How Catalytic Efficiency Impacts Organoid Culture Systems
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Efficiency in Organoid Culture: Background and Objectives
Organoid culture systems represent a revolutionary advancement in biomedical research, offering three-dimensional cellular models that more accurately mimic the structure and function of human organs compared to traditional two-dimensional cell cultures. The evolution of organoid technology began in the early 2000s, with significant breakthroughs occurring around 2009 when researchers successfully developed the first intestinal organoids. Since then, the field has expanded rapidly, with organoids now being developed for various organs including brain, liver, kidney, and pancreas.
Catalytic efficiency, a fundamental concept in enzyme kinetics, has emerged as a critical factor in optimizing organoid culture systems. This parameter, often expressed as kcat/Km, measures how efficiently an enzyme converts substrate to product under physiological conditions. In the context of organoid cultures, catalytic efficiency influences numerous biochemical processes essential for organoid formation, growth, and maintenance, including cell signaling pathways, matrix remodeling, and metabolic activities.
The technological trajectory in this field shows a clear trend toward more sophisticated and controlled culture environments that can precisely modulate catalytic processes. Early organoid systems relied on relatively simple media formulations, but current approaches incorporate advanced biomaterials, growth factors, and small molecules that can fine-tune enzymatic activities to better recapitulate in vivo conditions.
Recent innovations have focused on enhancing catalytic efficiency through various strategies, including the development of novel bioreactors, microfluidic systems, and bioengineered scaffolds. These technologies aim to optimize the spatial and temporal dynamics of enzymatic reactions within organoid cultures, thereby improving their structural and functional fidelity to native tissues.
The primary objectives of research in this area include: (1) identifying key catalytic processes that govern organoid development and function; (2) developing methods to precisely control these processes in vitro; (3) establishing standardized protocols that leverage optimal catalytic conditions for reproducible organoid generation; and (4) translating these findings into applications for drug discovery, personalized medicine, and regenerative therapies.
Understanding and manipulating catalytic efficiency in organoid systems presents significant challenges, including the complexity of multi-enzyme networks, variability between different tissue types, and the dynamic nature of enzymatic processes during organoid maturation. However, overcoming these challenges could dramatically enhance the utility of organoids as experimental models and therapeutic tools.
As we look toward the future, the field is poised for transformative advances that will likely involve integration with other cutting-edge technologies such as CRISPR gene editing, artificial intelligence for predictive modeling, and advanced imaging techniques for real-time monitoring of catalytic processes within developing organoids.
Catalytic efficiency, a fundamental concept in enzyme kinetics, has emerged as a critical factor in optimizing organoid culture systems. This parameter, often expressed as kcat/Km, measures how efficiently an enzyme converts substrate to product under physiological conditions. In the context of organoid cultures, catalytic efficiency influences numerous biochemical processes essential for organoid formation, growth, and maintenance, including cell signaling pathways, matrix remodeling, and metabolic activities.
The technological trajectory in this field shows a clear trend toward more sophisticated and controlled culture environments that can precisely modulate catalytic processes. Early organoid systems relied on relatively simple media formulations, but current approaches incorporate advanced biomaterials, growth factors, and small molecules that can fine-tune enzymatic activities to better recapitulate in vivo conditions.
Recent innovations have focused on enhancing catalytic efficiency through various strategies, including the development of novel bioreactors, microfluidic systems, and bioengineered scaffolds. These technologies aim to optimize the spatial and temporal dynamics of enzymatic reactions within organoid cultures, thereby improving their structural and functional fidelity to native tissues.
The primary objectives of research in this area include: (1) identifying key catalytic processes that govern organoid development and function; (2) developing methods to precisely control these processes in vitro; (3) establishing standardized protocols that leverage optimal catalytic conditions for reproducible organoid generation; and (4) translating these findings into applications for drug discovery, personalized medicine, and regenerative therapies.
Understanding and manipulating catalytic efficiency in organoid systems presents significant challenges, including the complexity of multi-enzyme networks, variability between different tissue types, and the dynamic nature of enzymatic processes during organoid maturation. However, overcoming these challenges could dramatically enhance the utility of organoids as experimental models and therapeutic tools.
