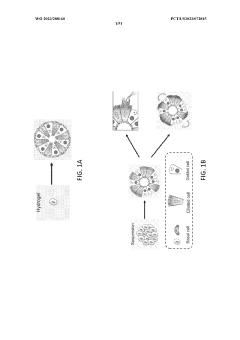
Organoid Culture Systems: Technical Mechanisms Unveiled
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Evolution and Research Objectives
Organoid technology has evolved significantly over the past decade, transforming from rudimentary cell culture techniques to sophisticated three-dimensional systems that accurately mimic organ functionality. The journey began with Hans Clevers' groundbreaking work in 2009, which demonstrated the first intestinal organoids derived from Lgr5+ stem cells. This pivotal discovery established the fundamental principles of organoid development: the capacity of stem cells to self-organize into complex structures when provided with appropriate biochemical and biophysical cues.
The technological progression has been marked by several key advancements. Initially, organoid cultures relied heavily on animal-derived matrices like Matrigel, which introduced variability and limited clinical applications. Recent innovations have focused on developing synthetic hydrogels with tunable properties, enabling more precise control over the microenvironment and improving reproducibility. Parallel developments in bioengineering have introduced microfluidic systems and bioprinting techniques, allowing for more complex organoid architectures and vascularization.
Current research objectives in organoid technology center around enhancing physiological relevance and addressing existing limitations. A primary goal is to improve organoid maturation, as many current models represent fetal or immature tissue states rather than adult functionality. Researchers are exploring various approaches, including co-culture systems with supporting cell types, mechanical stimulation, and electrical pacing for cardiac organoids, to achieve more mature phenotypes.
Another critical objective is standardization of protocols and materials. The field currently suffers from significant variability in methodologies, hampering reproducibility and clinical translation. Efforts are underway to establish reference standards, quality control metrics, and automated culture systems to address these challenges. The development of chemically defined, xeno-free culture conditions represents a particularly important goal for therapeutic applications.
Integration of advanced analytical techniques constitutes another major research direction. Single-cell RNA sequencing, spatial transcriptomics, and advanced imaging modalities are being incorporated to provide deeper insights into organoid development and function. These technologies enable more precise characterization of cellular heterogeneity and spatial organization within organoids, facilitating better comparison with native tissues.
The ultimate research objective remains the translation of organoid technology into clinical applications. This encompasses personalized medicine approaches using patient-derived organoids for drug screening and disease modeling, as well as regenerative medicine applications such as organoid transplantation. Achieving this goal requires addressing regulatory challenges, scaling up production, and developing preservation methods that maintain organoid viability and functionality during storage and transport.
The technological progression has been marked by several key advancements. Initially, organoid cultures relied heavily on animal-derived matrices like Matrigel, which introduced variability and limited clinical applications. Recent innovations have focused on developing synthetic hydrogels with tunable properties, enabling more precise control over the microenvironment and improving reproducibility. Parallel developments in bioengineering have introduced microfluidic systems and bioprinting techniques, allowing for more complex organoid architectures and vascularization.
Current research objectives in organoid technology center around enhancing physiological relevance and addressing existing limitations. A primary goal is to improve organoid maturation, as many current models represent fetal or immature tissue states rather than adult functionality. Researchers are exploring various approaches, including co-culture systems with supporting cell types, mechanical stimulation, and electrical pacing for cardiac organoids, to achieve more mature phenotypes.
Another critical objective is standardization of protocols and materials. The field currently suffers from significant variability in methodologies, hampering reproducibility and clinical translation. Efforts are underway to establish reference standards, quality control metrics, and automated culture systems to address these challenges. The development of chemically defined, xeno-free culture conditions represents a particularly important goal for therapeutic applications.
Integration of advanced analytical techniques constitutes another major research direction. Single-cell RNA sequencing, spatial transcriptomics, and advanced imaging modalities are being incorporated to provide deeper insights into organoid development and function. These technologies enable more precise characterization of cellular heterogeneity and spatial organization within organoids, facilitating better comparison with native tissues.
