Market Analysis of Organoid Culture Systems
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering. Since the first successful cultivation of intestinal organoids in 2009 by Hans Clevers' laboratory, this field has experienced exponential growth across multiple tissue types. Organoids are three-dimensional cellular structures that self-organize to mimic the architecture and functionality of native organs, providing unprecedented opportunities for studying human development, disease modeling, drug discovery, and personalized medicine.
The evolution of organoid technology has been marked by significant milestones, including the development of protocols for generating brain, liver, kidney, and pancreatic organoids. These advancements have been facilitated by our deepening understanding of stem cell biology, extracellular matrix interactions, and morphogenetic processes that guide tissue formation. The field has progressed from simple epithelial organoids to more complex structures incorporating multiple cell types and vascular elements.
Current technological trends in organoid research focus on enhancing physiological relevance through the integration of immune components, vascularization, and innervation. Additionally, there is growing emphasis on standardization of protocols, scaling up production, and developing more defined culture systems to reduce variability and increase reproducibility. The application of bioengineering approaches, including microfluidics and 3D bioprinting, is further expanding the capabilities and applications of organoid technology.
The primary objectives of organoid culture systems are multifaceted. First, they aim to provide more physiologically relevant models for studying human biology and disease mechanisms, addressing the limitations of traditional 2D cell cultures and animal models. Second, they seek to establish platforms for personalized medicine approaches, enabling patient-specific drug screening and therapy development. Third, they aspire to serve as sources for regenerative medicine applications, potentially providing transplantable tissues derived from patient cells.
Technical goals in the organoid field include developing fully defined, animal component-free culture systems that support long-term organoid growth while maintaining genetic stability. Researchers are working toward creating more complex organoid systems that better recapitulate organ functionality, including multi-organ systems or "body-on-a-chip" platforms. Additionally, there is a push to integrate advanced imaging and analytical technologies for real-time monitoring of organoid development and function.
The convergence of organoid technology with other cutting-edge fields, such as CRISPR gene editing, single-cell sequencing, and artificial intelligence, is expected to further accelerate progress and expand applications. As the field matures, addressing challenges related to scalability, standardization, and cost-effectiveness will be crucial for translating organoid technology from research tools to clinical applications.
The evolution of organoid technology has been marked by significant milestones, including the development of protocols for generating brain, liver, kidney, and pancreatic organoids. These advancements have been facilitated by our deepening understanding of stem cell biology, extracellular matrix interactions, and morphogenetic processes that guide tissue formation. The field has progressed from simple epithelial organoids to more complex structures incorporating multiple cell types and vascular elements.
Current technological trends in organoid research focus on enhancing physiological relevance through the integration of immune components, vascularization, and innervation. Additionally, there is growing emphasis on standardization of protocols, scaling up production, and developing more defined culture systems to reduce variability and increase reproducibility. The application of bioengineering approaches, including microfluidics and 3D bioprinting, is further expanding the capabilities and applications of organoid technology.
The primary objectives of organoid culture systems are multifaceted. First, they aim to provide more physiologically relevant models for studying human biology and disease mechanisms, addressing the limitations of traditional 2D cell cultures and animal models. Second, they seek to establish platforms for personalized medicine approaches, enabling patient-specific drug screening and therapy development. Third, they aspire to serve as sources for regenerative medicine applications, potentially providing transplantable tissues derived from patient cells.
Technical goals in the organoid field include developing fully defined, animal component-free culture systems that support long-term organoid growth while maintaining genetic stability. Researchers are working toward creating more complex organoid systems that better recapitulate organ functionality, including multi-organ systems or "body-on-a-chip" platforms. Additionally, there is a push to integrate advanced imaging and analytical technologies for real-time monitoring of organoid development and function.
The convergence of organoid technology with other cutting-edge fields, such as CRISPR gene editing, single-cell sequencing, and artificial intelligence, is expected to further accelerate progress and expand applications. As the field matures, addressing challenges related to scalability, standardization, and cost-effectiveness will be crucial for translating organoid technology from research tools to clinical applications.
