What Are the Industry Impacts of Organoid Culture Systems?
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering. These three-dimensional cellular structures mimic the architecture and functionality of native organs, providing unprecedented opportunities for studying human development, disease modeling, drug discovery, and regenerative medicine. The evolution of organoid technology can be traced back to the early 2000s, with significant breakthroughs occurring in 2009 when Sato and colleagues developed the first intestinal organoids from Lgr5+ stem cells.
The technological trajectory has since accelerated dramatically, with researchers successfully developing organoids representing various organ systems including brain, liver, kidney, pancreas, lung, and retina. This progression has been facilitated by advances in understanding stem cell biology, extracellular matrix components, and growth factor signaling pathways that regulate organogenesis. The integration of bioengineering approaches, including microfluidics and bioprinting, has further enhanced the complexity and physiological relevance of organoid systems.
Current technological objectives in the organoid field focus on addressing several key challenges. Researchers aim to improve the maturation and functional fidelity of organoids to better recapitulate adult organ physiology. This includes enhancing cellular diversity, establishing proper tissue architecture, and developing vascularization strategies to overcome size limitations and enable long-term culture. Additionally, there is significant emphasis on standardizing protocols to improve reproducibility and scalability for industrial applications.
Another critical objective involves integrating organoids with complementary technologies such as organ-on-chip platforms, advanced imaging techniques, and computational modeling. These integrative approaches promise to enhance the predictive power of organoid systems for drug screening and toxicity testing, potentially revolutionizing pharmaceutical development pipelines and reducing reliance on animal models.
The long-term vision for organoid technology extends beyond research applications to clinical translation. Objectives include developing personalized medicine approaches using patient-derived organoids for therapeutic testing, biobanking diverse organoid lines to represent population heterogeneity, and ultimately advancing organoid-based regenerative therapies. The potential for organoids to serve as transplantable tissue sources represents perhaps the most ambitious goal, requiring solutions to immunological challenges and the development of GMP-compliant manufacturing processes.
As organoid technology continues to mature, interdisciplinary collaboration between biologists, engineers, clinicians, and computational scientists will be essential to realize its full potential across research, pharmaceutical, and clinical domains.
The technological trajectory has since accelerated dramatically, with researchers successfully developing organoids representing various organ systems including brain, liver, kidney, pancreas, lung, and retina. This progression has been facilitated by advances in understanding stem cell biology, extracellular matrix components, and growth factor signaling pathways that regulate organogenesis. The integration of bioengineering approaches, including microfluidics and bioprinting, has further enhanced the complexity and physiological relevance of organoid systems.
Current technological objectives in the organoid field focus on addressing several key challenges. Researchers aim to improve the maturation and functional fidelity of organoids to better recapitulate adult organ physiology. This includes enhancing cellular diversity, establishing proper tissue architecture, and developing vascularization strategies to overcome size limitations and enable long-term culture. Additionally, there is significant emphasis on standardizing protocols to improve reproducibility and scalability for industrial applications.
Another critical objective involves integrating organoids with complementary technologies such as organ-on-chip platforms, advanced imaging techniques, and computational modeling. These integrative approaches promise to enhance the predictive power of organoid systems for drug screening and toxicity testing, potentially revolutionizing pharmaceutical development pipelines and reducing reliance on animal models.
The long-term vision for organoid technology extends beyond research applications to clinical translation. Objectives include developing personalized medicine approaches using patient-derived organoids for therapeutic testing, biobanking diverse organoid lines to represent population heterogeneity, and ultimately advancing organoid-based regenerative therapies. The potential for organoids to serve as transplantable tissue sources represents perhaps the most ambitious goal, requiring solutions to immunological challenges and the development of GMP-compliant manufacturing processes.
As organoid technology continues to mature, interdisciplinary collaboration between biologists, engineers, clinicians, and computational scientists will be essential to realize its full potential across research, pharmaceutical, and clinical domains.
