Regulatory Frameworks in Emerging Organoid Culture Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering. Since the first successful establishment of intestinal organoids in 2009 by the Clevers laboratory, this field has experienced exponential growth across multiple tissue types including brain, liver, kidney, and pancreas. Organoids are three-dimensional cellular structures that self-organize to recapitulate key structural and functional aspects of their corresponding organs, offering unprecedented opportunities for disease modeling, drug discovery, and personalized medicine.
The evolution of organoid technology has been marked by significant milestones in culture methodology, from simple Matrigel-based systems to more complex bioengineered matrices. Recent innovations have focused on improving organoid maturation, vascularization, and functional integration, addressing limitations of early-generation systems. The incorporation of microfluidic technologies and bioprinting has further enhanced the physiological relevance of these miniature organ models.
Current technical objectives in the organoid field center on establishing standardized protocols for reproducible organoid generation across laboratories. This standardization is crucial for translational applications and regulatory acceptance. Additionally, researchers aim to develop more defined culture conditions that eliminate animal-derived components like Matrigel, which introduce variability and complicate regulatory pathways for clinical applications.
A primary goal in organoid technology advancement is the creation of regulatory frameworks that can accommodate these novel biological entities. Unlike traditional cell cultures or animal models, organoids occupy a unique position that challenges existing regulatory paradigms. The development of appropriate quality control metrics, safety assessments, and validation standards represents a critical objective for the field's maturation.
The intersection of organoid technology with precision medicine constitutes another key objective, with efforts directed toward patient-derived organoids for personalized drug screening and therapeutic development. This application holds particular promise for rare diseases and cancer, where treatment responses vary significantly between individuals.
Long-term technical aspirations include the development of multi-organ systems or "body-on-a-chip" platforms that can model complex physiological interactions. These integrated systems aim to better predict systemic drug responses and toxicities, potentially reducing reliance on animal testing and improving clinical trial success rates.
As organoid technology continues to advance, establishing clear regulatory pathways will be essential for realizing its full potential in both research and clinical settings. The field's trajectory suggests a future where organoids become standard tools in drug development pipelines and potentially direct therapeutic applications, including transplantation and regenerative medicine.
The evolution of organoid technology has been marked by significant milestones in culture methodology, from simple Matrigel-based systems to more complex bioengineered matrices. Recent innovations have focused on improving organoid maturation, vascularization, and functional integration, addressing limitations of early-generation systems. The incorporation of microfluidic technologies and bioprinting has further enhanced the physiological relevance of these miniature organ models.
Current technical objectives in the organoid field center on establishing standardized protocols for reproducible organoid generation across laboratories. This standardization is crucial for translational applications and regulatory acceptance. Additionally, researchers aim to develop more defined culture conditions that eliminate animal-derived components like Matrigel, which introduce variability and complicate regulatory pathways for clinical applications.
A primary goal in organoid technology advancement is the creation of regulatory frameworks that can accommodate these novel biological entities. Unlike traditional cell cultures or animal models, organoids occupy a unique position that challenges existing regulatory paradigms. The development of appropriate quality control metrics, safety assessments, and validation standards represents a critical objective for the field's maturation.
The intersection of organoid technology with precision medicine constitutes another key objective, with efforts directed toward patient-derived organoids for personalized drug screening and therapeutic development. This application holds particular promise for rare diseases and cancer, where treatment responses vary significantly between individuals.
Long-term technical aspirations include the development of multi-organ systems or "body-on-a-chip" platforms that can model complex physiological interactions. These integrated systems aim to better predict systemic drug responses and toxicities, potentially reducing reliance on animal testing and improving clinical trial success rates.
As organoid technology continues to advance, establishing clear regulatory pathways will be essential for realizing its full potential in both research and clinical settings. The field's trajectory suggests a future where organoids become standard tools in drug development pipelines and potentially direct therapeutic applications, including transplantation and regenerative medicine.
Market Analysis of Organoid Culture Applications
The organoid culture systems market has witnessed substantial growth in recent years, driven primarily by increasing applications in drug discovery, personalized medicine, and disease modeling. The global organoid market was valued at approximately 1.3 billion USD in 2022 and is projected to reach 5.2 billion USD by 2030, representing a compound annual growth rate (CAGR) of 19.8% during the forecast period.