Market Demand Analysis for Organoid Culture Systems
The global organoid culture systems market is experiencing robust growth, driven by increasing adoption in drug discovery, personalized medicine, and disease modeling applications. Current market valuations indicate the sector reached approximately 1.3 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 22.5% through 2030, potentially reaching 6.8 billion USD by the end of the forecast period. This accelerated growth trajectory reflects the expanding utility of organoid technologies across multiple biomedical research domains.
Primary demand drivers include pharmaceutical companies seeking more physiologically relevant drug screening platforms to reduce late-stage clinical failures. These organizations are increasingly recognizing the limitations of traditional 2D cell cultures and animal models, turning to organoid systems that better recapitulate human tissue complexity and functionality. The cost-saving potential is substantial, with estimates suggesting that improved preclinical models could reduce drug development costs by 10-15% through earlier identification of ineffective or toxic compounds.
Academic and research institutions represent another significant market segment, with growing research funding allocated specifically to organoid technology development. Grant funding for organoid-related research has increased by approximately 35% over the past five years, indicating strong institutional interest in advancing these technologies.
The clinical application segment is emerging as a high-potential growth area, particularly in precision medicine. Healthcare providers are beginning to utilize patient-derived organoids for treatment response prediction, especially in oncology settings. Early clinical implementation studies suggest treatment optimization using organoid testing could improve response rates by 18-24% in certain cancer types.
Regionally, North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade, driven by increasing research investments in China, Japan, and South Korea, along with expanding biotechnology infrastructure.
Consumer demand patterns reveal growing interest in specialized organoid culture media and matrices that enhance reproducibility and standardization. End-users increasingly prioritize complete workflow solutions rather than individual components, creating opportunities for integrated system providers. Additionally, there is rising demand for automated culture systems that reduce labor requirements and improve consistency in organoid production.
Market challenges include the high cost of specialized culture media and matrices, technical complexity requiring specialized expertise, and standardization issues affecting reproducibility across laboratories. These factors currently limit broader adoption, particularly in smaller research institutions and emerging markets.
Primary demand drivers include pharmaceutical companies seeking more physiologically relevant drug screening platforms to reduce late-stage clinical failures. These organizations are increasingly recognizing the limitations of traditional 2D cell cultures and animal models, turning to organoid systems that better recapitulate human tissue complexity and functionality. The cost-saving potential is substantial, with estimates suggesting that improved preclinical models could reduce drug development costs by 10-15% through earlier identification of ineffective or toxic compounds.
Academic and research institutions represent another significant market segment, with growing research funding allocated specifically to organoid technology development. Grant funding for organoid-related research has increased by approximately 35% over the past five years, indicating strong institutional interest in advancing these technologies.
The clinical application segment is emerging as a high-potential growth area, particularly in precision medicine. Healthcare providers are beginning to utilize patient-derived organoids for treatment response prediction, especially in oncology settings. Early clinical implementation studies suggest treatment optimization using organoid testing could improve response rates by 18-24% in certain cancer types.
Regionally, North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade, driven by increasing research investments in China, Japan, and South Korea, along with expanding biotechnology infrastructure.
Consumer demand patterns reveal growing interest in specialized organoid culture media and matrices that enhance reproducibility and standardization. End-users increasingly prioritize complete workflow solutions rather than individual components, creating opportunities for integrated system providers. Additionally, there is rising demand for automated culture systems that reduce labor requirements and improve consistency in organoid production.
Market challenges include the high cost of specialized culture media and matrices, technical complexity requiring specialized expertise, and standardization issues affecting reproducibility across laboratories. These factors currently limit broader adoption, particularly in smaller research institutions and emerging markets.
Current Challenges in Organoid Culture Technology
Despite significant advancements in organoid culture technology, several critical challenges continue to impede broader adoption and commercialization. The primary technical hurdle remains reproducibility and standardization. Current protocols often yield organoids with high batch-to-batch variability, making experimental replication difficult and limiting their utility in drug screening applications. This inconsistency stems from the complex interplay of growth factors, extracellular matrix components, and culture conditions that have not been fully optimized across different tissue types.