Market Analysis for Organoid-Based Applications
The global organoid market is experiencing robust growth, valued at approximately $1.3 billion in 2022 and projected to reach $3.9 billion by 2027, representing a compound annual growth rate (CAGR) of 22.1%. This significant expansion is driven primarily by increasing applications in drug discovery and development, where organoids offer more physiologically relevant models than traditional 2D cell cultures, potentially reducing the high failure rates in clinical trials.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 60% of the current market share. These companies are increasingly adopting organoid technologies to improve preclinical testing efficiency and reduce development costs, which can exceed $2.6 billion per approved drug. The ability of organoids to recapitulate human tissue functionality makes them invaluable for toxicity screening and efficacy testing.
Personalized medicine represents the fastest-growing application segment, with an estimated CAGR of 25.3%. Patient-derived organoids enable tailored treatment approaches by allowing clinicians to test drug responses before administration to patients. This application is particularly promising in oncology, where treatment response varies significantly between individuals. Several cancer centers have begun implementing organoid-based drug sensitivity testing in clinical settings.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments and expanding biotechnology sectors in countries like China, Japan, and South Korea.
The regenerative medicine segment presents substantial long-term market potential. Organoids are being investigated for tissue replacement therapies, with early clinical trials underway for conditions such as inflammatory bowel disease and retinal disorders. Industry analysts project this segment could reach $1.2 billion by 2030 if current technical challenges in scaling and standardization are overcome.
Key market restraints include high production costs, with average expenses for maintaining organoid cultures approximately 3-5 times higher than conventional cell cultures. Additionally, regulatory uncertainties regarding the validation and standardization of organoid-based assays present challenges for widespread commercial adoption, particularly in clinical applications requiring regulatory approval.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 60% of the current market share. These companies are increasingly adopting organoid technologies to improve preclinical testing efficiency and reduce development costs, which can exceed $2.6 billion per approved drug. The ability of organoids to recapitulate human tissue functionality makes them invaluable for toxicity screening and efficacy testing.
Personalized medicine represents the fastest-growing application segment, with an estimated CAGR of 25.3%. Patient-derived organoids enable tailored treatment approaches by allowing clinicians to test drug responses before administration to patients. This application is particularly promising in oncology, where treatment response varies significantly between individuals. Several cancer centers have begun implementing organoid-based drug sensitivity testing in clinical settings.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing research investments and expanding biotechnology sectors in countries like China, Japan, and South Korea.
The regenerative medicine segment presents substantial long-term market potential. Organoids are being investigated for tissue replacement therapies, with early clinical trials underway for conditions such as inflammatory bowel disease and retinal disorders. Industry analysts project this segment could reach $1.2 billion by 2030 if current technical challenges in scaling and standardization are overcome.
Key market restraints include high production costs, with average expenses for maintaining organoid cultures approximately 3-5 times higher than conventional cell cultures. Additionally, regulatory uncertainties regarding the validation and standardization of organoid-based assays present challenges for widespread commercial adoption, particularly in clinical applications requiring regulatory approval.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid technology, several critical challenges continue to impede widespread industrial adoption and clinical translation. The primary technical hurdle remains standardization and reproducibility. Current organoid culture systems exhibit considerable batch-to-batch variability, with inconsistent growth patterns, morphology, and functionality even under seemingly identical conditions. This variability severely limits scalability for pharmaceutical applications and creates regulatory obstacles for clinical implementation.
Matrix composition represents another significant challenge, as most organoid systems rely on poorly defined animal-derived matrices like Matrigel. These natural matrices suffer from lot-to-lot variability and contain undefined growth factors that can influence experimental outcomes unpredictably. The development of synthetic alternatives has progressed but still fails to fully recapitulate the complex biochemical and mechanical properties of native extracellular matrices.
Cost-effectiveness remains a substantial barrier to industrial adoption. Current organoid culture protocols require expensive growth factors, specialized media components, and labor-intensive maintenance procedures. The high costs associated with establishing and maintaining organoid cultures make large-scale applications economically prohibitive for many potential industrial users, particularly in drug screening where thousands of compounds may need testing.
