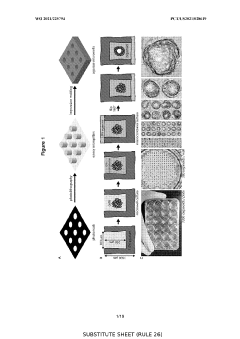
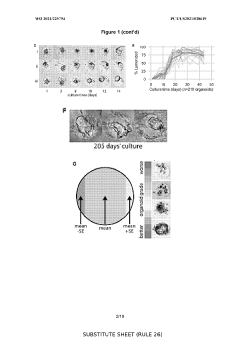
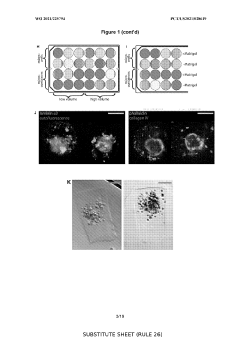
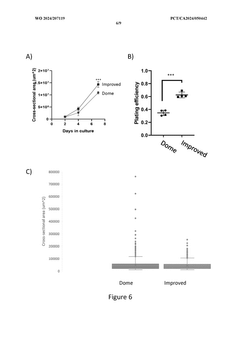
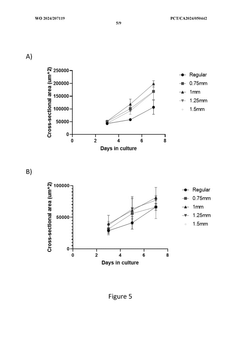
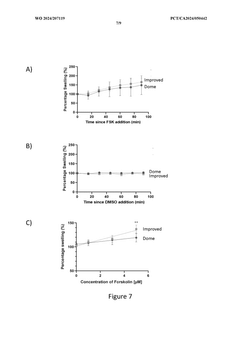
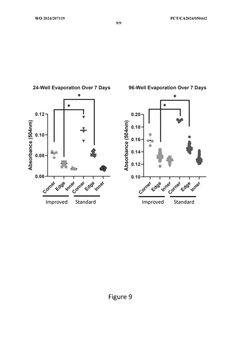
The Significance of Organoid Culture Systems in Biotechnology
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology represents a revolutionary advancement in biotechnology, emerging from the convergence of stem cell research, developmental biology, and tissue engineering. The evolution of this field can be traced back to the early 2000s when researchers first demonstrated the ability to generate three-dimensional cellular structures that mimic organ functionality. This technological progression was catalyzed by Clevers' groundbreaking work in 2009, which established intestinal organoids from Lgr5+ stem cells, marking a pivotal moment in organoid research.
The developmental trajectory of organoid technology has been characterized by rapid expansion across multiple organ systems. Initially limited to intestinal models, researchers have progressively developed organoids representing brain, liver, kidney, pancreas, and various other tissues. Each advancement has contributed to a more comprehensive understanding of organ development, function, and disease mechanisms at unprecedented levels of detail.
Technical innovations in extracellular matrix compositions, growth factor combinations, and culture methodologies have significantly enhanced organoid complexity and physiological relevance. The integration of bioengineering approaches, including microfluidics and bioprinting technologies, has further refined organoid systems, enabling better recapitulation of native tissue architecture and functionality.
The primary research objectives in the organoid field encompass several interconnected domains. First, researchers aim to improve organoid fidelity to native tissues by optimizing culture conditions and incorporating additional cell types to better represent the cellular heterogeneity found in vivo. Second, there is a focused effort to standardize organoid production protocols to enhance reproducibility across laboratories, a critical requirement for both research applications and potential clinical translation.
Third, the field is actively pursuing the integration of organoids with other technologies, such as organ-on-chip platforms and advanced imaging techniques, to create more sophisticated experimental systems. Fourth, researchers are working to scale up organoid production to meet the demands of high-throughput applications in drug discovery and toxicology testing.
Finally, a significant objective is the translation of organoid technology into clinical applications, including personalized medicine approaches where patient-derived organoids guide treatment decisions, and regenerative medicine strategies where organoids serve as a source of transplantable tissues. These objectives collectively drive the field toward more accurate modeling of human physiology and pathology, ultimately aiming to reduce reliance on animal models and accelerate therapeutic development.