The ultimate research objective remains the translation of organoid technology into clinical applications. This encompasses personalized medicine approaches using patient-derived organoids for drug screening and disease modeling, as well as regenerative medicine applications such as organoid transplantation. Achieving this goal requires addressing regulatory challenges, scaling up production, and developing preservation methods that maintain organoid viability and functionality during storage and transport.
Market Analysis for Organoid Culture Applications
The global organoid culture systems market is experiencing robust growth, valued at approximately $1.3 billion in 2023 with projections to reach $3.5 billion by 2028, representing a compound annual growth rate of 22.1%. This remarkable expansion is primarily driven by increasing applications in drug discovery and development, where organoids serve as more physiologically relevant models compared to traditional 2D cell cultures.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These entities are increasingly adopting organoid technologies to reduce drug attrition rates and accelerate the development pipeline. The cost of bringing a new drug to market exceeds $2.6 billion, with a significant portion attributed to failures in clinical trials due to poor translation from preclinical models. Organoid systems offer potential savings of 15-20% in development costs through improved predictive capabilities.
Academic research institutions represent the second-largest market segment at approximately 30%, focusing on fundamental biological research and disease modeling. The remaining market is distributed among contract research organizations, healthcare providers, and government institutions.
Geographically, North America dominates the market with a 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the fastest growth rate of 25% annually due to increasing research funding and expanding biotechnology sectors.
By application type, cancer research leads with 35% market share, followed by developmental biology (20%), regenerative medicine (15%), infectious disease research (10%), and others (20%). The oncology segment's dominance stems from the critical need for personalized treatment approaches and the ability of tumor organoids to recapitulate patient-specific disease characteristics.
Key market drivers include increasing R&D investments in precision medicine, growing prevalence of chronic diseases, and technological advancements in 3D cell culture techniques. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of reliable disease models for rapid therapeutic development.
Market challenges include high costs associated with organoid culture systems, technical complexities in maintaining long-term cultures, and standardization issues. The average cost per organoid culture ranges from $2,000 to $5,000, presenting accessibility barriers for smaller research institutions and companies in developing regions.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These entities are increasingly adopting organoid technologies to reduce drug attrition rates and accelerate the development pipeline. The cost of bringing a new drug to market exceeds $2.6 billion, with a significant portion attributed to failures in clinical trials due to poor translation from preclinical models. Organoid systems offer potential savings of 15-20% in development costs through improved predictive capabilities.
Academic research institutions represent the second-largest market segment at approximately 30%, focusing on fundamental biological research and disease modeling. The remaining market is distributed among contract research organizations, healthcare providers, and government institutions.
Geographically, North America dominates the market with a 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the fastest growth rate of 25% annually due to increasing research funding and expanding biotechnology sectors.
By application type, cancer research leads with 35% market share, followed by developmental biology (20%), regenerative medicine (15%), infectious disease research (10%), and others (20%). The oncology segment's dominance stems from the critical need for personalized treatment approaches and the ability of tumor organoids to recapitulate patient-specific disease characteristics.
Key market drivers include increasing R&D investments in precision medicine, growing prevalence of chronic diseases, and technological advancements in 3D cell culture techniques. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of reliable disease models for rapid therapeutic development.
Market challenges include high costs associated with organoid culture systems, technical complexities in maintaining long-term cultures, and standardization issues. The average cost per organoid culture ranges from $2,000 to $5,000, presenting accessibility barriers for smaller research institutions and companies in developing regions.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid culture systems, several critical challenges continue to impede progress in this rapidly evolving field. Matrix composition represents a fundamental obstacle, as current extracellular matrices like Matrigel suffer from batch-to-batch variability, undefined composition, and animal origin concerns. This inconsistency directly impacts experimental reproducibility and translational potential, particularly for clinical applications where standardization is paramount.
Scalability remains another significant hurdle, with current protocols struggling to produce organoids in quantities sufficient for high-throughput drug screening or therapeutic applications. The labor-intensive nature of organoid culture, coupled with limited automation capabilities, restricts industrial and clinical implementation. Additionally, the absence of standardized protocols across laboratories introduces variability that complicates cross-study comparisons and validation.