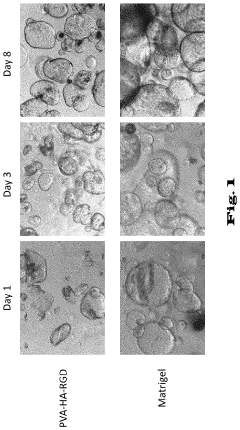
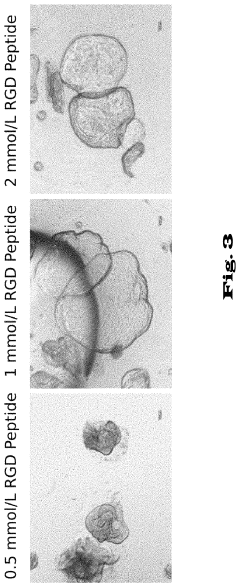
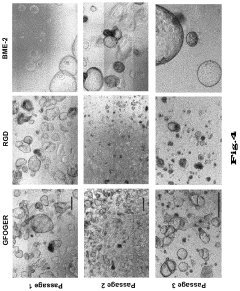
Matrix selection presents another significant challenge. Matrigel, the most commonly used matrix, is derived from mouse sarcoma cells, introducing animal components that create regulatory concerns for clinical applications. Its undefined composition and lot-to-batch variations further complicate standardization efforts. Alternative synthetic matrices are under development but have not yet achieved the versatility and effectiveness of Matrigel for supporting diverse organoid types.
Vascularization remains an unresolved technical limitation. Current organoid systems lack proper blood vessel networks, restricting nutrient and oxygen diffusion to approximately 200-400 micrometers. This size limitation prevents the development of larger, more complex organoids that would better recapitulate in vivo tissue architecture and function. Various bioengineering approaches, including microfluidic systems and 3D bioprinting, are being explored to address this constraint, but none have reached commercial maturity.
Cost factors significantly impact widespread adoption. The high price of specialized growth factors and matrices makes organoid culture prohibitively expensive for many research laboratories and industrial applications. A single experiment can cost hundreds to thousands of dollars in reagents alone, limiting scalability for high-throughput applications and routine use in pharmaceutical screening.
Long-term culture stability presents ongoing difficulties. Many organoid systems show phenotypic drift or deterioration after extended culture periods, limiting their utility for chronic disease modeling or longitudinal studies. This instability may result from accumulating genetic alterations, epigenetic changes, or suboptimal niche conditions that fail to maintain stem cell populations properly over time.
Integration with existing analytical platforms poses compatibility challenges. Many standard assays and imaging techniques were not designed for 3D structures, requiring adaptation or development of new methodologies. This technical gap slows data acquisition and analysis, particularly for high-content screening applications that demand rapid, automated assessment of organoid responses.
Addressing these interconnected challenges requires coordinated efforts across multiple disciplines, including materials science, bioengineering, molecular biology, and analytical chemistry. Progress in overcoming these limitations will determine the pace at which organoid technology transitions from specialized research tools to mainstream applications in drug development, personalized medicine, and regenerative therapies.
Matrix selection presents another significant challenge. Matrigel, the most commonly used matrix, is derived from mouse sarcoma cells, introducing animal components that create regulatory concerns for clinical applications. Its undefined composition and lot-to-batch variations further complicate standardization efforts. Alternative synthetic matrices are under development but have not yet achieved the versatility and effectiveness of Matrigel for supporting diverse organoid types.
Vascularization remains an unresolved technical limitation. Current organoid systems lack proper blood vessel networks, restricting nutrient and oxygen diffusion to approximately 200-400 micrometers. This size limitation prevents the development of larger, more complex organoids that would better recapitulate in vivo tissue architecture and function. Various bioengineering approaches, including microfluidic systems and 3D bioprinting, are being explored to address this constraint, but none have reached commercial maturity.
Cost factors significantly impact widespread adoption. The high price of specialized growth factors and matrices makes organoid culture prohibitively expensive for many research laboratories and industrial applications. A single experiment can cost hundreds to thousands of dollars in reagents alone, limiting scalability for high-throughput applications and routine use in pharmaceutical screening.