Drug discovery and development applications currently dominate the market, accounting for nearly 40% of the total market share. This segment's prominence stems from the pharmaceutical industry's growing adoption of organoid technologies to reduce drug attrition rates and development costs. Organoids provide more physiologically relevant models compared to traditional 2D cell cultures, enabling more accurate prediction of drug efficacy and toxicity in humans.
Personalized medicine represents the fastest-growing application segment, with an estimated CAGR of 22.3%. This growth is fueled by increasing interest in patient-derived organoids for treatment selection and monitoring therapeutic responses, particularly in oncology. Several academic medical centers and specialized companies now offer patient-derived organoid services for clinical decision support.
Regionally, North America holds the largest market share at approximately 42%, followed by Europe (30%) and Asia-Pacific (20%). The dominance of North America can be attributed to substantial research funding, presence of major pharmaceutical companies, and advanced healthcare infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing R&D investments, improving healthcare infrastructure, and growing awareness about precision medicine approaches.
Key end-user segments include pharmaceutical and biotechnology companies (45%), academic and research institutes (30%), and hospitals and diagnostic centers (25%). The pharmaceutical sector's significant share reflects the industry's substantial investment in adopting organoid technologies to enhance drug development processes.
Despite promising growth prospects, market penetration faces challenges related to regulatory uncertainties, high costs associated with organoid development and maintenance, and technical complexities in standardization. The lack of clear regulatory frameworks specifically addressing organoid technologies creates hesitancy among potential commercial adopters, particularly in clinical applications.
Emerging application areas showing significant potential include regenerative medicine, toxicology testing, and infectious disease research. The COVID-19 pandemic has particularly accelerated interest in using organoid systems for studying viral pathogenesis and screening potential therapeutic agents.
Drug discovery and development applications currently dominate the market, accounting for nearly 40% of the total market share. This segment's prominence stems from the pharmaceutical industry's growing adoption of organoid technologies to reduce drug attrition rates and development costs. Organoids provide more physiologically relevant models compared to traditional 2D cell cultures, enabling more accurate prediction of drug efficacy and toxicity in humans.
Personalized medicine represents the fastest-growing application segment, with an estimated CAGR of 22.3%. This growth is fueled by increasing interest in patient-derived organoids for treatment selection and monitoring therapeutic responses, particularly in oncology. Several academic medical centers and specialized companies now offer patient-derived organoid services for clinical decision support.
Regionally, North America holds the largest market share at approximately 42%, followed by Europe (30%) and Asia-Pacific (20%). The dominance of North America can be attributed to substantial research funding, presence of major pharmaceutical companies, and advanced healthcare infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing R&D investments, improving healthcare infrastructure, and growing awareness about precision medicine approaches.
Key end-user segments include pharmaceutical and biotechnology companies (45%), academic and research institutes (30%), and hospitals and diagnostic centers (25%). The pharmaceutical sector's significant share reflects the industry's substantial investment in adopting organoid technologies to enhance drug development processes.
Despite promising growth prospects, market penetration faces challenges related to regulatory uncertainties, high costs associated with organoid development and maintenance, and technical complexities in standardization. The lack of clear regulatory frameworks specifically addressing organoid technologies creates hesitancy among potential commercial adopters, particularly in clinical applications.
Emerging application areas showing significant potential include regenerative medicine, toxicology testing, and infectious disease research. The COVID-19 pandemic has particularly accelerated interest in using organoid systems for studying viral pathogenesis and screening potential therapeutic agents.
Current Regulatory Landscape and Technical Challenges
The regulatory landscape for organoid culture systems remains fragmented and evolving, with significant variations across different jurisdictions. In the United States, the FDA has not yet established specific regulatory frameworks dedicated to organoid technologies, instead applying existing regulations for biological products, medical devices, or combination products depending on the intended use. The European Medicines Agency has taken similar approaches, though with greater emphasis on ethical considerations through the European Group on Ethics in Science and New Technologies.