The evolution of organoid technology continues to be shaped by interdisciplinary collaboration, with contributions from molecular biologists, bioengineers, clinicians, and computational scientists. This convergence of expertise propels the field forward, addressing current limitations while expanding the potential applications of this transformative biotechnology.
The developmental trajectory of organoid technology has been characterized by rapid expansion across multiple organ systems. Initially limited to intestinal models, researchers have progressively developed organoids representing brain, liver, kidney, pancreas, and various other tissues. Each advancement has contributed to a more comprehensive understanding of organ development, function, and disease mechanisms at unprecedented levels of detail.
Technical innovations in extracellular matrix compositions, growth factor combinations, and culture methodologies have significantly enhanced organoid complexity and physiological relevance. The integration of bioengineering approaches, including microfluidics and bioprinting technologies, has further refined organoid systems, enabling better recapitulation of native tissue architecture and functionality.
The primary research objectives in the organoid field encompass several interconnected domains. First, researchers aim to improve organoid fidelity to native tissues by optimizing culture conditions and incorporating additional cell types to better represent the cellular heterogeneity found in vivo. Second, there is a focused effort to standardize organoid production protocols to enhance reproducibility across laboratories, a critical requirement for both research applications and potential clinical translation.
Third, the field is actively pursuing the integration of organoids with other technologies, such as organ-on-chip platforms and advanced imaging techniques, to create more sophisticated experimental systems. Fourth, researchers are working to scale up organoid production to meet the demands of high-throughput applications in drug discovery and toxicology testing.
Finally, a significant objective is the translation of organoid technology into clinical applications, including personalized medicine approaches where patient-derived organoids guide treatment decisions, and regenerative medicine strategies where organoids serve as a source of transplantable tissues. These objectives collectively drive the field toward more accurate modeling of human physiology and pathology, ultimately aiming to reduce reliance on animal models and accelerate therapeutic development.
The evolution of organoid technology continues to be shaped by interdisciplinary collaboration, with contributions from molecular biologists, bioengineers, clinicians, and computational scientists. This convergence of expertise propels the field forward, addressing current limitations while expanding the potential applications of this transformative biotechnology.
Market Analysis for Organoid-Based Applications
The global organoid market is experiencing robust growth, valued at approximately $1.3 billion in 2022 and projected to reach $3.9 billion by 2027, representing a compound annual growth rate (CAGR) of 22.1%. This significant expansion is driven by several key factors, including increasing research activities in regenerative medicine, rising prevalence of chronic diseases, and growing adoption of personalized medicine approaches.
The pharmaceutical industry represents the largest market segment for organoid technologies, accounting for nearly 40% of the total market share. Drug discovery and toxicity testing applications are particularly prominent, as organoids provide more physiologically relevant models compared to traditional 2D cell cultures, potentially reducing the high attrition rates in drug development pipelines. Several major pharmaceutical companies have established dedicated organoid research divisions or partnerships with specialized biotechnology firms.
Cancer research constitutes another substantial market segment, with organoid models being increasingly utilized for patient-derived tumor studies, precision oncology applications, and development of novel therapeutic approaches. The ability to create patient-specific tumor organoids allows for personalized drug screening and treatment optimization, driving adoption in clinical settings.
Regionally, North America dominates the organoid market with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate during the forecast period, primarily due to increasing research investments, improving healthcare infrastructure, and growing biotechnology sectors in countries like China, Japan, and South Korea.
Key market challenges include high production costs, technical complexities in maintaining consistent organoid cultures, and regulatory uncertainties regarding clinical applications. The average cost of developing and maintaining organoid cultures remains significantly higher than traditional cell culture methods, limiting widespread adoption in resource-constrained settings.
Emerging application areas showing promising growth potential include regenerative medicine, where organoids serve as models for tissue engineering and transplantation research; infectious disease modeling, particularly accelerated by the COVID-19 pandemic; and developmental biology studies. The intestinal organoid segment currently holds the largest market share among organoid types, followed by cerebral, liver, and kidney organoids.
Commercial opportunities are expanding through service-based business models, including contract research services, customized organoid development, and specialized culture media and equipment. Several startups focused exclusively on organoid technologies have secured substantial venture capital funding in recent years, indicating strong investor confidence in the market's growth trajectory.