Long-term culture stability presents ongoing difficulties. Many organoid systems show phenotypic drift or deterioration after extended culture periods, limiting their utility for chronic disease modeling or longitudinal studies. This instability may result from accumulating genetic alterations, epigenetic changes, or suboptimal niche conditions that fail to maintain stem cell populations properly over time.
Integration with existing analytical platforms poses compatibility challenges. Many standard assays and imaging techniques were not designed for 3D structures, requiring adaptation or development of new methodologies. This technical gap slows data acquisition and analysis, particularly for high-content screening applications that demand rapid, automated assessment of organoid responses.
Addressing these interconnected challenges requires coordinated efforts across multiple disciplines, including materials science, bioengineering, molecular biology, and analytical chemistry. Progress in overcoming these limitations will determine the pace at which organoid technology transitions from specialized research tools to mainstream applications in drug development, personalized medicine, and regenerative therapies.
Current Organoid Culture System Solutions
01 3D organoid culture methods and systems
Three-dimensional organoid culture systems that mimic in vivo tissue architecture and function. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and development of tissue-specific functions that are not achievable in traditional 2D cultures.- 3D organoid culture methods and systems: Three-dimensional organoid culture systems that mimic in vivo tissue architecture and function. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The 3D environment allows for proper cell-cell interactions, differentiation, and development of tissue-specific functions that are not achievable in traditional 2D cultures.
- Extracellular matrix components for organoid culture: Specific extracellular matrix components and hydrogels that support organoid growth and development. These matrices provide structural support, biochemical cues, and mechanical properties necessary for proper organoid formation. They often contain proteins like laminin, collagen, and growth factors that mimic the native tissue environment and promote cell adhesion, migration, and differentiation into functional organoids.
- Bioreactor systems for organoid culture: Specialized bioreactor systems designed for the cultivation and maintenance of organoids. These systems provide controlled environments for organoid growth, including regulation of temperature, pH, oxygen levels, and nutrient supply. Advanced bioreactors may incorporate perfusion systems, mechanical stimulation, or other features that enhance organoid development and functionality by mimicking physiological conditions.
- Disease modeling using organoid culture systems: Applications of organoid culture systems for modeling human diseases and testing therapeutic interventions. Patient-derived organoids can recapitulate disease phenotypes, enabling studies of disease mechanisms and personalized medicine approaches. These systems are particularly valuable for studying genetic disorders, cancer, infectious diseases, and developmental abnormalities in a physiologically relevant context.
- Growth factors and signaling molecules for organoid development: Specific growth factors, morphogens, and signaling molecules that regulate organoid formation and differentiation. These bioactive compounds are added to culture media to direct stem cell fate, promote tissue-specific differentiation, and maintain organoid homeostasis. Optimized combinations of these factors are essential for generating organoids that accurately represent the cellular composition and function of native organs.