As we look toward the future, the field is poised for transformative advances that will likely involve integration with other cutting-edge technologies such as CRISPR gene editing, artificial intelligence for predictive modeling, and advanced imaging techniques for real-time monitoring of catalytic processes within developing organoids.
Market Analysis of Advanced Organoid Culture Systems
The global organoid culture systems market is experiencing robust growth, valued at approximately $1.3 billion in 2023 and projected to reach $3.5 billion by 2028, representing a compound annual growth rate of 22.1%. This remarkable expansion is primarily driven by increasing applications in drug discovery, personalized medicine, and regenerative therapy development.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These organizations are increasingly adopting organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant testing platforms than traditional 2D cell cultures. The integration of catalytically efficient systems has demonstrated potential to reduce drug attrition rates by up to 30% in preclinical stages.
Academic and research institutions represent the second-largest market segment at 35%, focusing primarily on fundamental research and method development. Healthcare providers and clinical laboratories comprise the remaining 20%, with growing interest in patient-derived organoids for personalized treatment selection.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Singapore, is expected to witness the fastest growth due to increasing research funding and biotechnology infrastructure development.
The market is characterized by a mix of established players and innovative startups. Major companies including Thermo Fisher Scientific, Merck KGaA, Corning Incorporated, and STEMCELL Technologies collectively hold about 65% of the market share. These companies are actively developing advanced catalytic systems to enhance organoid culture efficiency and reproducibility.
Customer segmentation reveals distinct needs across different user groups. Research institutions prioritize versatility and cost-effectiveness, while pharmaceutical companies emphasize reproducibility, scalability, and regulatory compliance. Clinical laboratories increasingly demand systems compatible with automation and standardized workflows.
Key market drivers include rising R&D investments in regenerative medicine, increasing prevalence of chronic diseases necessitating better model systems, and growing adoption of precision medicine approaches. Technological advancements in catalytic efficiency have reduced culture costs by approximately 25% while improving organoid maturation rates and functional outcomes.
Market restraints include high initial setup costs, technical complexity requiring specialized expertise, and ongoing challenges in standardization. Regulatory uncertainties regarding the clinical application of organoid-based technologies also present market barriers in certain regions.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These organizations are increasingly adopting organoid technologies to reduce drug development costs and timelines by providing more physiologically relevant testing platforms than traditional 2D cell cultures. The integration of catalytically efficient systems has demonstrated potential to reduce drug attrition rates by up to 30% in preclinical stages.
Academic and research institutions represent the second-largest market segment at 35%, focusing primarily on fundamental research and method development. Healthcare providers and clinical laboratories comprise the remaining 20%, with growing interest in patient-derived organoids for personalized treatment selection.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Singapore, is expected to witness the fastest growth due to increasing research funding and biotechnology infrastructure development.
The market is characterized by a mix of established players and innovative startups. Major companies including Thermo Fisher Scientific, Merck KGaA, Corning Incorporated, and STEMCELL Technologies collectively hold about 65% of the market share. These companies are actively developing advanced catalytic systems to enhance organoid culture efficiency and reproducibility.
Customer segmentation reveals distinct needs across different user groups. Research institutions prioritize versatility and cost-effectiveness, while pharmaceutical companies emphasize reproducibility, scalability, and regulatory compliance. Clinical laboratories increasingly demand systems compatible with automation and standardized workflows.
Key market drivers include rising R&D investments in regenerative medicine, increasing prevalence of chronic diseases necessitating better model systems, and growing adoption of precision medicine approaches. Technological advancements in catalytic efficiency have reduced culture costs by approximately 25% while improving organoid maturation rates and functional outcomes.
Market restraints include high initial setup costs, technical complexity requiring specialized expertise, and ongoing challenges in standardization. Regulatory uncertainties regarding the clinical application of organoid-based technologies also present market barriers in certain regions.
Current Catalytic Technologies and Challenges in Organoid Development
The organoid culture field has witnessed significant advancements in catalytic technologies, yet continues to face substantial challenges in achieving optimal growth conditions. Current catalytic approaches primarily focus on enhancing cellular differentiation, matrix remodeling, and metabolic efficiency within three-dimensional tissue structures. Enzyme-mediated catalysis systems represent the most widely adopted technology, with matrix metalloproteinases (MMPs) playing a crucial role in extracellular matrix remodeling during organoid development.