The pharmaceutical industry represents the largest market segment for organoid technologies, accounting for nearly 40% of the total market share. Drug discovery and toxicity testing applications are particularly prominent, as organoids provide more physiologically relevant models compared to traditional 2D cell cultures, potentially reducing the high attrition rates in drug development pipelines. Several major pharmaceutical companies have established dedicated organoid research divisions or partnerships with specialized biotechnology firms.
Cancer research constitutes another substantial market segment, with organoid models being increasingly utilized for patient-derived tumor studies, precision oncology applications, and development of novel therapeutic approaches. The ability to create patient-specific tumor organoids allows for personalized drug screening and treatment optimization, driving adoption in clinical settings.
Regionally, North America dominates the organoid market with approximately 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate during the forecast period, primarily due to increasing research investments, improving healthcare infrastructure, and growing biotechnology sectors in countries like China, Japan, and South Korea.
Key market challenges include high production costs, technical complexities in maintaining consistent organoid cultures, and regulatory uncertainties regarding clinical applications. The average cost of developing and maintaining organoid cultures remains significantly higher than traditional cell culture methods, limiting widespread adoption in resource-constrained settings.
Emerging application areas showing promising growth potential include regenerative medicine, where organoids serve as models for tissue engineering and transplantation research; infectious disease modeling, particularly accelerated by the COVID-19 pandemic; and developmental biology studies. The intestinal organoid segment currently holds the largest market share among organoid types, followed by cerebral, liver, and kidney organoids.
Commercial opportunities are expanding through service-based business models, including contract research services, customized organoid development, and specialized culture media and equipment. Several startups focused exclusively on organoid technologies have secured substantial venture capital funding in recent years, indicating strong investor confidence in the market's growth trajectory.
Current Organoid Culture Systems and Technical Barriers
Organoid culture systems have evolved significantly over the past decade, with several established methodologies now widely adopted in research laboratories and biotechnology companies. The most prevalent systems include Matrigel-based 3D cultures, which provide a rich extracellular matrix environment that supports organoid growth and differentiation. These systems have been successfully applied to develop organoids representing various tissues including intestine, liver, pancreas, brain, and kidney.
Another prominent approach involves the use of synthetic hydrogels as scaffolds, which offer better batch-to-batch consistency compared to naturally derived matrices. Air-liquid interface cultures have gained traction particularly for respiratory and skin organoids, allowing for more physiologically relevant exposure to air on the apical surface while maintaining nutrient supply from the basal side.
Microfluidic organ-on-chip platforms represent a more sophisticated culture system, integrating organoids with controlled fluid flow to mimic physiological conditions more accurately. These systems enable the study of dynamic processes such as drug absorption and metabolism under conditions that better recapitulate in vivo environments.
Despite these advances, significant technical barriers persist in organoid culture technology. Reproducibility remains a major challenge, with high variability observed between batches and laboratories. This variability stems from inconsistencies in starting materials, differences in culture protocols, and the intrinsic heterogeneity of stem cell populations used to generate organoids.
Scalability presents another substantial hurdle, particularly for pharmaceutical applications requiring high-throughput screening. Current methods are labor-intensive and difficult to automate, limiting their utility in industrial settings. The cost of specialized media components and growth factors further compounds this issue, making large-scale organoid culture economically challenging.
Vascularization represents perhaps the most significant biological barrier in organoid technology. Most current organoids lack functional blood vessels, limiting their size due to diffusion constraints and preventing the accurate modeling of drug distribution and systemic responses. This absence of vasculature also restricts nutrient and oxygen delivery to inner cell layers, often resulting in necrotic cores in larger organoids.
Maturation is another persistent challenge, as many organoids fail to achieve the full functional capacity of their in vivo counterparts. This limitation is particularly evident in more complex tissues like brain organoids, which lack the complete cellular diversity and neural circuit formation seen in developed brains. The absence of immune components in most organoid systems further limits their ability to model inflammatory responses and immune-mediated pathologies.
Another prominent approach involves the use of synthetic hydrogels as scaffolds, which offer better batch-to-batch consistency compared to naturally derived matrices. Air-liquid interface cultures have gained traction particularly for respiratory and skin organoids, allowing for more physiologically relevant exposure to air on the apical surface while maintaining nutrient supply from the basal side.