02 Extracellular matrix components for organoid culture
Specialized extracellular matrix formulations that support organoid growth and development. These matrices provide structural support and biochemical cues necessary for stem cell differentiation and organoid formation. Components may include basement membrane extracts, collagen, laminin, fibronectin, and other proteins that mimic the native tissue environment and promote proper organoid development.Expand Specific Solutions03 Growth factors and signaling molecules for organoid development
Specific combinations of growth factors and signaling molecules that direct stem cell differentiation and organoid formation. These bioactive compounds are added to culture media to activate developmental pathways and promote tissue-specific differentiation. Different organoid types require unique combinations of factors to recapitulate the signaling environment of the developing organ.Expand Specific Solutions04 Bioreactor systems for organoid culture
Specialized bioreactor systems designed for the large-scale production and maintenance of organoids. These systems provide controlled environments for organoid growth, including regulation of temperature, pH, oxygen levels, and nutrient delivery. Advanced bioreactors may incorporate perfusion systems, mechanical stimulation, or other features that enhance organoid development and functionality.Expand Specific Solutions05 Disease modeling and drug screening applications of organoid systems
Applications of organoid culture systems for modeling human diseases and screening therapeutic compounds. Patient-derived organoids can recapitulate disease phenotypes, enabling personalized medicine approaches. These systems provide platforms for drug efficacy and toxicity testing that better predict in vivo responses compared to traditional cell culture methods, potentially reducing animal testing requirements in pharmaceutical development.Expand Specific Solutions
Key Industry Players in Organoid Research
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research and clinical applications. The market size is expanding rapidly, driven by advancements in personalized medicine and drug discovery, with projections indicating substantial growth over the next decade. Technologically, the field shows varying maturity levels, with companies like STEMCELL Technologies, Molecular Devices, and Xilis leading innovation in standardized culture systems. Academic institutions including Wuhan University and Johns Hopkins University contribute significant research advancements, while emerging players like Organoidsciences and Shanghai Ruiyu Biotech are developing specialized applications. The competitive landscape features a mix of established biotechnology firms and specialized startups focusing on automation, scalability, and clinical translation of organoid technologies.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive organoid culture systems including their flagship IntestiCult™ and PancreaCult™ platforms. Their technology provides defined, serum-free media formulations specifically optimized for the growth and differentiation of intestinal, pancreatic, and other organ-specific organoids. The company's systems incorporate specialized extracellular matrix components and growth factors that mimic the native stem cell niche, enabling long-term expansion of organoids while maintaining physiological relevance. Their platforms support both mouse and human-derived organoids with demonstrated genetic stability over multiple passages. STEMCELL has also pioneered complementary technologies for organoid harvesting, passaging, and cryopreservation, creating an integrated workflow solution that addresses the entire organoid culture lifecycle.
Strengths: Comprehensive product portfolio covering multiple organ types; highly standardized and reproducible culture conditions; strong technical support infrastructure. Weaknesses: Higher cost compared to home-brew media formulations; proprietary formulations may limit customization for specialized research applications.
Xilis, Inc.
Technical Solution: Xilis has developed the Micro-Organosphere Technology (MOT) platform, a revolutionary approach to patient-derived organoid culture that dramatically accelerates the timeline from biopsy to functional organoid. Their system enables the generation of thousands of uniform micro-organoids from patient samples within days rather than weeks or months required by conventional methods. The technology incorporates proprietary media formulations and culture protocols that maintain the native tumor microenvironment, including stromal and immune components. Xilis has integrated their organoid platform with AI-powered image analysis to predict patient-specific drug responses, positioning their technology as a precision medicine tool for clinical decision support. Their system has demonstrated particular success with colorectal, pancreatic, and breast cancer organoids, with clinical validation studies showing high concordance between organoid drug responses and patient outcomes.
Strengths: Rapid turnaround time enabling clinical applications; preservation of tumor microenvironment complexity; AI integration for predictive analytics. Weaknesses: Relatively new technology with more limited publication history; primarily focused on cancer applications rather than normal tissue models.
Critical Patents and Innovations in Organoid Culture
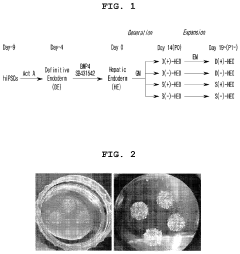
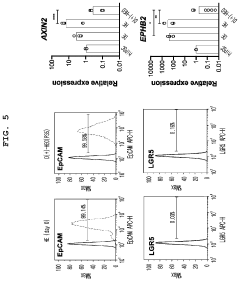
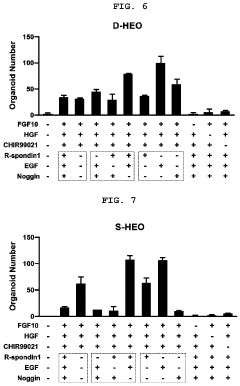
Method for constructing human pluripotent stem cell-derived liver organoid having enhanced drug metabolic potential and liver organoid constructed by same method
PatentActiveUS20240141289A1
Innovation
- A method for constructing human pluripotent stem cell-derived liver organoids by differentiating cells into definitive endoderm, hepatic endoderm, and finally liver organoids, using specific culture media without R-Spondin-1, Noggin, and EGF, which enhances drug metabolic capabilities and toxicity evaluation.