Long-term culture stability presents ongoing difficulties, with many organoid systems showing phenotypic drift, altered gene expression profiles, or decreased functionality over extended culture periods. This instability compromises the reliability of long-term studies and limits applications requiring consistent performance over time, such as chronic toxicity testing or disease modeling.
Vascularization deficiency constitutes a fundamental limitation, as most organoids lack functional blood vessels. Without proper vascularization, organoids typically develop necrotic cores as they grow beyond 300-400 micrometers in diameter, restricting their size and complexity. This limitation particularly affects applications requiring larger tissue constructs or those modeling interactions between multiple tissue types.
Technical expertise requirements further constrain widespread adoption, as successful organoid culture demands specialized knowledge and skills not commonly available in standard industrial settings. The steep learning curve and need for experienced personnel increase implementation barriers across various sectors.
Regulatory uncertainty compounds these challenges, with unclear pathways for validation and approval of organoid-based technologies in pharmaceutical development and clinical applications. The absence of established regulatory frameworks creates hesitancy among industrial stakeholders to invest heavily in organoid technologies despite their potential advantages over traditional models.
Matrix composition represents another significant challenge, as most organoid systems rely on poorly defined animal-derived matrices like Matrigel. These natural matrices suffer from lot-to-lot variability and contain undefined growth factors that can influence experimental outcomes unpredictably. The development of synthetic alternatives has progressed but still fails to fully recapitulate the complex biochemical and mechanical properties of native extracellular matrices.
Cost-effectiveness remains a substantial barrier to industrial adoption. Current organoid culture protocols require expensive growth factors, specialized media components, and labor-intensive maintenance procedures. The high costs associated with establishing and maintaining organoid cultures make large-scale applications economically prohibitive for many potential industrial users, particularly in drug screening where thousands of compounds may need testing.
Long-term culture stability presents ongoing difficulties, with many organoid systems showing phenotypic drift, altered gene expression profiles, or decreased functionality over extended culture periods. This instability compromises the reliability of long-term studies and limits applications requiring consistent performance over time, such as chronic toxicity testing or disease modeling.
Vascularization deficiency constitutes a fundamental limitation, as most organoids lack functional blood vessels. Without proper vascularization, organoids typically develop necrotic cores as they grow beyond 300-400 micrometers in diameter, restricting their size and complexity. This limitation particularly affects applications requiring larger tissue constructs or those modeling interactions between multiple tissue types.
Technical expertise requirements further constrain widespread adoption, as successful organoid culture demands specialized knowledge and skills not commonly available in standard industrial settings. The steep learning curve and need for experienced personnel increase implementation barriers across various sectors.
Regulatory uncertainty compounds these challenges, with unclear pathways for validation and approval of organoid-based technologies in pharmaceutical development and clinical applications. The absence of established regulatory frameworks creates hesitancy among industrial stakeholders to invest heavily in organoid technologies despite their potential advantages over traditional models.
State-of-the-Art Organoid Culture Methodologies
01 3D organoid culture systems and methods
Three-dimensional organoid culture systems that mimic in vivo tissue architecture and function. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and development of tissue-specific functions. These culture systems are valuable for studying organ development, disease modeling, and drug testing in a more physiologically relevant context than traditional 2D cultures.- 3D Organoid Culture Methods: Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The methods include specific growth factors, extracellular matrix components, and culture conditions that promote organoid development and maintenance, enabling more physiologically relevant models for research and drug testing.
- Stem Cell-Derived Organoids: Techniques for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These methods involve specific differentiation protocols to direct stem cells toward particular lineages before allowing self-organization into organoids. The resulting structures can model various organs such as brain, liver, intestine, and kidney, providing valuable tools for studying development, disease modeling, and regenerative medicine applications.
- Organoid Culture Media and Supplements: Specialized media formulations and supplements designed to support organoid growth and differentiation. These include defined combinations of growth factors, hormones, small molecules, and other bioactive compounds that promote cell proliferation, differentiation, and self-organization. The media compositions are often tissue-specific and may include components such as Wnt agonists, BMP inhibitors, EGF, FGF, and R-spondin to maintain stem cell niches and direct proper organoid development.