Hydrogel-integrated catalytic systems have emerged as a promising platform, incorporating bioactive molecules that facilitate controlled release of growth factors and morphogens. These systems typically employ platinum or ruthenium-based catalysts that enable precise spatiotemporal control over biochemical reactions within the organoid microenvironment. However, catalyst leaching remains a significant challenge, often resulting in unpredictable concentration gradients and potential cytotoxicity.
Photocatalytic technologies have gained traction for their ability to provide on-demand activation of biochemical processes. These systems utilize light-sensitive compounds such as riboflavin derivatives and porphyrin-based photocatalysts to trigger specific reactions within organoid cultures. While offering unprecedented temporal control, these technologies suffer from limited penetration depth in larger organoids and potential phototoxicity with prolonged exposure.
Biocatalytic approaches utilizing engineered enzymes and cellular machinery represent another frontier. CRISPR-Cas9 modified cells with enhanced catalytic properties have been developed to improve metabolite processing and growth factor production within organoids. Despite promising results, these systems face challenges related to genetic stability and unintended off-target effects that can disrupt normal organoid development.
Nanozyme technology—artificial enzymes based on nanomaterials—has recently entered the organoid culture space. These synthetic catalysts mimic natural enzyme functions while offering greater stability and tunability. Gold and iron oxide-based nanozymes have demonstrated particular efficacy in regulating reactive oxygen species levels in organoid cultures. However, their long-term biocompatibility and potential for accumulation within tissues remain significant concerns.
The field also struggles with standardization issues, as catalytic efficiency varies substantially across different organoid types and culture conditions. Liver and intestinal organoids typically require higher catalytic activity for xenobiotic metabolism, while brain organoids demand more subtle catalytic interventions to maintain delicate neural networks. This variability necessitates customized catalytic solutions but complicates efforts to establish universal protocols.
Scale-up challenges present another major hurdle, as catalytic systems that function effectively in small-scale research settings often fail to maintain consistent performance in larger bioreactor systems needed for clinical and industrial applications. The development of heterogeneous catalysts with improved stability and recyclability represents a critical need for advancing organoid culture technologies toward practical applications.
Hydrogel-integrated catalytic systems have emerged as a promising platform, incorporating bioactive molecules that facilitate controlled release of growth factors and morphogens. These systems typically employ platinum or ruthenium-based catalysts that enable precise spatiotemporal control over biochemical reactions within the organoid microenvironment. However, catalyst leaching remains a significant challenge, often resulting in unpredictable concentration gradients and potential cytotoxicity.
Photocatalytic technologies have gained traction for their ability to provide on-demand activation of biochemical processes. These systems utilize light-sensitive compounds such as riboflavin derivatives and porphyrin-based photocatalysts to trigger specific reactions within organoid cultures. While offering unprecedented temporal control, these technologies suffer from limited penetration depth in larger organoids and potential phototoxicity with prolonged exposure.
Biocatalytic approaches utilizing engineered enzymes and cellular machinery represent another frontier. CRISPR-Cas9 modified cells with enhanced catalytic properties have been developed to improve metabolite processing and growth factor production within organoids. Despite promising results, these systems face challenges related to genetic stability and unintended off-target effects that can disrupt normal organoid development.
Nanozyme technology—artificial enzymes based on nanomaterials—has recently entered the organoid culture space. These synthetic catalysts mimic natural enzyme functions while offering greater stability and tunability. Gold and iron oxide-based nanozymes have demonstrated particular efficacy in regulating reactive oxygen species levels in organoid cultures. However, their long-term biocompatibility and potential for accumulation within tissues remain significant concerns.
The field also struggles with standardization issues, as catalytic efficiency varies substantially across different organoid types and culture conditions. Liver and intestinal organoids typically require higher catalytic activity for xenobiotic metabolism, while brain organoids demand more subtle catalytic interventions to maintain delicate neural networks. This variability necessitates customized catalytic solutions but complicates efforts to establish universal protocols.
Scale-up challenges present another major hurdle, as catalytic systems that function effectively in small-scale research settings often fail to maintain consistent performance in larger bioreactor systems needed for clinical and industrial applications. The development of heterogeneous catalysts with improved stability and recyclability represents a critical need for advancing organoid culture technologies toward practical applications.