Hydrogels for cultivating pancreatic organoids
PatentInactiveUS20190367869A1
Innovation
- A chemically defined hydrogel composition with a reduced shear modulus, composed of functionalized polymer molecules and linker molecules, and low-molecular peptides with cell adhesion motifs, which allows for the cultivation and expansion of pancreatic cells into stable and functional 3D organoids without natural extracellular matrix proteins.
Regulatory Framework for Organoid Applications
The regulatory landscape for organoid technologies is complex and evolving rapidly as these advanced cellular models gain prominence in research and clinical applications. Currently, organoid applications fall under multiple regulatory frameworks depending on their intended use, with significant variations across different regions. In the United States, the FDA oversees organoid applications through different centers: drug development applications through the Center for Drug Evaluation and Research (CDER), medical device applications via the Center for Devices and Radiological Health (CDRH), and biological applications through the Center for Biologics Evaluation and Research (CBER).
The European Medicines Agency (EMA) has established specific guidelines for advanced therapy medicinal products (ATMPs) that encompass certain organoid applications, particularly those intended for therapeutic use. Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine, provides an accelerated pathway for regenerative medicine products that may benefit organoid-based therapies.
Quality control standards represent a critical regulatory challenge, as standardization of organoid production remains inconsistent. Regulatory bodies increasingly require demonstration of batch-to-batch reproducibility, genetic stability, and absence of contamination. The International Society for Stem Cell Research (ISSCR) has published guidelines specifically addressing organoid research ethics and quality standards that are becoming reference points for regulators.
Ethical considerations form another significant regulatory dimension, particularly regarding brain organoids and organoids derived from human embryonic tissues. Several countries have established specialized ethics committees to evaluate research protocols involving human-derived organoids, with particular scrutiny applied to cerebral organoids that demonstrate complex neural activity.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, impact organoid research when patient-derived materials are used. These frameworks mandate specific consent procedures and data protection measures that researchers must implement throughout the organoid development pipeline.
Looking forward, regulatory harmonization efforts are underway through initiatives like the International Coalition of Medicines Regulatory Authorities (ICMRA), which aims to develop consistent approaches to novel technologies including organoids. Industry stakeholders anticipate that specialized regulatory pathways for organoid-based products will emerge within the next 3-5 years as applications move closer to clinical implementation.
For companies developing commercial organoid platforms, early engagement with regulatory authorities through programs like the FDA's Breakthrough Devices Program or the EMA's Innovation Task Force is becoming essential to navigate this evolving regulatory landscape effectively.
The European Medicines Agency (EMA) has established specific guidelines for advanced therapy medicinal products (ATMPs) that encompass certain organoid applications, particularly those intended for therapeutic use. Japan's regulatory framework, revised under the Act on the Safety of Regenerative Medicine, provides an accelerated pathway for regenerative medicine products that may benefit organoid-based therapies.
Quality control standards represent a critical regulatory challenge, as standardization of organoid production remains inconsistent. Regulatory bodies increasingly require demonstration of batch-to-batch reproducibility, genetic stability, and absence of contamination. The International Society for Stem Cell Research (ISSCR) has published guidelines specifically addressing organoid research ethics and quality standards that are becoming reference points for regulators.
Ethical considerations form another significant regulatory dimension, particularly regarding brain organoids and organoids derived from human embryonic tissues. Several countries have established specialized ethics committees to evaluate research protocols involving human-derived organoids, with particular scrutiny applied to cerebral organoids that demonstrate complex neural activity.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, impact organoid research when patient-derived materials are used. These frameworks mandate specific consent procedures and data protection measures that researchers must implement throughout the organoid development pipeline.
Looking forward, regulatory harmonization efforts are underway through initiatives like the International Coalition of Medicines Regulatory Authorities (ICMRA), which aims to develop consistent approaches to novel technologies including organoids. Industry stakeholders anticipate that specialized regulatory pathways for organoid-based products will emerge within the next 3-5 years as applications move closer to clinical implementation.
