput screening at costs approaching traditional cell-based assays when operated at scale. Recent technological advancements have reduced per-sample testing costs by approximately 40% over the past five years.
The return on investment timeline remains a critical consideration. While traditional models offer immediate operational familiarity, organoid systems typically require 12-18 months to achieve operational efficiency but subsequently deliver superior cost-effectiveness over the product development lifecycle. Industry projections suggest that comprehensive integration of organoid technologies could reduce overall drug development costs by 15-20% while simultaneously increasing success rates.
However, the long-term economic benefits become increasingly apparent when considering the pharmaceutical development pipeline. Traditional animal models and 2D cell cultures demonstrate a concerning 90% failure rate of drug candidates in clinical trials, with each failed drug costing pharmaceutical companies approximately $800 million to $1.4 billion. Organoid systems have demonstrated improved predictive validity, potentially reducing late-stage clinical failures by 25-30% according to recent industry analyses.
The resource efficiency of organoid systems further enhances their economic value proposition. These systems require smaller quantities of test compounds, reducing material costs by up to 60% compared to animal testing. Additionally, the accelerated testing timeline—approximately 2-3 weeks for organoid assays versus 2-3 months for animal studies—translates to significant time-cost savings in the development process.
Ethical considerations also factor into the cost-benefit equation. Regulatory pressures to reduce animal testing have financial implications, with organoid systems offering compliance advantages that may prevent costly regulatory delays. Several pharmaceutical companies report saving $2-5 million per drug development program through reduced animal testing requirements.
Scalability represents another economic dimension, with automated organoid platforms demonstrating potential for high-throughput screening at costs approaching traditional cell-based assays when operated at scale. Recent technological advancements have reduced per-sample testing costs by approximately 40% over the past five years.
The return on investment timeline remains a critical consideration. While traditional models offer immediate operational familiarity, organoid systems typically require 12-18 months to achieve operational efficiency but subsequently deliver superior cost-effectiveness over the product development lifecycle. Industry projections suggest that comprehensive integration of organoid technologies could reduce overall drug development costs by 15-20% while simultaneously increasing success rates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!