Japan's regulatory system offers a potential model through its conditional approval pathway for regenerative medicine, which could accelerate organoid-based therapies while collecting post-market data. This approach balances innovation with safety concerns that are particularly relevant to organoid technologies.
A primary technical challenge in the regulatory space is standardization. Current organoid culture systems exhibit significant batch-to-batch variability, making it difficult to establish consistent quality control parameters required by regulatory bodies. This variability stems from multiple factors including heterogeneous starting materials, complex extracellular matrix components, and stochastic developmental processes inherent to organoid formation.
Validation protocols represent another significant hurdle. Traditional validation methods developed for 2D cell cultures or animal models are often inadequate for 3D organoid systems. Regulatory agencies require demonstration of reproducibility and predictive value, yet the complex nature of organoids makes establishing these parameters technically challenging.
The use of animal-derived components in many organoid culture systems presents additional regulatory complications. Matrigel, the most commonly used matrix for organoid culture, contains undefined animal components that introduce variability and potential safety concerns from a regulatory perspective. Developing chemically defined alternatives remains a critical technical challenge.
Data standardization across different organoid platforms also presents significant obstacles. The lack of harmonized reporting standards for organoid characteristics, functional assessments, and experimental conditions hampers regulatory evaluation and cross-study comparisons.
Ethical considerations further complicate the regulatory landscape, particularly for brain organoids and reproductive tissue models. Current frameworks struggle to address questions regarding the moral status of increasingly complex organoid systems, consent for donor materials, and appropriate limits for organoid development.
The intersection of these technical and regulatory challenges necessitates collaborative approaches between researchers, industry stakeholders, and regulatory bodies to develop appropriate frameworks that ensure safety while enabling scientific progress in this rapidly evolving field.
Japan's regulatory system offers a potential model through its conditional approval pathway for regenerative medicine, which could accelerate organoid-based therapies while collecting post-market data. This approach balances innovation with safety concerns that are particularly relevant to organoid technologies.
A primary technical challenge in the regulatory space is standardization. Current organoid culture systems exhibit significant batch-to-batch variability, making it difficult to establish consistent quality control parameters required by regulatory bodies. This variability stems from multiple factors including heterogeneous starting materials, complex extracellular matrix components, and stochastic developmental processes inherent to organoid formation.
Validation protocols represent another significant hurdle. Traditional validation methods developed for 2D cell cultures or animal models are often inadequate for 3D organoid systems. Regulatory agencies require demonstration of reproducibility and predictive value, yet the complex nature of organoids makes establishing these parameters technically challenging.
The use of animal-derived components in many organoid culture systems presents additional regulatory complications. Matrigel, the most commonly used matrix for organoid culture, contains undefined animal components that introduce variability and potential safety concerns from a regulatory perspective. Developing chemically defined alternatives remains a critical technical challenge.
Data standardization across different organoid platforms also presents significant obstacles. The lack of harmonized reporting standards for organoid characteristics, functional assessments, and experimental conditions hampers regulatory evaluation and cross-study comparisons.
Ethical considerations further complicate the regulatory landscape, particularly for brain organoids and reproductive tissue models. Current frameworks struggle to address questions regarding the moral status of increasingly complex organoid systems, consent for donor materials, and appropriate limits for organoid development.
The intersection of these technical and regulatory challenges necessitates collaborative approaches between researchers, industry stakeholders, and regulatory bodies to develop appropriate frameworks that ensure safety while enabling scientific progress in this rapidly evolving field.
Current Compliance Approaches for Organoid Systems
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve embedding stem cells or tissue-derived cells in extracellular matrix components to support self-organization into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and development of tissue architecture that better represents in vivo conditions compared to traditional 2D cultures.- 3D organoid culture systems and methods: Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems involve culturing stem cells or progenitor cells in specific conditions that allow them to self-organize into organ-like structures. The methods include providing appropriate extracellular matrix components, growth factors, and other signaling molecules to support organoid development. These 3D culture systems are valuable for studying organ development, disease modeling, and drug screening.