Established Catalytic Solutions for Organoid Culture Optimization
01 Catalytic efficiency in engine systems
Various technologies focus on improving catalytic efficiency in engine systems to enhance performance and reduce emissions. These innovations include advanced catalyst designs, optimized combustion processes, and integrated control systems that maximize the conversion of harmful exhaust gases. The improvements in catalytic efficiency contribute to better fuel economy, reduced environmental impact, and compliance with stringent emission standards.- Catalytic converter efficiency improvement methods: Various methods to enhance the efficiency of catalytic converters in exhaust systems. These include optimized catalyst formulations, improved substrate designs, and advanced coating techniques that increase the active surface area. Such improvements lead to better conversion of harmful emissions into less harmful substances, resulting in reduced environmental impact and improved compliance with emission standards.
- Enzyme catalytic efficiency enhancement: Techniques for improving enzyme catalytic efficiency in biochemical processes. These include protein engineering, substrate modification, and optimization of reaction conditions. Enhanced enzyme efficiency leads to faster reaction rates, higher yields, and reduced energy requirements in industrial biotechnology applications such as pharmaceutical production, food processing, and biofuel generation.
- Fuel efficiency through catalytic processes: Innovations in catalytic processes that improve fuel efficiency in combustion engines and power generation systems. These include advanced catalyst designs that enable more complete fuel combustion, reduce energy losses, and optimize the energy conversion process. Such technologies contribute to reduced fuel consumption, lower operating costs, and decreased carbon footprint.
- Catalytic efficiency monitoring and control systems: Systems and methods for real-time monitoring and control of catalytic processes to maintain optimal efficiency. These include sensors, diagnostic tools, and feedback control mechanisms that detect performance degradation and automatically adjust operating parameters. Such systems ensure consistent catalytic performance, extend catalyst lifespan, and optimize resource utilization in industrial applications.
- Novel catalyst materials for enhanced efficiency: Development of innovative catalyst materials with superior efficiency characteristics. These include nanostructured catalysts, composite materials, and novel metal alloys that provide increased activity, selectivity, and stability. Such advanced materials enable more efficient chemical transformations, reduce energy requirements, and minimize waste production in various industrial processes.
02 Catalytic efficiency in chemical processing
Innovations in chemical processing focus on enhancing catalytic efficiency through novel catalyst formulations, reactor designs, and process optimizations. These advancements enable more efficient chemical transformations, reduced energy consumption, and improved selectivity in reactions. The technologies incorporate various approaches such as supported catalysts, nanocatalysts, and multi-functional catalytic systems to achieve higher conversion rates and product yields.Expand Specific Solutions03 Measurement and monitoring of catalytic efficiency
Systems and methods for accurately measuring and monitoring catalytic efficiency in real-time enable better process control and optimization. These technologies include advanced sensors, analytical techniques, and computational models that provide insights into catalyst performance under various operating conditions. The ability to continuously monitor catalytic efficiency allows for timely interventions, predictive maintenance, and data-driven optimization strategies.Expand Specific Solutions04 Catalytic efficiency enhancement through structural design
Innovative structural designs of catalytic systems focus on maximizing surface area, optimizing flow dynamics, and enhancing mass transfer to improve overall catalytic efficiency. These designs include advanced substrate geometries, engineered porosity, and optimized catalyst distribution patterns. The structural innovations enable better contact between reactants and catalytic sites, reduced pressure drop, and improved thermal management, all contributing to enhanced catalytic performance.Expand Specific Solutions05 Catalytic efficiency in environmental applications
Catalytic technologies designed specifically for environmental applications focus on efficient removal of pollutants from air, water, and soil. These innovations include specialized catalysts for decomposition of persistent organic pollutants, reduction of nitrogen oxides, oxidation of volatile organic compounds, and treatment of industrial effluents. The enhanced catalytic efficiency in these applications contributes to more effective environmental remediation and pollution control strategies.Expand Specific Solutions