Vascularization deficiency represents perhaps the most physiologically limiting factor in current organoid systems. Without proper blood vessel networks, organoids typically develop necrotic cores as they exceed 200-300μm in diameter, preventing the formation of larger, more complex structures. This limitation restricts nutrient and oxygen diffusion, ultimately constraining organoid size and longevity.
The microenvironmental complexity challenge manifests in difficulties recreating the intricate cellular and molecular interactions present in native tissues. Most current systems lack immune components, stromal elements, and mechanical forces that significantly influence organ development and function. This simplification limits the physiological relevance of organoid models, particularly for studying complex disease processes and immune responses.
Maturation barriers constitute another significant challenge, as organoids often remain in fetal-like states rather than achieving adult tissue functionality. This developmental arrest limits their utility for modeling adult-onset diseases and age-related conditions. The temporal aspects of development remain difficult to accelerate or manipulate in vitro, creating a fundamental disconnect between model systems and clinical reality.
Technical reproducibility issues persist across laboratories, with variations in starting cell populations, culture conditions, and analytical endpoints generating inconsistent results. The absence of standardized quality control metrics further complicates cross-laboratory validation and clinical translation. These challenges collectively highlight the need for more robust, defined, and scalable culture systems to realize the full potential of organoid technology in research and therapeutic applications.
Scalability remains another significant hurdle, with current protocols struggling to produce organoids in quantities sufficient for high-throughput drug screening or therapeutic applications. The labor-intensive nature of organoid culture, coupled with limited automation capabilities, restricts industrial and clinical implementation. Additionally, the absence of standardized protocols across laboratories introduces variability that complicates cross-study comparisons and validation.
Vascularization deficiency represents perhaps the most physiologically limiting factor in current organoid systems. Without proper blood vessel networks, organoids typically develop necrotic cores as they exceed 200-300μm in diameter, preventing the formation of larger, more complex structures. This limitation restricts nutrient and oxygen diffusion, ultimately constraining organoid size and longevity.
The microenvironmental complexity challenge manifests in difficulties recreating the intricate cellular and molecular interactions present in native tissues. Most current systems lack immune components, stromal elements, and mechanical forces that significantly influence organ development and function. This simplification limits the physiological relevance of organoid models, particularly for studying complex disease processes and immune responses.
Maturation barriers constitute another significant challenge, as organoids often remain in fetal-like states rather than achieving adult tissue functionality. This developmental arrest limits their utility for modeling adult-onset diseases and age-related conditions. The temporal aspects of development remain difficult to accelerate or manipulate in vitro, creating a fundamental disconnect between model systems and clinical reality.
Technical reproducibility issues persist across laboratories, with variations in starting cell populations, culture conditions, and analytical endpoints generating inconsistent results. The absence of standardized quality control metrics further complicates cross-laboratory validation and clinical translation. These challenges collectively highlight the need for more robust, defined, and scalable culture systems to realize the full potential of organoid technology in research and therapeutic applications.
Established Protocols and Culture Methodologies
01 3D Organoid Culture Techniques
Three-dimensional organoid culture systems that mimic the structure and function of organs. These systems utilize specialized matrices, scaffolds, and bioreactors to support the growth and differentiation of stem cells into organ-like structures. The techniques enable long-term culture maintenance and provide more physiologically relevant models compared to traditional 2D cell cultures for studying organ development, disease modeling, and drug testing.- 3D Organoid Culture Methods: Three-dimensional organoid culture systems that mimic the structure and function of organs in vitro. These systems typically involve culturing stem cells or progenitor cells in specialized matrices that support self-organization into organ-like structures. The methods include specific growth factors, extracellular matrix components, and culture conditions that promote organoid development, enabling more physiologically relevant models for research and drug testing.
- Stem Cell-Derived Organoids: Techniques for generating organoids from various types of stem cells, including pluripotent stem cells, adult stem cells, and tissue-specific progenitor cells. These methods involve specific differentiation protocols that guide stem cells through developmental pathways to form organ-specific structures. The resulting organoids can recapitulate key aspects of organ development, structure, and function, providing valuable tools for studying human development and disease.