- Stem cell-derived organoid technologies: Technologies for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve directing stem cell differentiation toward specific lineages and then supporting their self-organization into organoids. The technologies include specific culture conditions, growth factor combinations, and temporal regulation of signaling pathways to recapitulate developmental processes in vitro.
- Specialized culture media and supplements for organoid growth: Formulations of culture media and supplements specifically designed to support the growth and maintenance of organoids. These formulations include defined combinations of growth factors, hormones, small molecules, and nutrients that promote organoid formation, growth, and functional maturation. The media compositions are tailored to specific organoid types and can be optimized for long-term culture and preservation of tissue-specific functions.
- Organoid culture platforms and devices: Specialized platforms, devices, and bioreactors designed for organoid culture. These include microfluidic systems, perfusion bioreactors, and other engineered platforms that provide controlled environments for organoid growth. Such systems can incorporate features like controlled fluid flow, mechanical stimulation, and gradient formation to better mimic physiological conditions. These platforms enhance organoid development, maturation, and functionality while allowing for real-time monitoring and analysis.
- Disease modeling and drug screening using organoid systems: Applications of organoid culture systems for modeling diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, allowing for personalized medicine approaches. These systems enable high-throughput drug screening, toxicity testing, and efficacy assessment in physiologically relevant models. The methods include generating disease-specific organoids, introducing genetic modifications, and developing assays to evaluate compound effects on organoid growth, morphology, and function.
02 Growth Media and Supplements for Organoid Culture
Specialized growth media formulations and supplements that support organoid development and maintenance. These include defined combinations of growth factors, hormones, and small molecules that promote stem cell proliferation, differentiation, and self-organization into organoids. The media compositions are often tissue-specific and designed to recapitulate the signaling environment of the native organ.Expand Specific Solutions03 Disease Modeling with Organoid Systems
Applications of organoid culture systems for modeling human diseases. Patient-derived organoids can be used to study disease mechanisms, progression, and response to treatments. These models are particularly valuable for personalized medicine approaches, allowing for testing of therapeutic interventions on patient-specific tissues outside the body. Disease models include cancer, genetic disorders, and infectious diseases.Expand Specific Solutions04 Bioengineered Scaffolds and Matrices for Organoid Culture
Advanced biomaterials and scaffolds designed to support organoid growth and development. These include natural and synthetic hydrogels, microfluidic devices, and bioprinted structures that provide mechanical support and biochemical cues for organoid formation. The scaffolds can be engineered to control stiffness, porosity, and degradation rates to optimize organoid culture conditions.Expand Specific Solutions05 Organoid Preservation and Banking Technologies
Methods for long-term preservation and banking of organoids for research and clinical applications. These technologies include cryopreservation protocols, vitrification techniques, and storage systems that maintain organoid viability and functionality. Preservation methods enable the creation of biobanks of patient-derived organoids that can be used for drug screening, personalized medicine, and regenerative therapy development.Expand Specific Solutions
Key Stakeholders in Organoid Technology Regulation
The regulatory landscape for organoid culture systems is evolving rapidly as this emerging technology transitions from early research to clinical applications. The market is experiencing significant growth, projected to expand as organoids gain traction in drug discovery, personalized medicine, and regenerative therapies. Technologically, the field shows varying maturity levels across different applications. Leading academic institutions like Peking University, Johns Hopkins University, and EPFL are driving fundamental research, while companies such as STEMCELL Technologies, Mimetas, Cell Microsystems, and Organoidsciences are commercializing enabling technologies. Biotechnology firms including Tempus AI and Ardelyx are exploring clinical applications, with regulatory frameworks still developing to address the unique challenges of these complex 3D cellular systems, particularly regarding standardization, safety assessment, and ethical considerations.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has pioneered comprehensive regulatory frameworks for their organoid culture systems, focusing on standardization and reproducibility. Their approach includes the development of defined, animal component-free culture media (such as IntestiCult™ and CerebralCult™) that meet stringent regulatory requirements for consistency and safety. The company has implemented a robust quality management system that encompasses all aspects of organoid culture, from source material validation to final product characterization, ensuring compliance with international standards including ISO 13485. STEMCELL has developed detailed documentation protocols that address traceability concerns in organoid research, providing researchers with standardized reporting templates that satisfy regulatory requirements across different jurisdictions. They actively collaborate with regulatory agencies to establish consensus guidelines for organoid applications in drug discovery and personalized medicine, particularly addressing the complex ethical considerations surrounding patient-derived organoids.