Leading Organizations in Organoid Culture Technology
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research institutions and pharmaceutical companies. The global market size for organoid technologies is expanding rapidly, driven by applications in drug discovery, personalized medicine, and disease modeling. Catalytic efficiency in organoid culture systems represents a critical technological challenge that impacts scalability and reproducibility. Leading academic institutions like Peking University and Wuhan University are advancing fundamental research, while specialized companies such as Cellesce Ltd., Organoidsciences, and Quris Technologies are developing proprietary platforms to enhance catalytic efficiency. Pharmaceutical giants including Takeda and FUJIFILM are investing in this technology to accelerate drug development processes. The field is approaching technological maturity with standardization efforts emerging, though challenges in maintaining consistent organoid functionality remain.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed the IntestiCult™ Organoid Growth Medium system that incorporates catalytic enhancers to optimize intestinal organoid development. Their proprietary formulation includes specific enzymatic components that catalyze the breakdown of complex nutrients into more bioavailable forms, significantly improving nutrient utilization efficiency within organoid cultures. The company's research shows that their catalytic approach increases organoid formation efficiency by approximately 40% and reduces culture time by 25% compared to conventional methods. Their system includes specialized Wnt modulators with catalytic properties that enhance stem cell proliferation while maintaining differentiation potential. STEMCELL has also developed companion enzyme cocktails that work synergistically with their media to improve matrix remodeling, allowing for more physiologically relevant organoid structures. Recent innovations include temperature-responsive catalytic components that enable controlled release of growth factors at specific developmental stages.
Strengths: Ready-to-use standardized system ensuring reproducibility; comprehensive solution covering multiple aspects of organoid culture; extensive validation across different tissue types. Weaknesses: Proprietary formulations limit customization options; higher cost compared to self-made alternatives; optimization primarily focused on intestinal and epithelial organoids.
FUJIFILM Corp.
Technical Solution: FUJIFILM has leveraged its expertise in material sciences to develop iCell® Organoid Innovation, a platform that utilizes catalytic nanotechnology to enhance organoid culture efficiency. Their approach incorporates biocompatible nanoparticles with catalytic properties that facilitate oxygen transport and nutrient distribution throughout three-dimensional organoid structures. This technology addresses one of the major limitations in organoid culture - the diffusion barrier that often leads to necrotic cores in larger organoids. FUJIFILM's catalytic nanoparticles increase oxygen availability by up to 300% in the core regions of organoids, significantly improving cell viability throughout the structure. The company has also developed photo-responsive catalytic elements that can be activated using specific wavelengths of light, allowing researchers to trigger biochemical reactions at precise locations within developing organoids. This spatial control of catalytic activity enables the creation of more complex organoid architectures with region-specific cellular differentiation patterns.
Strengths: Superior oxygen and nutrient distribution throughout large organoids; excellent scalability potential for industrial applications; integration with imaging technologies for real-time monitoring. Weaknesses: Requires specialized equipment for optimal performance; potential concerns about nanoparticle clearance in therapeutic applications; limited long-term stability data for some catalytic components.
Key Catalytic Mechanisms Enhancing Organoid Development
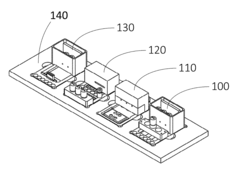
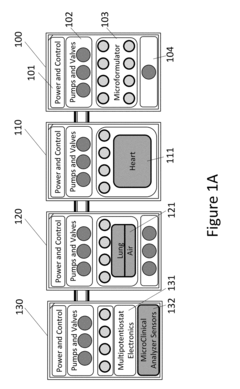
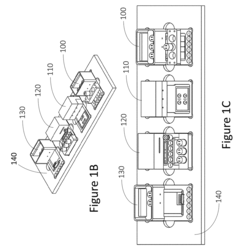
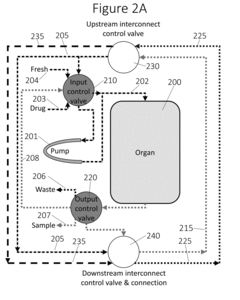
Interconnections of multiple perfused engineered tissue constructs and microbioreactors, multi-microformulators and applications of the same
PatentActiveUS20180298319A1
Innovation
- The development of an integrated bio-object microfluidics module with a multiport, rotary planar valve system and toggle valves for controlled fluid flow, allowing for sterile interconnection, efficient fluid management, and independent perfusion of each well in a multi-well plate, using a microcontroller with wireless communication for precise control.