Microfluidic organ-on-chip platforms represent a more sophisticated culture system, integrating organoids with controlled fluid flow to mimic physiological conditions more accurately. These systems enable the study of dynamic processes such as drug absorption and metabolism under conditions that better recapitulate in vivo environments.
Despite these advances, significant technical barriers persist in organoid culture technology. Reproducibility remains a major challenge, with high variability observed between batches and laboratories. This variability stems from inconsistencies in starting materials, differences in culture protocols, and the intrinsic heterogeneity of stem cell populations used to generate organoids.
Scalability presents another substantial hurdle, particularly for pharmaceutical applications requiring high-throughput screening. Current methods are labor-intensive and difficult to automate, limiting their utility in industrial settings. The cost of specialized media components and growth factors further compounds this issue, making large-scale organoid culture economically challenging.
Vascularization represents perhaps the most significant biological barrier in organoid technology. Most current organoids lack functional blood vessels, limiting their size due to diffusion constraints and preventing the accurate modeling of drug distribution and systemic responses. This absence of vasculature also restricts nutrient and oxygen delivery to inner cell layers, often resulting in necrotic cores in larger organoids.
Maturation is another persistent challenge, as many organoids fail to achieve the full functional capacity of their in vivo counterparts. This limitation is particularly evident in more complex tissues like brain organoids, which lack the complete cellular diversity and neural circuit formation seen in developed brains. The absence of immune components in most organoid systems further limits their ability to model inflammatory responses and immune-mediated pathologies.
Contemporary Organoid Culture Methodologies
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems mimic the in vivo environment more accurately than traditional 2D cultures. These systems utilize specialized matrices, scaffolds, and bioreactors to support the growth and differentiation of cells into organ-like structures. The 3D environment allows for proper cell-cell interactions, spatial organization, and the development of tissue-specific architecture, resulting in organoids that better recapitulate the structure and function of native organs.- 3D organoid culture systems and methods: Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems involve culturing stem cells or progenitor cells in specific conditions that allow them to self-organize into organ-like structures. The methods include providing appropriate extracellular matrix components, growth factors, and other signaling molecules to support organoid development. These 3D culture systems are valuable for studying organ development, disease modeling, and drug screening.
- Stem cell-derived organoid technologies: Technologies for generating organoids from various types of stem cells, including embryonic stem cells, induced pluripotent stem cells, and adult stem cells. These approaches involve specific differentiation protocols to guide stem cells toward particular lineages before organoid formation. The technologies enable the production of diverse organoid types representing different organs such as intestine, brain, liver, and kidney. Stem cell-derived organoids provide valuable tools for personalized medicine and regenerative therapy research.
- Culture media and supplements for organoid growth: Specialized culture media formulations and supplements designed to support the growth and maintenance of organoids. These include defined media containing specific growth factors, hormones, and small molecules that promote organoid formation and maturation. The compositions may also include extracellular matrix components like Matrigel or synthetic hydrogels that provide structural support. Optimized media compositions are crucial for achieving reproducible organoid cultures with physiologically relevant characteristics.
- Microfluidic and bioreactor systems for organoid culture: Advanced culture platforms incorporating microfluidic technology or bioreactor systems for improved organoid culture. These systems provide controlled delivery of nutrients and oxygen while removing waste products, creating more physiological conditions for organoid growth. Some platforms include perfusion systems that mimic blood circulation or mechanical stimulation to enhance organoid maturation. These technologies enable long-term maintenance of organoids and facilitate more complex experimental manipulations for disease modeling and drug testing.
- Disease modeling and drug screening using organoids: Applications of organoid culture systems for modeling human diseases and screening potential therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, allowing for studies of disease mechanisms and progression. High-throughput screening platforms using organoids enable testing of drug efficacy and toxicity in more physiologically relevant models compared to traditional 2D cell cultures. These approaches are particularly valuable for personalized medicine, where patient-specific organoids can be used to predict individual responses to treatments.