Strengths: Comprehensive quality control systems ensure batch-to-batch consistency of culture components, addressing a major regulatory challenge in organoid research. Their established global presence facilitates navigation of diverse international regulatory requirements. Weaknesses: Their focus on standardized commercial reagents may limit customization options needed for certain specialized organoid applications, potentially restricting innovation in emerging research areas.
Organoidsciences Ltd.
Technical Solution: Organoidsciences has developed a specialized regulatory framework focused on clinical applications of organoid technology. Their approach centers on establishing a regulatory pathway for organoid-based diagnostic and therapeutic applications, with particular emphasis on personalized medicine applications. The company has implemented a comprehensive validation system that addresses the specific challenges of organoid-based diagnostics, including standardized protocols for sample processing, organoid generation, and functional characterization that meet clinical laboratory standards. Their regulatory strategy includes detailed documentation systems that establish traceability from patient sample to diagnostic result, creating audit trails that satisfy healthcare regulatory requirements. Organoidsciences has pioneered quality control metrics specifically designed for patient-derived organoids, including genomic fidelity assessment, morphological characterization, and functional validation protocols that align with emerging regulatory guidance. They actively engage with regulatory agencies to develop frameworks for the validation and approval of organoid-based companion diagnostics, particularly in oncology applications.
Strengths: Specialized focus on clinical applications provides depth in addressing the most stringent regulatory requirements for medical use of organoids. Their established quality control metrics for patient-derived organoids address a critical gap in the regulatory landscape. Weaknesses: Their narrow focus on clinical applications may limit broader applicability of their regulatory frameworks to basic research contexts, and as a smaller company, they may have limited resources for influencing global regulatory standards.
Critical Regulatory Guidelines and Scientific Literature
Engineering of organoid culture for enhanced organogenesis in a dish
PatentPendingUS20240026261A1
Innovation
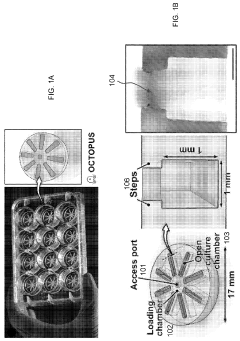
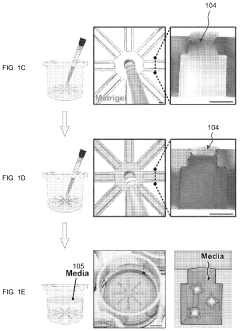
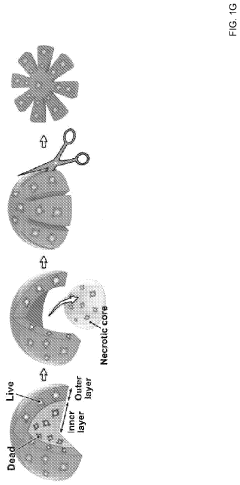
- The OCTOPUS device provides a 3D culture system with radially arranged culture chambers that reduce diffusion limitations by allowing unrestricted access to nutrients and oxygen, enabling continuous culture of organoids for extended periods without passaging, using a simple and scalable design compatible with standard cell culture plates.