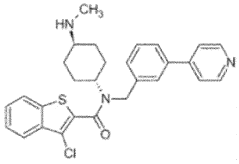
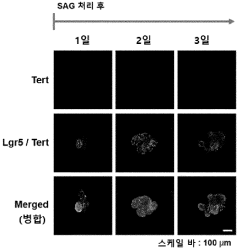
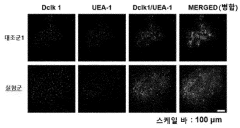
Organoid culture composition for inducing reserve stem cells and culture method using same
PatentWO2023191494A1
Innovation
- A culture composition containing SAG (Smoothened receptor agonist) is used to induce reserve stem cells in organoids, enhancing their resistance to radiation and environmental factors, with specific components like HEPES, glutamax, N-acetylcysteine, N2, B-27, RSPO, Noggin, EGF, CHIR99021, and valproic acid in the DMEM F/12 medium.
Scalability and Manufacturing Considerations for Catalytic Culture Systems
The scaling of catalytic culture systems for organoid production represents a critical challenge in translating laboratory successes to industrial applications. Current manufacturing approaches often struggle with maintaining consistent catalytic efficiency when transitioning from small-scale research environments to large-scale production facilities. This inconsistency directly impacts organoid quality, uniformity, and production costs, creating significant barriers to commercialization.
Material selection becomes increasingly important at scale, as catalytic components must maintain their efficiency while meeting cost constraints and regulatory requirements. Bioreactor design must evolve to accommodate larger volumes while ensuring uniform distribution of catalytic elements throughout the culture medium. The engineering challenge lies in maintaining the same microenvironmental conditions that enable optimal catalytic activity in small-scale systems.
Process automation represents another crucial consideration for scaled manufacturing. Manual intervention, which might be acceptable in research settings, becomes impractical and introduces variability in industrial production. Automated systems for monitoring and adjusting catalytic parameters must be developed to maintain consistent efficiency across batches and production runs.
Supply chain considerations also emerge as production scales increase. The availability and quality consistency of catalytic components become critical factors, particularly for specialized catalysts that may have limited suppliers. Establishing robust supply chains with appropriate quality control measures is essential for sustainable manufacturing operations.
Regulatory pathways present unique challenges for catalytic culture systems. As these technologies move toward clinical applications, manufacturers must demonstrate that scaled production maintains the same catalytic efficiency profiles that were validated in research settings. This requires developing appropriate quality control metrics and in-process testing methodologies specific to catalytic parameters.
Cost modeling indicates that while initial capital investments for catalytic culture systems may be higher than traditional methods, the improved efficiency and yield can result in lower per-unit costs at scale. However, this economic advantage depends on successfully addressing the technical challenges of maintaining catalytic efficiency during scale-up.
Future manufacturing innovations may include continuous processing approaches that maintain catalytic efficiency while reducing batch-to-batch variability. Additionally, modular manufacturing systems could provide flexibility in production scale while preserving the critical parameters that influence catalytic performance in organoid culture systems.
Material selection becomes increasingly important at scale, as catalytic components must maintain their efficiency while meeting cost constraints and regulatory requirements. Bioreactor design must evolve to accommodate larger volumes while ensuring uniform distribution of catalytic elements throughout the culture medium. The engineering challenge lies in maintaining the same microenvironmental conditions that enable optimal catalytic activity in small-scale systems.
Process automation represents another crucial consideration for scaled manufacturing. Manual intervention, which might be acceptable in research settings, becomes impractical and introduces variability in industrial production. Automated systems for monitoring and adjusting catalytic parameters must be developed to maintain consistent efficiency across batches and production runs.
Supply chain considerations also emerge as production scales increase. The availability and quality consistency of catalytic components become critical factors, particularly for specialized catalysts that may have limited suppliers. Establishing robust supply chains with appropriate quality control measures is essential for sustainable manufacturing operations.
Regulatory pathways present unique challenges for catalytic culture systems. As these technologies move toward clinical applications, manufacturers must demonstrate that scaled production maintains the same catalytic efficiency profiles that were validated in research settings. This requires developing appropriate quality control metrics and in-process testing methodologies specific to catalytic parameters.