- Organoid Culture Media and Supplements: Specialized media formulations and supplements designed to support organoid growth and maintenance. These include defined combinations of growth factors, hormones, small molecules, and other bioactive compounds that promote cell proliferation, differentiation, and self-organization. The media compositions are often tissue-specific and may include components such as Wnt agonists, R-spondin, EGF, FGF, and BMP inhibitors to recapitulate the native signaling environment of specific organs.
- Extracellular Matrix for Organoid Culture: Various extracellular matrix (ECM) components and scaffolds used to support organoid formation and growth. These include natural matrices like Matrigel, collagen, and laminin, as well as synthetic hydrogels with defined compositions. The ECM provides structural support, facilitates cell-cell interactions, and presents biochemical cues that influence cell behavior and organoid development, allowing for more accurate modeling of tissue architecture and function.
- Applications of Organoid Technology: Diverse applications of organoid culture systems in research, drug development, personalized medicine, and regenerative therapy. Organoids are used for disease modeling, particularly for cancer and genetic disorders, high-throughput drug screening, toxicity testing, and personalized drug response prediction. They also serve as platforms for studying host-pathogen interactions, tissue development, and as potential sources for transplantation and regenerative medicine approaches.
02 Stem Cell-Derived Organoid Systems
Methods for generating organoids from various types of stem cells, including embryonic, induced pluripotent, and adult stem cells. These approaches involve specific growth factors and signaling molecules to direct stem cell differentiation into specialized organoids representing different tissues and organs. The resulting organoids can self-organize to recapitulate key aspects of organ development and function, providing valuable tools for regenerative medicine research.Expand Specific Solutions03 Disease Modeling with Organoid Cultures
Applications of organoid culture systems for modeling human diseases, particularly cancer, genetic disorders, and infectious diseases. Patient-derived organoids can be established from diseased tissues to study disease mechanisms, progression, and response to treatments. These personalized organoid models enable precision medicine approaches by allowing drug screening and identification of effective therapeutic strategies tailored to individual patients.Expand Specific Solutions04 Culture Media and Growth Factor Formulations
Specialized media formulations and growth factor combinations essential for establishing and maintaining organoid cultures. These formulations typically include tissue-specific growth factors, morphogens, and small molecules that support organoid formation, growth, and differentiation. Optimized media compositions are crucial for preserving the stem cell niche, promoting cellular differentiation, and maintaining the structural and functional properties of organoids over extended culture periods.Expand Specific Solutions05 High-Throughput Organoid Screening Platforms
Advanced technologies and platforms for high-throughput screening using organoid cultures. These systems integrate automated culture maintenance, imaging, and analysis to enable large-scale drug screening, toxicity testing, and functional studies. The platforms may incorporate microfluidic devices, specialized culture vessels, and computational tools for data analysis, facilitating efficient screening of compounds and biological agents using physiologically relevant organoid models.Expand Specific Solutions
Leading Research Institutions and Biotech Companies
The organoid culture systems market is currently in a growth phase, characterized by increasing adoption across research and clinical applications. The global market size is expanding rapidly, driven by advancements in personalized medicine and drug discovery. Technologically, the field shows moderate maturity with established protocols but significant room for innovation. Leading players include Molecular Devices, which provides advanced imaging and analysis platforms, Cell Microsystems with its single-cell isolation technologies, and STEMCELL Technologies offering specialized culture media. Academic institutions like Johns Hopkins University and Memorial Sloan Kettering Cancer Center contribute significant research advancements, while companies like Tempus AI are integrating AI capabilities. The competitive landscape features both specialized biotech firms and larger pharmaceutical companies like Takeda, indicating the technology's growing commercial importance across multiple healthcare sectors.