Automated cell passaging
PatentWO2024196827A1
Innovation
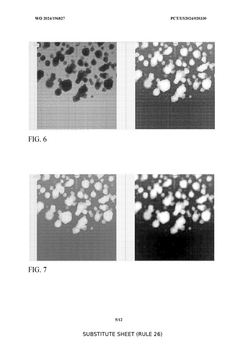
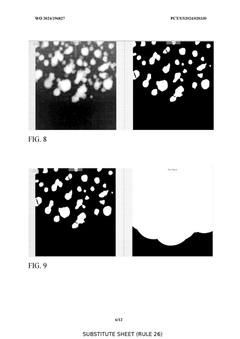
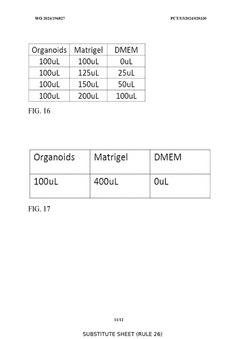
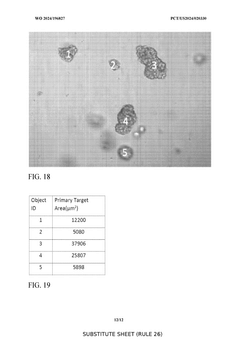
- A substantially automated process for identifying and disrupting extracellular matrix structures using a liquid handler with pipette tips, involving image processing to determine x,y coordinates and patterns for disrupting the ECM, allowing for precise removal and fragmentation of cells, and subsequent seeding in new culture plates.
Ethical Considerations in Organoid Research
The ethical landscape surrounding organoid research presents complex challenges that require careful consideration as regulatory frameworks continue to evolve. Organoids—three-dimensional cellular structures that mimic organ functionality—raise profound questions about human dignity, consent, and the boundaries of scientific exploration.
Patient consent and donor rights constitute a primary ethical concern in organoid research. When human cells are used to develop organoids, questions arise regarding the extent to which donors should maintain control over their biological materials after donation. Current practices vary significantly across jurisdictions, with some regions requiring ongoing consent for new research applications while others implement one-time consent models.
The potential development of cerebral organoids introduces particularly sensitive ethical considerations. As these brain-like structures become increasingly sophisticated, researchers and ethicists debate whether such entities might eventually develop rudimentary consciousness or sentience. This possibility necessitates the establishment of clear boundaries regarding the permissible complexity of cerebral organoid development.
Commercialization presents another significant ethical dimension. The patenting of organoid technologies and cell lines derived from patient samples raises questions about equitable benefit sharing. When therapeutic applications emerge from organoid research using donated tissues, determining fair compensation or acknowledgment for donors becomes ethically imperative.
Cultural and religious perspectives further complicate the ethical landscape. Various traditions hold differing views on the moral status of human tissues and embryonic materials often used in organoid development. Regulatory frameworks must navigate these diverse perspectives while establishing scientifically sound guidelines.
Data privacy concerns also emerge as organoids derived from specific patients may contain genetic information with implications beyond the individual donor. The potential for organoids to reveal familial genetic traits necessitates robust privacy protections within regulatory structures.
International harmonization of ethical standards represents a critical challenge. The global nature of scientific research means that inconsistent ethical frameworks across countries could lead to "ethics shopping," where researchers conduct controversial work in jurisdictions with less stringent oversight. Developing consensus-based international guidelines would help prevent such practices while promoting responsible advancement of organoid technologies.
Patient consent and donor rights constitute a primary ethical concern in organoid research. When human cells are used to develop organoids, questions arise regarding the extent to which donors should maintain control over their biological materials after donation. Current practices vary significantly across jurisdictions, with some regions requiring ongoing consent for new research applications while others implement one-time consent models.
The potential development of cerebral organoids introduces particularly sensitive ethical considerations. As these brain-like structures become increasingly sophisticated, researchers and ethicists debate whether such entities might eventually develop rudimentary consciousness or sentience. This possibility necessitates the establishment of clear boundaries regarding the permissible complexity of cerebral organoid development.
Commercialization presents another significant ethical dimension. The patenting of organoid technologies and cell lines derived from patient samples raises questions about equitable benefit sharing. When therapeutic applications emerge from organoid research using donated tissues, determining fair compensation or acknowledgment for donors becomes ethically imperative.
Cultural and religious perspectives further complicate the ethical landscape. Various traditions hold differing views on the moral status of human tissues and embryonic materials often used in organoid development. Regulatory frameworks must navigate these diverse perspectives while establishing scientifically sound guidelines.
Data privacy concerns also emerge as organoids derived from specific patients may contain genetic information with implications beyond the individual donor. The potential for organoids to reveal familial genetic traits necessitates robust privacy protections within regulatory structures.