02 Stem Cell-Derived Organoid Systems
Organoid culture systems often utilize stem cells, including embryonic stem cells, induced pluripotent stem cells (iPSCs), and adult stem cells as starting material. These stem cells are directed to differentiate into specific cell types through carefully controlled culture conditions and signaling molecules. The resulting organoids can model development, disease, and tissue regeneration, providing valuable tools for research and potential therapeutic applications.Expand Specific Solutions03 Extracellular Matrix Components for Organoid Culture
The composition of the extracellular matrix (ECM) is crucial for successful organoid culture. Various natural and synthetic ECM components, such as Matrigel, collagen, laminin, and hydrogels, provide the necessary physical support and biochemical cues for organoid formation. These matrices can be modified to control stiffness, porosity, and bioactive properties, influencing organoid development, differentiation, and functionality.Expand Specific Solutions04 Growth Factors and Signaling Molecules in Organoid Culture
Specific combinations of growth factors and signaling molecules are essential for directing organoid formation from stem cells or tissue-derived progenitors. These include Wnt agonists, R-spondin, EGF, FGF, BMP inhibitors, and tissue-specific factors. The precise temporal and spatial presentation of these factors guides cell fate decisions, patterning, and maturation of organoids, allowing researchers to generate diverse organ-specific structures under controlled conditions.Expand Specific Solutions05 Applications of Organoid Culture Systems
Organoid culture systems have diverse applications in biomedical research and medicine. They serve as models for studying organ development, disease pathogenesis, and drug responses. Patient-derived organoids enable personalized medicine approaches, including drug screening and toxicity testing. Additionally, organoids are being explored for regenerative medicine applications, such as tissue replacement therapy and bioengineered organ development, offering potential solutions for organ transplantation challenges.Expand Specific Solutions
Leading Organizations in Organoid Research and Development
The organoid culture systems market is in a growth phase, characterized by increasing adoption across biotechnology and pharmaceutical sectors. The market size is expanding rapidly due to rising applications in drug discovery, personalized medicine, and disease modeling. Technologically, the field is advancing from early-stage development toward greater standardization and scalability. Leading players include established companies like STEMCELL Technologies and Molecular Devices, which provide specialized culture media and imaging platforms, alongside innovative startups such as Xilis and Organoidsciences focused on precision medicine applications. Academic institutions including Johns Hopkins University and University of Washington contribute significantly to technological advancement. The competitive landscape features increasing collaboration between industry and academia, with companies like Cell Microsystems and CYTOO developing novel microenvironment control technologies to enhance organoid culture reproducibility and functionality.
Cell Microsystems, Inc.
Technical Solution: Cell Microsystems has developed the CellRaft® Technology, an innovative platform for isolating, culturing, and analyzing single cells and organoids. Their system utilizes microwell arrays containing thousands of individual "rafts" where organoids can be cultured in isolation, allowing for clonal derivation and tracking of individual organoid development. The technology incorporates a proprietary magnetic retrieval system that enables selective isolation of specific organoids based on morphological criteria or functional characteristics without disturbing neighboring structures. Their AIR™ System (Automated Imaging and Retrieval) combines high-resolution imaging with machine learning algorithms to identify and select organoids meeting user-defined criteria, enabling automated workflows for organoid isolation and expansion. The platform supports various extracellular matrix formulations and is compatible with a wide range of organoid types including intestinal, pancreatic, and tumor-derived models. Cell Microsystems' technology addresses key challenges in organoid heterogeneity by enabling selection and expansion of organoids with specific characteristics, improving experimental reproducibility.
Strengths: Unparalleled single organoid isolation and tracking capabilities; non-disruptive selection of specific organoid populations; compatibility with various matrix formulations; reduced cross-contamination between organoid lines. Weaknesses: Limited throughput compared to some bulk culture systems; specialized equipment requirements; higher per-organoid costs; learning curve for optimal system utilization.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed the IntestiCult™ Organoid Growth Medium system, a specialized culture platform for generating and maintaining intestinal organoids from various species including human, mouse, and pig. Their technology utilizes defined serum-free media formulations containing precise combinations of growth factors (including R-spondin, Noggin, and EGF) that support the expansion of LGR5+ intestinal stem cells and their differentiation into functional intestinal epithelium. The company has expanded their organoid platform to include specialized media for hepatic, pancreatic, and cerebral organoid generation, each optimized for tissue-specific development. Their systems incorporate proprietary extracellular matrix components that enhance organoid formation efficiency and structural organization. STEMCELL's platforms are designed for scalability, allowing researchers to transition from basic research to drug screening applications while maintaining consistent organoid characteristics across experiments.