The Regents of the University of California
Technical Solution: The University of California system has developed multiple innovative organoid culture technologies across its campuses. UC San Diego researchers pioneered brain organoid systems incorporating microfluidic perfusion to overcome size limitations caused by diffusion constraints, enabling development of organoids exceeding 4mm in diameter with reduced necrotic cores. At UCSF, scientists established air-liquid interface cerebral organoids with enhanced cortical development through optimized extracellular matrix composition and growth factor gradients. UCLA teams developed gastrointestinal organoid platforms featuring modular extracellular matrix components that allow independent tuning of stiffness and biochemical properties. Their approach includes specialized differentiation protocols that achieve region-specific intestinal phenotypes through temporal modulation of BMP, Wnt, and Notch signaling pathways. UC Berkeley researchers implemented micropattern-guided organoid formation techniques that enhance reproducibility by controlling initial cell positioning and subsequent morphogenesis, reducing batch-to-batch variability in organoid structure and function.
Strengths: Cutting-edge engineering approaches to overcome fundamental limitations in organoid culture; strong interdisciplinary collaboration between biological and physical sciences; extensive intellectual property portfolio. Weaknesses: Technologies often remain in academic development stage with limited commercialization; protocols can be complex and require specialized expertise; some approaches have limited validation across multiple tissue types.
STEMCELL Technologies Canada, Inc.
Technical Solution: STEMCELL Technologies has developed comprehensive organoid culture systems featuring specialized extracellular matrices and defined media formulations. Their IntestiCult™ Organoid Growth Medium (OGM) provides essential growth factors and small molecules that mimic the intestinal stem cell niche, enabling long-term expansion of intestinal organoids. The company's technology incorporates Matrigel-based 3D culture systems with optimized Wnt, R-spondin, and Noggin signaling pathways to maintain stemness while promoting differentiation into functional epithelial cell types. Their PneumaCult™ platform similarly supports airway organoid development through precise modulation of Notch signaling and specialized air-liquid interface techniques. STEMCELL has also pioneered cryopreservation protocols specifically optimized for organoid recovery, maintaining genomic stability and functional characteristics across multiple passages.
Strengths: Industry-leading defined media formulations with batch-to-batch consistency; comprehensive product ecosystem spanning isolation to analysis; extensive validation across multiple organoid types. Weaknesses: Relatively high cost compared to lab-made alternatives; dependence on animal-derived matrices limits clinical translation; proprietary formulations create potential vendor lock-in for researchers.
Key Biomaterials and Signaling Pathways
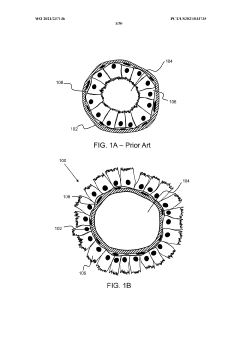
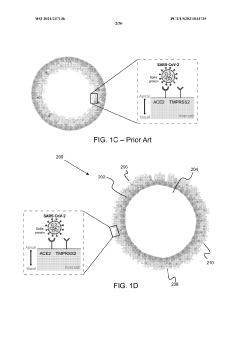
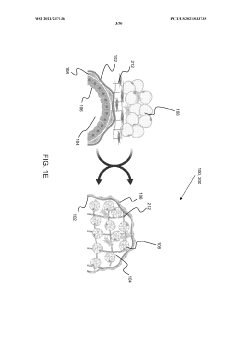
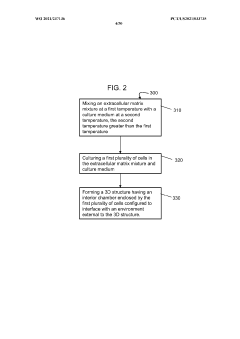
Stably-inverted organoids and methods of producing and using the same
PatentWO2021237136A2
Innovation
- The development of stably-inverted organoids with a hollow lumen and epithelial cells exposed to the exterior surface, allowing easier access for invasion studies, is achieved by creating a 3D structure with a tissue layer and an opposing surface where the first plurality of cells, including epithelial cells, interface with the environment externally, and an extracellular matrix mixture forms the interior chamber.
Methods for engineering and use of ciliated organoids having native-like, apical-out polarity
PatentWO2023288160A2
Innovation
- Culturing basal cell clusters in suspension without extracellular matrix support to generate apical-out ciliated organoids, which allows for reversed polarity and enables direct interaction with respiratory pathogens and environmental agents, facilitating investigation of lung biology and respiratory pathology.