For companies developing commercial organoid platforms, early engagement with regulatory authorities through programs like the FDA's Breakthrough Devices Program or the EMA's Innovation Task Force is becoming essential to navigate this evolving regulatory landscape effectively.
Cost-Benefit Analysis of Organoid Systems
The economic evaluation of organoid culture systems reveals a complex cost-benefit landscape that organizations must navigate when implementing these advanced biological models. Initial investment costs for organoid systems are substantial, with specialized equipment requirements including bioreactors, incubators with precise environmental controls, and advanced imaging systems often totaling $100,000-$500,000 for a comprehensive laboratory setup. Additionally, recurring operational expenses for high-quality growth factors, extracellular matrix components, and specialized media formulations can range from $2,000-$5,000 per month for maintaining multiple organoid lines.
When comparing these costs against traditional 2D cell culture systems, organoids represent a 3-5 fold increase in direct expenses. However, this financial analysis must be contextualized within the superior biological relevance that organoids provide. The enhanced predictive capacity of organoid models in drug discovery pipelines demonstrates significant long-term economic advantages, with studies indicating up to 25% improvement in predictive accuracy for drug efficacy and toxicity compared to conventional models.
The return on investment timeline typically extends between 18-36 months, with pharmaceutical applications showing the most rapid financial returns. Academic research settings may experience longer payback periods but benefit from increased grant competitiveness and publication impact. Notably, organizations implementing organoid technologies report an average 30% reduction in late-stage drug development failures, representing potential savings of millions in development costs per compound.
Scalability considerations present significant economic challenges, as the transition from research-scale to production-scale organoid systems often requires substantial additional investment. Current commercial systems demonstrate variable cost efficiency, with automated platforms reducing labor costs by 40-60% but requiring initial investments of $250,000-$750,000. This creates a significant barrier for smaller organizations and research institutions.
Risk assessment reveals that while the upfront costs are considerable, the greatest financial risk lies in underutilization of established systems due to technical challenges or insufficient staff expertise. Organizations report that comprehensive staff training programs, though representing an additional 5-10% of initial investment, significantly improve utilization rates and accelerate return on investment.
The long-term economic sustainability of organoid systems depends heavily on technological advancements in automation, standardization, and reduced reagent costs. Industry projections suggest that continued innovation could reduce operational costs by 30-50% within the next five years, substantially improving the cost-benefit ratio and expanding accessibility across diverse research and clinical applications.
When comparing these costs against traditional 2D cell culture systems, organoids represent a 3-5 fold increase in direct expenses. However, this financial analysis must be contextualized within the superior biological relevance that organoids provide. The enhanced predictive capacity of organoid models in drug discovery pipelines demonstrates significant long-term economic advantages, with studies indicating up to 25% improvement in predictive accuracy for drug efficacy and toxicity compared to conventional models.
The return on investment timeline typically extends between 18-36 months, with pharmaceutical applications showing the most rapid financial returns. Academic research settings may experience longer payback periods but benefit from increased grant competitiveness and publication impact. Notably, organizations implementing organoid technologies report an average 30% reduction in late-stage drug development failures, representing potential savings of millions in development costs per compound.
Scalability considerations present significant economic challenges, as the transition from research-scale to production-scale organoid systems often requires substantial additional investment. Current commercial systems demonstrate variable cost efficiency, with automated platforms reducing labor costs by 40-60% but requiring initial investments of $250,000-$750,000. This creates a significant barrier for smaller organizations and research institutions.
Risk assessment reveals that while the upfront costs are considerable, the greatest financial risk lies in underutilization of established systems due to technical challenges or insufficient staff expertise. Organizations report that comprehensive staff training programs, though representing an additional 5-10% of initial investment, significantly improve utilization rates and accelerate return on investment.
The long-term economic sustainability of organoid systems depends heavily on technological advancements in automation, standardization, and reduced reagent costs. Industry projections suggest that continued innovation could reduce operational costs by 30-50% within the next five years, substantially improving the cost-benefit ratio and expanding accessibility across diverse research and clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!