Strengths: Comprehensive suite of tissue-specific organoid culture systems; highly standardized reagents ensuring experimental reproducibility; extensive validation across multiple research applications; ready-to-use formulations reducing technical barriers. Weaknesses: Relatively high cost for long-term studies; dependence on proprietary components; may require supplementation for specialized applications; limited customization options compared to fully custom systems.
Key Innovations in Extracellular Matrix and Growth Factors
Methods for organoids production
PatentWO2021225794A1
Innovation
- A method involving microcontainers with hydrogel walls and lids that allow cells to self-organize without exogenous extracellular matrix, using a culturing medium with biological colloids to achieve a higher density than the hydrogel lid, enabling the formation of organoids that exhibit contractility and physiological differentiation.
Cell culture apparatus and methods of using same
PatentWO2024207119A1
Innovation
- A cell culture apparatus with a well design featuring multiple sidewall segments and ledges that confine and control the geometry of the extracellular matrix, allowing for uniform polymerization and reduced variability in mass transfer, enhancing the formation and growth of multicellular aggregates or organoids.
Regulatory Framework for Organoid-Based Drug Testing
The regulatory landscape for organoid-based drug testing is evolving rapidly as these three-dimensional cellular models gain prominence in pharmaceutical research and development. Currently, there exists a patchwork of guidelines rather than a unified global framework, with regulatory bodies such as the FDA, EMA, and PMDA taking different approaches to the validation and acceptance of organoid data in drug approval processes.
In the United States, the FDA has begun incorporating organoid testing data as supplementary evidence in preclinical studies through its Predictive Toxicology Roadmap initiative. This framework encourages the use of novel methodologies including organoids, but stops short of accepting them as standalone replacements for traditional animal testing models. The FDA's position emphasizes the need for standardization of organoid protocols before full regulatory acceptance can be achieved.
The European Medicines Agency has taken a more progressive stance through its Innovation Task Force, which specifically addresses advanced in vitro models including organoids. Under Directive 2010/63/EU, which promotes the 3Rs principle (Replacement, Reduction, Refinement) of animal testing, organoid technologies are gaining regulatory support as potential alternatives to conventional testing methods.
Standardization remains a critical challenge in the regulatory acceptance of organoid systems. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working to develop consensus guidelines for the validation of alternative testing methods, including specific parameters for organoid culture systems such as reproducibility metrics, genetic stability assessments, and functional characterization benchmarks.
Ethical and legal considerations further complicate the regulatory framework, particularly for human-derived organoids. Issues surrounding informed consent, data privacy, and intellectual property rights have prompted organizations like the International Society for Stem Cell Research (ISSCR) to publish guidelines specifically addressing the ethical implications of organoid research and applications in drug testing.
Looking forward, regulatory acceptance will likely follow a tiered approach, with organoid systems first being accepted for specific applications where they demonstrate clear advantages over existing models, such as neurotoxicity testing or rare disease drug development. Full integration into regulatory frameworks will require continued collaboration between industry stakeholders, academic researchers, and regulatory authorities to establish robust validation protocols and quality control standards.
In the United States, the FDA has begun incorporating organoid testing data as supplementary evidence in preclinical studies through its Predictive Toxicology Roadmap initiative. This framework encourages the use of novel methodologies including organoids, but stops short of accepting them as standalone replacements for traditional animal testing models. The FDA's position emphasizes the need for standardization of organoid protocols before full regulatory acceptance can be achieved.
The European Medicines Agency has taken a more progressive stance through its Innovation Task Force, which specifically addresses advanced in vitro models including organoids. Under Directive 2010/63/EU, which promotes the 3Rs principle (Replacement, Reduction, Refinement) of animal testing, organoid technologies are gaining regulatory support as potential alternatives to conventional testing methods.
Standardization remains a critical challenge in the regulatory acceptance of organoid systems. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working to develop consensus guidelines for the validation of alternative testing methods, including specific parameters for organoid culture systems such as reproducibility metrics, genetic stability assessments, and functional characterization benchmarks.
Ethical and legal considerations further complicate the regulatory framework, particularly for human-derived organoids. Issues surrounding informed consent, data privacy, and intellectual property rights have prompted organizations like the International Society for Stem Cell Research (ISSCR) to publish guidelines specifically addressing the ethical implications of organoid research and applications in drug testing.