Ethical and Regulatory Framework
The rapid advancement of organoid culture systems has necessitated the development of comprehensive ethical and regulatory frameworks to guide research and applications in this field. As these three-dimensional cellular structures increasingly mimic human organs' functionality, they raise profound ethical questions regarding consent, ownership, and the boundaries of research. Current regulatory approaches vary significantly across jurisdictions, with some countries implementing specific guidelines for organoid research while others rely on broader bioethical frameworks originally designed for stem cell research.
The informed consent process represents a critical ethical cornerstone in organoid research, particularly when human-derived tissues are utilized. Donors must receive clear information about potential commercial applications, long-term storage, and the possibility of generating brain organoids that may develop rudimentary neural activity. Several international bioethics committees have proposed standardized consent protocols specifically tailored to organoid research, emphasizing transparency regarding genetic modification procedures and potential clinical applications.
Data privacy considerations have emerged as another significant regulatory challenge, especially as organoid biobanks expand globally. These repositories must implement robust safeguards to protect donor identities while facilitating scientific collaboration. The European Union's General Data Protection Regulation (GDPR) has become an influential model for managing sensitive biological data associated with organoid research, though harmonization across international boundaries remains problematic.
The ethical implications of cerebral organoids demand particular regulatory attention due to their potential to develop primitive neural networks. Current scientific consensus maintains that existing cerebral organoids lack consciousness or sentience, yet regulatory frameworks increasingly incorporate precautionary principles as technology advances. Several jurisdictions have established specialized ethics review boards specifically for evaluating research involving neural organoids, with mandatory periodic reassessments as these models become more sophisticated.
Commercial applications of organoid technology have introduced additional regulatory complexities regarding intellectual property rights and commercialization pathways. Patent landscapes surrounding organoid technologies remain contentious, with ongoing debates about whether naturally occurring biological structures should be patentable. Regulatory agencies like the FDA and EMA are developing specialized approval pathways for organoid-based drug screening platforms, focusing on validation standards and reproducibility requirements.
Looking forward, the establishment of international harmonization efforts will be crucial for addressing regulatory fragmentation in this rapidly evolving field. Organizations such as the International Society for Stem Cell Research have begun developing global guidelines specifically addressing organoid research ethics, aiming to balance innovation with appropriate safeguards for human dignity and welfare.
The informed consent process represents a critical ethical cornerstone in organoid research, particularly when human-derived tissues are utilized. Donors must receive clear information about potential commercial applications, long-term storage, and the possibility of generating brain organoids that may develop rudimentary neural activity. Several international bioethics committees have proposed standardized consent protocols specifically tailored to organoid research, emphasizing transparency regarding genetic modification procedures and potential clinical applications.
Data privacy considerations have emerged as another significant regulatory challenge, especially as organoid biobanks expand globally. These repositories must implement robust safeguards to protect donor identities while facilitating scientific collaboration. The European Union's General Data Protection Regulation (GDPR) has become an influential model for managing sensitive biological data associated with organoid research, though harmonization across international boundaries remains problematic.
The ethical implications of cerebral organoids demand particular regulatory attention due to their potential to develop primitive neural networks. Current scientific consensus maintains that existing cerebral organoids lack consciousness or sentience, yet regulatory frameworks increasingly incorporate precautionary principles as technology advances. Several jurisdictions have established specialized ethics review boards specifically for evaluating research involving neural organoids, with mandatory periodic reassessments as these models become more sophisticated.
Commercial applications of organoid technology have introduced additional regulatory complexities regarding intellectual property rights and commercialization pathways. Patent landscapes surrounding organoid technologies remain contentious, with ongoing debates about whether naturally occurring biological structures should be patentable. Regulatory agencies like the FDA and EMA are developing specialized approval pathways for organoid-based drug screening platforms, focusing on validation standards and reproducibility requirements.
Looking forward, the establishment of international harmonization efforts will be crucial for addressing regulatory fragmentation in this rapidly evolving field. Organizations such as the International Society for Stem Cell Research have begun developing global guidelines specifically addressing organoid research ethics, aiming to balance innovation with appropriate safeguards for human dignity and welfare.