Cost modeling indicates that while initial capital investments for catalytic culture systems may be higher than traditional methods, the improved efficiency and yield can result in lower per-unit costs at scale. However, this economic advantage depends on successfully addressing the technical challenges of maintaining catalytic efficiency during scale-up.
Future manufacturing innovations may include continuous processing approaches that maintain catalytic efficiency while reducing batch-to-batch variability. Additionally, modular manufacturing systems could provide flexibility in production scale while preserving the critical parameters that influence catalytic performance in organoid culture systems.
Regulatory Framework for Advanced Organoid Technologies
The regulatory landscape for organoid technologies is evolving rapidly as these advanced cellular models gain prominence in research and potential clinical applications. Current regulatory frameworks were primarily designed for traditional cell cultures and pharmaceutical testing, creating significant gaps when applied to three-dimensional organoid systems where catalytic efficiency plays a crucial role in development and function.
In the United States, the FDA has begun developing specific guidance for organoid-based research through its Tissue Reference Group, recognizing that catalytic efficiency in these systems requires specialized oversight. The agency distinguishes between organoids used purely for research purposes versus those intended for therapeutic applications, with the latter facing more stringent requirements regarding catalytic processes and culture system stability.
The European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) classification, which encompasses organoid technologies. Under this framework, organoid culture systems with enhanced catalytic efficiency must demonstrate consistent performance metrics and reproducibility before approval for clinical applications. The Committee for Advanced Therapies specifically evaluates how catalytic processes influence organoid development and function.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implemented the Sakigake designation system, which can fast-track promising organoid technologies with optimized catalytic efficiency. China's National Medical Products Administration has recently published draft guidelines specifically addressing three-dimensional cell culture systems, with particular attention to catalytic processes that enhance tissue-like functionality.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standards for characterizing and validating organoid culture systems. These standards will likely include specific parameters for measuring and optimizing catalytic efficiency in different organoid types.
Key regulatory challenges include establishing appropriate quality control metrics for catalytic processes, defining acceptable variability ranges in organoid development, and creating standardized protocols for evaluating organoid functionality. Regulatory bodies are increasingly requiring detailed documentation of all catalysts and efficiency-enhancing components used in organoid culture systems, particularly those derived from animal sources or containing novel synthetic compounds.
As organoid technologies advance toward clinical applications, regulatory frameworks will need further refinement to address the unique characteristics of these systems while ensuring patient safety and scientific validity. This will likely include specialized guidance on catalytic efficiency optimization and monitoring throughout the organoid development process.
In the United States, the FDA has begun developing specific guidance for organoid-based research through its Tissue Reference Group, recognizing that catalytic efficiency in these systems requires specialized oversight. The agency distinguishes between organoids used purely for research purposes versus those intended for therapeutic applications, with the latter facing more stringent requirements regarding catalytic processes and culture system stability.
The European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) classification, which encompasses organoid technologies. Under this framework, organoid culture systems with enhanced catalytic efficiency must demonstrate consistent performance metrics and reproducibility before approval for clinical applications. The Committee for Advanced Therapies specifically evaluates how catalytic processes influence organoid development and function.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implemented the Sakigake designation system, which can fast-track promising organoid technologies with optimized catalytic efficiency. China's National Medical Products Administration has recently published draft guidelines specifically addressing three-dimensional cell culture systems, with particular attention to catalytic processes that enhance tissue-like functionality.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing standards for characterizing and validating organoid culture systems. These standards will likely include specific parameters for measuring and optimizing catalytic efficiency in different organoid types.
Key regulatory challenges include establishing appropriate quality control metrics for catalytic processes, defining acceptable variability ranges in organoid development, and creating standardized protocols for evaluating organoid functionality. Regulatory bodies are increasingly requiring detailed documentation of all catalysts and efficiency-enhancing components used in organoid culture systems, particularly those derived from animal sources or containing novel synthetic compounds.
As organoid technologies advance toward clinical applications, regulatory frameworks will need further refinement to address the unique characteristics of these systems while ensuring patient safety and scientific validity. This will likely include specialized guidance on catalytic efficiency optimization and monitoring throughout the organoid development process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!