Looking forward, regulatory acceptance will likely follow a tiered approach, with organoid systems first being accepted for specific applications where they demonstrate clear advantages over existing models, such as neurotoxicity testing or rare disease drug development. Full integration into regulatory frameworks will require continued collaboration between industry stakeholders, academic researchers, and regulatory authorities to establish robust validation protocols and quality control standards.
Ethical Implications of Organoid Research
The ethical landscape surrounding organoid research presents complex challenges that require careful consideration as this technology advances. Organoid culture systems, which replicate human organ functionality in vitro, raise profound questions about the moral status of these entities. Unlike traditional cell cultures, organoids possess a degree of self-organization and functionality that blurs the line between tissue samples and rudimentary organs, necessitating new ethical frameworks.
Consent and ownership issues stand at the forefront of ethical concerns. When human tissues are used to develop organoids, questions arise regarding appropriate informed consent procedures. Donors may not fully comprehend how their cells might be used to create long-lasting organoid lines that could be commercialized, raising issues of tissue ownership, intellectual property rights, and potential profit-sharing arrangements.
Brain organoids present particularly challenging ethical dilemmas. As these structures become increasingly complex, concerns emerge about the potential development of consciousness or sentience. While current brain organoids lack the structural complexity for consciousness, advancing technology may eventually create entities capable of rudimentary sensory experiences, demanding careful ethical boundaries and monitoring protocols.
The use of organoids in drug testing and personalized medicine introduces questions about data privacy and discrimination. Patient-derived organoids contain genetic information that could potentially be used in ways that compromise privacy or lead to genetic discrimination. Regulatory frameworks must evolve to protect individuals while enabling scientific progress.
Cultural and religious perspectives add another dimension to ethical considerations. Various traditions hold different views on the manipulation of human tissues and the creation of organ-like structures, which must be respected in research governance. Inclusive ethical frameworks that acknowledge diverse cultural viewpoints are essential for global acceptance of organoid technologies.
Responsible governance requires balancing scientific advancement with ethical considerations. International collaboration on regulatory standards is crucial, as inconsistent regulations across countries could lead to "ethics tourism" where researchers conduct controversial work in less regulated environments. Transparent reporting mechanisms and ongoing ethical review processes must be established to ensure responsible research practices.
Public engagement represents a critical component of ethical organoid research. Educating the public about the nature, limitations, and potential of organoid technology can foster informed societal discussions about acceptable boundaries and applications. This dialogue should include diverse stakeholders to ensure that organoid research advances in alignment with broader societal values and priorities.
Consent and ownership issues stand at the forefront of ethical concerns. When human tissues are used to develop organoids, questions arise regarding appropriate informed consent procedures. Donors may not fully comprehend how their cells might be used to create long-lasting organoid lines that could be commercialized, raising issues of tissue ownership, intellectual property rights, and potential profit-sharing arrangements.
Brain organoids present particularly challenging ethical dilemmas. As these structures become increasingly complex, concerns emerge about the potential development of consciousness or sentience. While current brain organoids lack the structural complexity for consciousness, advancing technology may eventually create entities capable of rudimentary sensory experiences, demanding careful ethical boundaries and monitoring protocols.
The use of organoids in drug testing and personalized medicine introduces questions about data privacy and discrimination. Patient-derived organoids contain genetic information that could potentially be used in ways that compromise privacy or lead to genetic discrimination. Regulatory frameworks must evolve to protect individuals while enabling scientific progress.
Cultural and religious perspectives add another dimension to ethical considerations. Various traditions hold different views on the manipulation of human tissues and the creation of organ-like structures, which must be respected in research governance. Inclusive ethical frameworks that acknowledge diverse cultural viewpoints are essential for global acceptance of organoid technologies.
Responsible governance requires balancing scientific advancement with ethical considerations. International collaboration on regulatory standards is crucial, as inconsistent regulations across countries could lead to "ethics tourism" where researchers conduct controversial work in less regulated environments. Transparent reporting mechanisms and ongoing ethical review processes must be established to ensure responsible research practices.
Public engagement represents a critical component of ethical organoid research. Educating the public about the nature, limitations, and potential of organoid technology can foster informed societal discussions about acceptable boundaries and applications. This dialogue should include diverse stakeholders to ensure that organoid research advances in alignment with broader societal values and priorities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!