- Organoid Matrices and Scaffolds: Materials and structures that provide physical support and biochemical cues for organoid formation. These include natural extracellular matrix components like Matrigel, collagen, and laminin, as well as synthetic hydrogels with tunable properties. Advanced scaffolds may incorporate bioprinting technologies, micropatterning, or microfluidic systems to create more complex and organized structures that better recapitulate tissue architecture and function.
- Applications of Organoid Technology: Diverse applications of organoid culture systems in research, drug development, and personalized medicine. Organoids are used for disease modeling, particularly for cancer and genetic disorders, enabling patient-specific drug screening and toxicity testing. They also serve as platforms for studying host-pathogen interactions, developmental processes, and organ regeneration. Advanced applications include biobanking of patient-derived organoids and integration with other technologies like organ-on-chip systems for more complex tissue modeling.
02 Extracellular matrix components for organoid culture
Specialized extracellular matrix (ECM) components and scaffolds that support organoid growth and development. These include natural matrices like Matrigel, collagen, and fibronectin, as well as synthetic hydrogels with defined compositions. The ECM provides structural support, facilitates cell adhesion, and delivers biochemical and biomechanical cues that guide organoid formation. Optimized ECM formulations can enhance organoid viability, promote proper differentiation, and improve reproducibility in organoid culture systems.Expand Specific Solutions03 Growth factors and signaling molecules for organoid development
Specific growth factors, morphogens, and signaling molecules that regulate organoid formation and maturation. These bioactive compounds are added to culture media to activate key developmental pathways and direct cell fate decisions. Different combinations of factors are used depending on the desired organoid type, such as Wnt activators for intestinal organoids or FGF for brain organoids. Optimizing the concentration, timing, and sequence of these factors is crucial for generating functional organoids that accurately represent their in vivo counterparts.Expand Specific Solutions04 Bioreactor systems for organoid culture
Specialized bioreactor systems designed for large-scale, reproducible organoid culture. These systems provide controlled environments with optimized conditions for oxygen delivery, nutrient exchange, and waste removal. Bioreactors can incorporate features such as perfusion, mechanical stimulation, or electrical stimulation to enhance organoid development and functionality. Advanced bioreactor designs enable long-term culture maintenance, improved standardization, and potential scale-up for applications in regenerative medicine and drug screening.Expand Specific Solutions05 Disease modeling and drug screening using organoid systems
Applications of organoid culture systems for modeling human diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, enabling personalized medicine approaches. These systems are particularly valuable for studying genetic disorders, cancer, and infectious diseases. High-throughput screening platforms using organoids provide more physiologically relevant data than traditional cell culture models. The integration of organoid technology with advanced imaging and analysis methods enhances their utility for drug discovery and development.Expand Specific Solutions
Leading Organizations in Organoid Research
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research and clinical applications. The global market size is expanding rapidly, driven by advancements in personalized medicine and drug discovery applications. Technologically, the field shows varying maturity levels, with companies like STEMCELL Technologies and Molecular Devices leading with established product lines, while innovative startups like Cellesce and Organoidsciences are developing specialized bioprocessing technologies. Academic-industry partnerships are prominent, with institutions like University of Pennsylvania and Oxford University collaborating with commercial entities. Companies such as Chuangxin International Biotechnology and Hefei Zhongke Presheng are advancing the clinical translation of organoid technologies, particularly in cancer research and precision medicine applications, indicating a competitive landscape that balances established players with emerging specialists.
Cell Microsystems, Inc.