Scalability and Standardization Issues
Scalability and standardization remain critical bottlenecks in the widespread adoption of organoid culture systems across research and clinical applications. Current organoid protocols exhibit significant batch-to-batch variability, with success rates often dependent on technician expertise rather than standardized procedures. This inconsistency poses substantial challenges for pharmaceutical companies seeking reliable drug screening platforms and clinical laboratories requiring reproducible diagnostic tools.
The fundamental challenge lies in the inherent complexity of organoid formation processes. Unlike traditional cell culture systems, organoids develop through intricate self-organization mechanisms that remain partially understood. Environmental factors including matrix composition, growth factor concentrations, and physical parameters all influence organoid development in ways that are difficult to precisely control across different laboratories and production batches.
Matrix components present particular standardization challenges. Commonly used matrices like Matrigel derive from mouse sarcoma cells, introducing inherent batch variability and raising regulatory concerns for clinical applications. Synthetic alternatives have emerged but struggle to replicate the complex biochemical and mechanical properties that natural matrices provide for proper organoid development.
Growth factor delivery systems similarly lack standardization. Current protocols typically involve periodic media changes with freshly added factors, creating fluctuating concentration gradients rather than the steady-state conditions found in vivo. Advanced microfluidic and controlled-release technologies show promise but remain technically complex and difficult to implement at scale.
Scale-up efforts face additional hurdles related to oxygen and nutrient diffusion limitations. As organoids grow beyond certain dimensions (typically 300-500μm), central necrosis occurs due to diffusion constraints. Various engineering solutions including spinner flasks, perfusion bioreactors, and microfluidic devices have been developed, but each introduces new variables that complicate standardization efforts.
Automated systems for organoid culture represent a promising approach to addressing both scalability and standardization challenges. Robotic platforms can perform consistent seeding, media changes, and monitoring with minimal human intervention. However, these systems require substantial capital investment and specialized expertise, limiting their accessibility to well-resourced institutions.
Quality control metrics present another standardization gap. Unlike conventional cell lines, organoids lack universally accepted parameters for defining "normal" versus "abnormal" development. Emerging technologies including high-content imaging, metabolomic profiling, and single-cell sequencing offer potential solutions but have yet to be integrated into standardized quality control workflows accessible to most laboratories.
The fundamental challenge lies in the inherent complexity of organoid formation processes. Unlike traditional cell culture systems, organoids develop through intricate self-organization mechanisms that remain partially understood. Environmental factors including matrix composition, growth factor concentrations, and physical parameters all influence organoid development in ways that are difficult to precisely control across different laboratories and production batches.
Matrix components present particular standardization challenges. Commonly used matrices like Matrigel derive from mouse sarcoma cells, introducing inherent batch variability and raising regulatory concerns for clinical applications. Synthetic alternatives have emerged but struggle to replicate the complex biochemical and mechanical properties that natural matrices provide for proper organoid development.
Growth factor delivery systems similarly lack standardization. Current protocols typically involve periodic media changes with freshly added factors, creating fluctuating concentration gradients rather than the steady-state conditions found in vivo. Advanced microfluidic and controlled-release technologies show promise but remain technically complex and difficult to implement at scale.
Scale-up efforts face additional hurdles related to oxygen and nutrient diffusion limitations. As organoids grow beyond certain dimensions (typically 300-500μm), central necrosis occurs due to diffusion constraints. Various engineering solutions including spinner flasks, perfusion bioreactors, and microfluidic devices have been developed, but each introduces new variables that complicate standardization efforts.
Automated systems for organoid culture represent a promising approach to addressing both scalability and standardization challenges. Robotic platforms can perform consistent seeding, media changes, and monitoring with minimal human intervention. However, these systems require substantial capital investment and specialized expertise, limiting their accessibility to well-resourced institutions.
Quality control metrics present another standardization gap. Unlike conventional cell lines, organoids lack universally accepted parameters for defining "normal" versus "abnormal" development. Emerging technologies including high-content imaging, metabolomic profiling, and single-cell sequencing offer potential solutions but have yet to be integrated into standardized quality control workflows accessible to most laboratories.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!