International harmonization of ethical standards represents a critical challenge. The global nature of scientific research means that inconsistent ethical frameworks across countries could lead to "ethics shopping," where researchers conduct controversial work in jurisdictions with less stringent oversight. Developing consensus-based international guidelines would help prevent such practices while promoting responsible advancement of organoid technologies.
International Harmonization of Organoid Standards
The global landscape of organoid research and application necessitates coordinated international efforts to establish harmonized standards. Currently, significant disparities exist between regulatory frameworks across different regions, creating barriers to collaborative research and clinical translation. The European Union has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which provides specific guidelines for organoid development, while the United States FDA has adopted a more case-by-case approach through its Tissue Reference Group.
These regulatory divergences have created challenges for multinational research collaborations and commercial development of organoid technologies. Research institutions and biotechnology companies operating across borders must navigate complex and sometimes contradictory requirements, increasing development costs and delaying innovation. The International Society for Stem Cell Research (ISSCR) has recognized this challenge and initiated working groups focused on developing consensus guidelines for organoid research and applications.
Key areas requiring harmonization include standardized protocols for organoid derivation, characterization criteria for functional assessment, quality control parameters, and ethical frameworks for donor consent and data privacy. The Human Cell Atlas consortium has proposed a unified classification system for organoid models that could serve as a foundation for international standardization efforts. This system incorporates molecular signatures, functional characteristics, and structural features to enable consistent categorization across research groups.
Recent progress has emerged through collaborative initiatives like the Organoid Standards Working Group, which brings together representatives from regulatory bodies, academic institutions, and industry partners from North America, Europe, and Asia. Their 2023 white paper outlined a roadmap for establishing common technical standards and ethical guidelines that could be adopted across jurisdictions while respecting regional regulatory sovereignty.
The International Organization for Standardization (ISO) has also established a technical committee focused on biotechnology standards (ISO/TC 276) that is developing reference materials and methodologies specifically for 3D cellular models including organoids. These efforts aim to create globally recognized benchmarks for organoid characterization and quality assessment that can be referenced by regulatory frameworks worldwide.
Achieving international harmonization will require continued dialogue between stakeholders and a flexible approach that acknowledges legitimate regional differences while establishing core principles and technical standards. The establishment of international reference laboratories and proficiency testing networks could further support standardization efforts by providing independent validation of organoid models across different research settings.
These regulatory divergences have created challenges for multinational research collaborations and commercial development of organoid technologies. Research institutions and biotechnology companies operating across borders must navigate complex and sometimes contradictory requirements, increasing development costs and delaying innovation. The International Society for Stem Cell Research (ISSCR) has recognized this challenge and initiated working groups focused on developing consensus guidelines for organoid research and applications.
Key areas requiring harmonization include standardized protocols for organoid derivation, characterization criteria for functional assessment, quality control parameters, and ethical frameworks for donor consent and data privacy. The Human Cell Atlas consortium has proposed a unified classification system for organoid models that could serve as a foundation for international standardization efforts. This system incorporates molecular signatures, functional characteristics, and structural features to enable consistent categorization across research groups.
Recent progress has emerged through collaborative initiatives like the Organoid Standards Working Group, which brings together representatives from regulatory bodies, academic institutions, and industry partners from North America, Europe, and Asia. Their 2023 white paper outlined a roadmap for establishing common technical standards and ethical guidelines that could be adopted across jurisdictions while respecting regional regulatory sovereignty.
The International Organization for Standardization (ISO) has also established a technical committee focused on biotechnology standards (ISO/TC 276) that is developing reference materials and methodologies specifically for 3D cellular models including organoids. These efforts aim to create globally recognized benchmarks for organoid characterization and quality assessment that can be referenced by regulatory frameworks worldwide.
Achieving international harmonization will require continued dialogue between stakeholders and a flexible approach that acknowledges legitimate regional differences while establishing core principles and technical standards. The establishment of international reference laboratories and proficiency testing networks could further support standardization efforts by providing independent validation of organoid models across different research settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!