Technical Solution: Cell Microsystems has developed the CellRaft® Technology, an innovative platform for organoid culture that enables single-cell isolation and clonal organoid development. Their system utilizes arrays of microfabricated cell culture sites (CellRafts®) on which individual cells or small cell clusters can be cultured within extracellular matrix. The technology incorporates a proprietary magnetic retrieval system that allows for selective isolation of specific organoids based on morphological criteria or fluorescent markers without disturbing neighboring structures [1]. This capability addresses a major challenge in organoid research by enabling precise tracking of clonal populations from single cells through full organoid development. Their platform supports time-lapse imaging for monitoring developmental trajectories of individual organoids. Cell Microsystems has demonstrated particular success with patient-derived tumor organoids, where their technology enables isolation of rare subpopulations with distinct drug response profiles. The company has also developed specialized culture surfaces with micropatterned features that guide organoid formation and architecture, improving reproducibility across experiments [2]. Their system has been validated for applications in personalized medicine, where selective isolation of organoids with specific genetic mutations enables targeted therapeutic testing.
Strengths: Unparalleled single-cell resolution and selective retrieval capabilities; excellent for heterogeneity studies and clonal analysis. Weaknesses: Lower throughput compared to some competing platforms; requires specialized equipment and training for optimal results.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive organoid culture systems including their flagship IntestiCult™ and PancreaCult™ platforms. Their technology utilizes defined, serum-free media formulations that maintain stem cell populations while promoting differentiation into functional organoids. The company's approach incorporates specialized extracellular matrix components and growth factor combinations that mimic the native tissue microenvironment. Their systems enable long-term culture maintenance with preserved genetic stability across multiple passages [1]. STEMCELL has also pioneered automated organoid processing workflows that standardize culture conditions, reducing variability between experiments. Their technology supports both research and pharmaceutical applications, with validated protocols for drug screening and toxicity testing that demonstrate high reproducibility and physiological relevance compared to traditional 2D cultures [2].
Strengths: Industry-leading standardization of reagents and protocols enabling consistent organoid generation across laboratories; extensive validation data supporting reproducibility. Weaknesses: Higher cost compared to home-made media formulations; proprietary formulations limit customization options for specialized research applications.
Key Patents and Breakthroughs in Organoid Systems
Stably-inverted organoids and methods of producing and using the same
PatentWO2021237136A2
Innovation
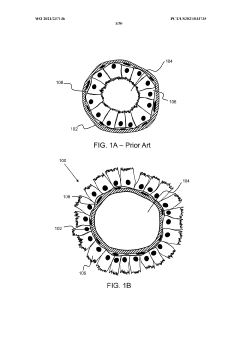
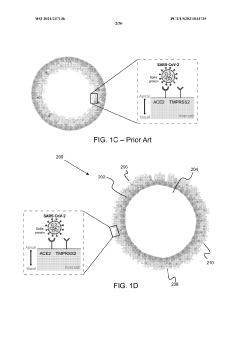
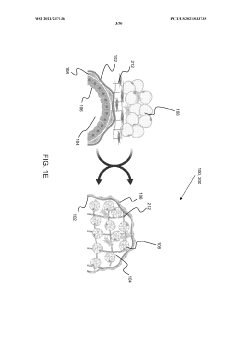
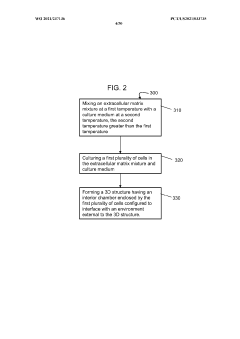
- The development of stably-inverted organoids with a hollow lumen and epithelial cells exposed to the exterior surface, allowing easier access for invasion studies, is achieved by creating a 3D structure with a tissue layer and an opposing surface where the first plurality of cells, including epithelial cells, interface with the environment externally, and an extracellular matrix mixture forms the interior chamber.
Derivation of liver organoids from human pluripotent stem cells
PatentInactiveUS20200199538A1
Innovation
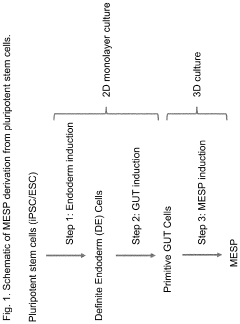
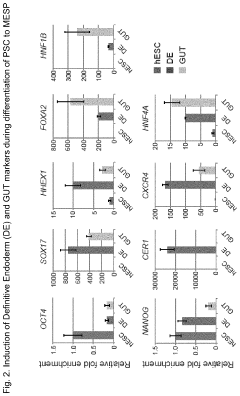
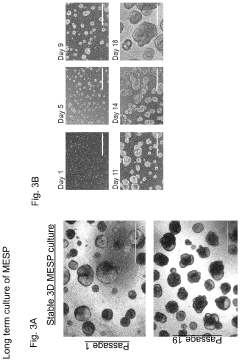
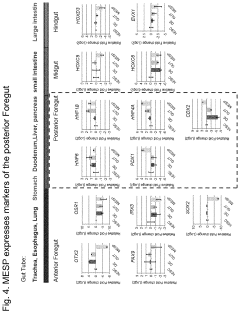
- Development of multipotent endoderm spheroid progenitor cells (MESPs) that can self-renew and differentiate into hepatic organoids containing both hepatocytes and cholangiocytes, using CRISPR/Cas technology to create a scalable and cost-effective liver organoid model capable of mimicking human liver tissue and responding to statins, and generating organoids from adult stem cells with similar structures and functions.
Regulatory Framework for Organoid Research
The regulatory landscape for organoid research is evolving rapidly as this technology advances from laboratory experiments to potential clinical applications. Currently, organoid research operates within a complex framework of existing regulations that were not specifically designed for these three-dimensional cellular structures. In the United States, the FDA has begun addressing organoids through its framework for regenerative medicine advanced therapies (RMAT), while the European Medicines Agency (EMA) considers them under advanced therapy medicinal products (ATMPs) regulations.
Ethical considerations form a critical component of the regulatory framework, particularly regarding consent for human tissue use in organoid development. The 2018 International Society for Stem Cell Research (ISSCR) guidelines provide important reference points, emphasizing informed consent processes that specifically address the potential for long-term cultivation and commercialization of tissue-derived organoids.
Data privacy regulations such as GDPR in Europe and HIPAA in the US significantly impact organoid research, as these systems often involve genetic information from tissue donors. Researchers must navigate these requirements while maintaining scientific utility of their work, creating tension between regulatory compliance and research advancement.
Intellectual property protection presents another regulatory challenge, with patent offices worldwide still developing consistent approaches to organoid-related innovations. The patentability of organoids derived from natural tissues remains contentious, with different jurisdictions taking varying positions on what constitutes patentable subject matter in this field.
Quality control standards are emerging as organoids move toward clinical applications. Organizations including the International Organization for Standardization (ISO) and the International Council for Harmonisation (ICH) are working to establish standardized protocols for organoid development, characterization, and validation.
Cross-border research collaborations face particular regulatory challenges, as material transfer agreements must address the varying legal frameworks governing human tissue use across different countries. This has led to calls for international harmonization of regulations specific to organoid research.
Industry stakeholders are increasingly engaging with regulatory bodies through public-private partnerships to develop appropriate frameworks that balance innovation with safety considerations. These collaborative efforts aim to create regulatory pathways that acknowledge the unique characteristics of organoid systems while ensuring appropriate oversight.
Ethical considerations form a critical component of the regulatory framework, particularly regarding consent for human tissue use in organoid development. The 2018 International Society for Stem Cell Research (ISSCR) guidelines provide important reference points, emphasizing informed consent processes that specifically address the potential for long-term cultivation and commercialization of tissue-derived organoids.
Data privacy regulations such as GDPR in Europe and HIPAA in the US significantly impact organoid research, as these systems often involve genetic information from tissue donors. Researchers must navigate these requirements while maintaining scientific utility of their work, creating tension between regulatory compliance and research advancement.
Intellectual property protection presents another regulatory challenge, with patent offices worldwide still developing consistent approaches to organoid-related innovations. The patentability of organoids derived from natural tissues remains contentious, with different jurisdictions taking varying positions on what constitutes patentable subject matter in this field.
Quality control standards are emerging as organoids move toward clinical applications. Organizations including the International Organization for Standardization (ISO) and the International Council for Harmonisation (ICH) are working to establish standardized protocols for organoid development, characterization, and validation.
Cross-border research collaborations face particular regulatory challenges, as material transfer agreements must address the varying legal frameworks governing human tissue use across different countries. This has led to calls for international harmonization of regulations specific to organoid research.
Industry stakeholders are increasingly engaging with regulatory bodies through public-private partnerships to develop appropriate frameworks that balance innovation with safety considerations. These collaborative efforts aim to create regulatory pathways that acknowledge the unique characteristics of organoid systems while ensuring appropriate oversight.
Ethical Implications of Organoid Applications
The ethical landscape surrounding organoid applications presents complex challenges that require careful consideration as this technology advances. The development of three-dimensional cellular structures that mimic human organs raises profound questions about personhood, consent, and the boundaries of research. As organoids increasingly resemble functional human tissues, the philosophical question of when these entities might deserve moral consideration becomes increasingly relevant.
Consent issues form a critical ethical dimension, particularly regarding the sourcing of cells for organoid development. When patient-derived cells are used to create organoids for personalized medicine applications, clear informed consent protocols must address not only immediate research purposes but also potential future applications, commercial uses, and data privacy concerns. The current regulatory frameworks often lag behind technological capabilities in this rapidly evolving field.
The potential for developing cerebral organoids that exhibit neural activity introduces particularly sensitive ethical questions. While current brain organoids remain far from consciousness, their increasing complexity necessitates ongoing ethical assessment regarding the moral status of these entities. This becomes especially important as researchers work toward more sophisticated neural organoids that might eventually demonstrate primitive cognitive functions.
Privacy and ownership considerations present another significant ethical challenge. Patient-derived organoids contain the complete genetic information of their donors, raising questions about who owns these biological entities and the associated intellectual property. The potential commercialization of organoid technologies further complicates these ownership questions, particularly when considering equitable access to therapeutic benefits derived from organoid research.
Cultural and religious perspectives add another layer of complexity to the ethical discourse. Different cultural traditions and religious viewpoints may interpret the creation and manipulation of organoid systems in fundamentally different ways, affecting public acceptance and regulatory approaches across global contexts. These diverse perspectives must be acknowledged in developing ethical frameworks for organoid applications.
The potential for organoid misuse also warrants ethical scrutiny. While organoids offer tremendous benefits for drug testing and disease modeling, they could potentially be weaponized for biological warfare or used in ways that violate human dignity. Establishing robust governance structures and international oversight mechanisms represents a crucial step in preventing such misuse while enabling beneficial applications to flourish.
Consent issues form a critical ethical dimension, particularly regarding the sourcing of cells for organoid development. When patient-derived cells are used to create organoids for personalized medicine applications, clear informed consent protocols must address not only immediate research purposes but also potential future applications, commercial uses, and data privacy concerns. The current regulatory frameworks often lag behind technological capabilities in this rapidly evolving field.
The potential for developing cerebral organoids that exhibit neural activity introduces particularly sensitive ethical questions. While current brain organoids remain far from consciousness, their increasing complexity necessitates ongoing ethical assessment regarding the moral status of these entities. This becomes especially important as researchers work toward more sophisticated neural organoids that might eventually demonstrate primitive cognitive functions.
Privacy and ownership considerations present another significant ethical challenge. Patient-derived organoids contain the complete genetic information of their donors, raising questions about who owns these biological entities and the associated intellectual property. The potential commercialization of organoid technologies further complicates these ownership questions, particularly when considering equitable access to therapeutic benefits derived from organoid research.
Cultural and religious perspectives add another layer of complexity to the ethical discourse. Different cultural traditions and religious viewpoints may interpret the creation and manipulation of organoid systems in fundamentally different ways, affecting public acceptance and regulatory approaches across global contexts. These diverse perspectives must be acknowledged in developing ethical frameworks for organoid applications.
The potential for organoid misuse also warrants ethical scrutiny. While organoids offer tremendous benefits for drug testing and disease modeling, they could potentially be weaponized for biological warfare or used in ways that violate human dignity. Establishing robust governance structures and international oversight mechanisms represents a crucial step in preventing such misuse while enabling beneficial applications to flourish.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!